Published online Aug 19, 2020. doi: 10.5498/wjp.v10.i8.175
Peer-review started: February 29, 2020
First decision: April 29, 2020
Revised: May 31, 2020
Accepted: June 27, 2020
Article in press: June 27, 2020
Published online: August 19, 2020
Processing time: 166 Days and 0.1 Hours
Major depressive disorder (MDD) is a global health issue that affects 350 million people of all ages. Although between 2% and 5.6% of affected individuals are adolescents, research on young patients is limited. The inflammatory response contributes to the onset of depression, and in adult MDD patients, symptom severity has been linked to chemokine levels.
To determine the differences in circulatory levels of chemokines in healthy volunteers (HVs) and adolescents with MDD, and assess the changes induced by fluoxetine consume.
The 22 adolescents with MDD were monitored during the first 8 wk of clinical follow-up and clinical psychiatric evaluation was done using the Hamilton depresión rating scale (HDRS). The serum levels of monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein (MIP)-1α, MIP-1β, interleukin (IL)-8, interferon gamma-induced protein (IP)-10, and eotaxin were measured in patients and HVs.
In all cases, significant differences were detected in circulating chemokine levels between patients before treatment and HVs (P < 0.0001). All chemokines decreased at 4 wk, but only MCP-1 and IL-8 significantly differed (P < 0.05) between 0 wk and 4 wk. In the patients, all chemokines rose to their initial concentrations by 8 wk vs 0 wk, but only IP-10 did so significantly (P < 0.05). All patients experienced a significant decrease in HDRS scores at 4 wk (P < 0.0001) and 8 wk (P < 0.0001) compared with 0 wk.
Despite the consumption of fluoxetine, patients had significantly higher chemokine levels, even after considering the improvement in HDRS score. The high levels of eotaxin, IP-10, and IL-8 partially explain certain aspects that are affected in MDD such as cognition, memory, and learning.
Core tip: Major depressive disorder affects 350 million persons, of whom approximately 6% are adolescents. This study determined the differences in circulatory levels of chemokines including eotaxin, interleukin-8, induced protein 10, monocyte chemoattractant protein-1, macrophage inflammatory protein-1α, and macrophage inflammatory protein-1β in healthy volunteers and adolescents with major depression. In addition, the changes induced by antidepressants consumed during 8 wk of clinical follow-up were assessed, which is the minimum time to observe the therapeutic efficacy of selective serotonin reuptake inhibitors, such as fluoxetine in adolescents. Our results showed significant elevation in serum chemokine levels in adolescents with major depression despite treatment with fluoxetine, and an improvement in scores on the Hamilton depresión rating scale.
- Citation: de la Peña FR, Cruz-Fuentes C, Palacios L, Girón-Pérez MI, Medina-Rivero E, Ponce-Regalado MD, Alvarez-Herrera S, Pérez-Sánchez G, Becerril-Villanueva E, Maldonado-García JL, Jiménez-Martínez MC, Pavón L. Serum levels of chemokines in adolescents with major depression treated with fluoxetine. World J Psychiatr 2020; 10(8): 175-186
- URL: https://www.wjgnet.com/2220-3206/full/v10/i8/175.htm
- DOI: https://dx.doi.org/10.5498/wjp.v10.i8.175
Major depressive disorder (MDD) is a significant international health problem affecting 350 million people of all ages globally[1]. Studies worldwide have reported prevalence rates of depression between 2.8% and 5.6% in adolescents[2], yet research on this clinical condition is limited. The depressive symptomatology in adolescents can be confused with other psychiatric disorders, in turn affecting the proper diagnosis and normal development of patients. MDD in adolescents is associated with several negative outcomes including other psychiatric disorders later in life, educational impairment, self-injury, and suicide[2,3].
The inflammatory response contributes to the onset of depression, as demonstrated by several meta-analyses[4,5]. Three pathways interacting with the central nervous system (CNS) have been described through the peripheral inflammatory response[6,7]. The first are the inflammatory factors that directly diffuse into the brain through leakage of the blood-brain barrier; the second pathway is when circulatory inflammatory factors indirectly act to stimulate the secretion of central inflammatory factors through vagal afferent nerves; the last pathway is when CNS cells directly produce inflammatory molecules and express their receptors. The first two possible ways help explain that the peripheral administration of cytokines can produce depressive-like symptoms, while the third way suggests that high levels of cytokines in the CNS may be strongly associated with the development of MDD, and that the circulatory cytokines levels correlate with cytokine levels in the CNS[8].
These findings have prompted interest in soluble inflammatory molecules, called chemokines[9]. Chemokines are considered a subtype of cytokines based on their functions during inflammation, although they differ structurally from cytokines. Canonically, chemokines favor the migration of leukocytes towards inflamed tissues along a concentration gradient, modulating cellular inflammatory responses[10]. In addition, clinical evidence has demonstrated that chemokines are produced by astrocytes, microglia, and neurons[11]. Chemokines and their receptors have significant functions in the CNS, for example, in brain development, plasticity, cell-to-cell communication, neurotransmission, neuroendocrinology, inflammatory processes, and behavioral regulation[9,12].
A wide range of chemokines have been implicated in the develop of depression and depression-like behavior[9,12-15]. Vogelzangs et al[16] reported an association between interleukin (IL)-8, monocyte chemoattractant protein-1 (MCP-1), and matrix metalloproteinase-2 serum levels and depressive symptom severity in 591 patients. Nevertheless, few studies have evaluated serum chemokine levels in adolescents with MDD before and during clinical follow-up. Therefore, these inflammatory molecules have been proposed to be biological markers of MDD by several groups[17,18].
The objective of this work was to determine the differences in circulatory levels of eotaxin or C-C motif chemokine ligand 11 (CCL11), IL-8 or C-X-C motif chemokine ligand 8 (CXCL8), IP-10 or CXCL10, MCP-1 or CCL2, macrophage inflammatory protein (MIP)-1α or CCL3, and MIP-1β or CCL4 in healthy volunteers (HVs) and adolescents with MDD; and to assess the changes induced by antidepressants consumed during 8 wk of clinical follow-up, which is the minimum time to observe the therapeutic efficacy of selective serotonin reuptake inhibitors (SSRIs), as fluoxetine, in adolescents[19].
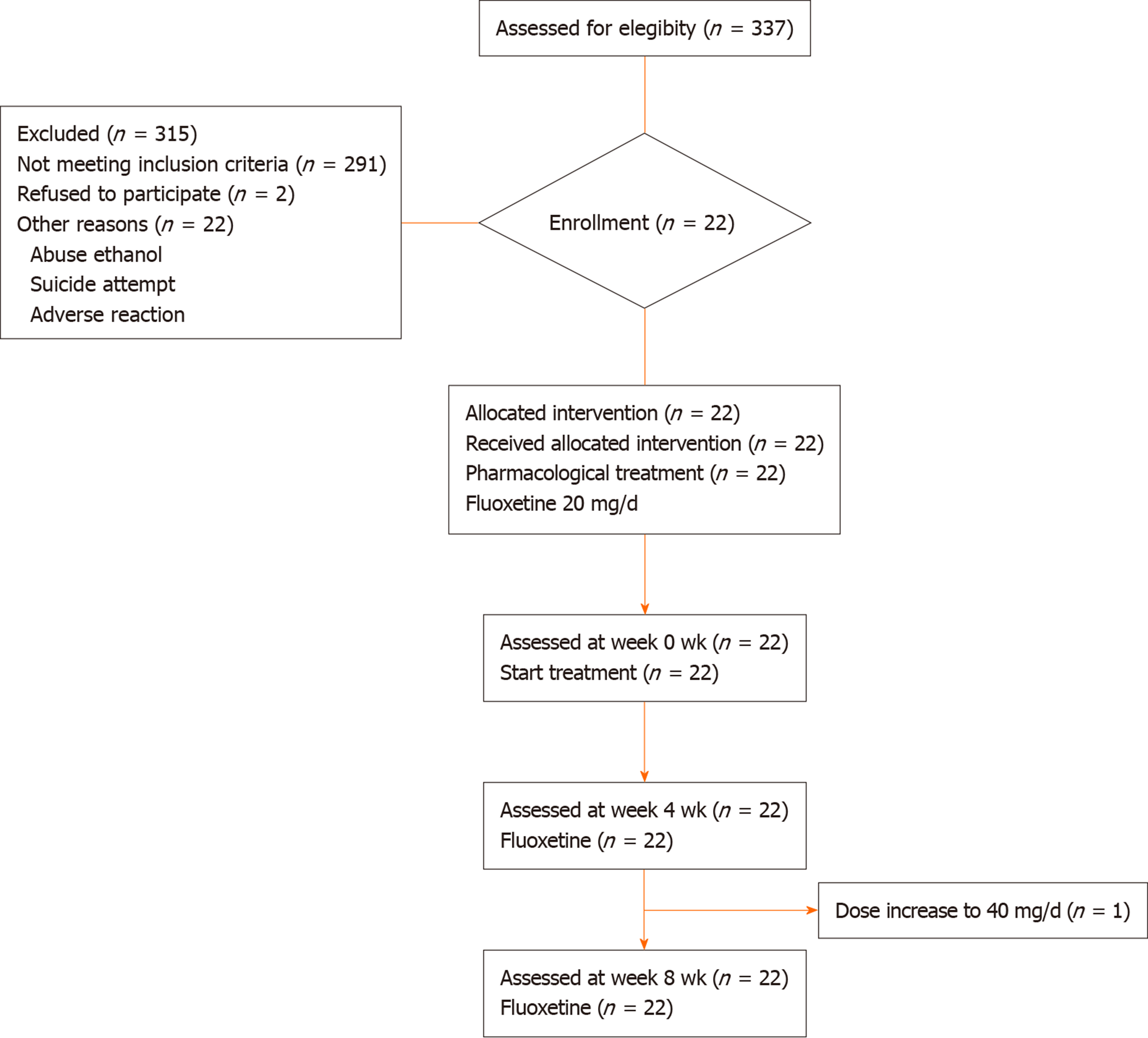
Patients: The Adolescent Clinic of Instituto Nacional de Psiquiatría “Ramón de Fuente,” Mexico City, assessed 337 individuals and recruited 22 Mexican patients who met the inclusion criteria from January 2006 to December 2008. The patients were men and women aged 14 to 19 years, who met the diagnostic criteria for MDD per the Diagnostic and Statistical Manual of Mental Disorders IV - text revision, and whose episodes at the moment of the study were moderate. They had no history of treatment for MDD with SSRIs, the duration of their depressive episodes was no longer than 2 years, and their minimum baseline score on the Hamilton depresión rating scale (HDRS) was ≥ 14.
All included participants received an explanation and signed informed consent forms to participate in this study (INPRF-2035). They were recruited per the clinical follow-up in the INPRF-2035 research protocol, as approved by the ethics committee of Instituto Nacional de Psiquiatría, “Ramón de la Fuente Muñiz” Mexico.
Healthy volunteers: Eighteen HVs were recruited from the general population between January 2006 and December 2008. Clinical parameters of HVs were within normal reference ranges (data not shown). The Mini International Neuropsychiatric Interview confirmed that HVs did not suffer from any mental disorder, and all had been free of any medication use by 3 wk before blood sampling. Demographic data of this group are shown in Table 1.
| HVs, n = 18 | Patients, n = 22 | |
| Age in yr | 18.9 ± 1.2 | 17.1 ± 2.3 |
| Gender, male/female | 4/14 | 4/18 |
| BMI in kg/m2 | 23.2 ± 2.1 | 23.1 ± 2.1 |
| Education in yr | 12.9 ± 1.2 | 11.5 ± 2.6 |
| Family history, yes/no | 3/15 | 8/14 |
| First episode | N/A | 8 |
| Recurrent episode | N/A | 14 |
Psychiatrists diagnosed all subjects, and the clinical status of adolescents with MDD was determined using the validated Spanish version of the 21-item HDRS-21. Patients were free of antidepressants by 3 wk before the study and signed written consent forms only after receiving a detailed explanation of the study aims. All participants were treated with SSRIs. At the screening visit, after being administered the HDRS test, each participant underwent a laboratory examination to rule out any medical illness. Participants were evaluated every month along with clinical follow-up by their psychiatrists, who applied the HDRS test. Figure 1 represents a summary of the number of participants, the pharmacological treatment, and the changes to the protocol.
Fluoxetine dose was 20 mg/d, which was established and adjusted by the psychiatrists. The medications used by patients were paid for by themselves.
Blood samples (10 mL) were collected by venipuncture from the cubital vein using Vacutainer® SST™ tubes with gel for serum separation (REF: 367988; BD Vacutainer System, Franklin Lakes, NJ, United States). Then samples were centrifuged immediately (1.125 × g) at 4°C for 15 min to obtain serum. Serum samples were aliquoted and stored at -80°C until use.
The serum levels of eotaxin, IL-8, IP-10, MCP-1, MIP-1α, and MIP-1β were measured in serum using the Bio-Plex Pro™ Human Cytokine 27-Plex Assay Kit per the manufacturer’s instructions. Detection was performed using streptavidin-phycoerythrin and quantified on the Bio-Plex MAGPIX™ Multiplex Reader (Bio-Rad Laboratories Inc., Hercules, CA, United States). Chemokine concentrations were determined by interpolation using standard curves in Bio-Plex Manager™ (version 6.1; Bio-Rad Laboratories Inc.). The ranges of detection (pg/mL) reported by the manufacturer were as follows: Eotaxin: 2.1-24390, IL-8: 1.9-22233, IP-10: 3.0-34730, MCP-1: 2.0-23446, MIP-1α: 1.5-1773, and MIP-1β: 1.1-13533.
Statistical analysis was performed using GraphPad Prism, version 6.00 for MAC OS X (GraphPad Software, La Jolla, CA, United States). Homogeneity of variance and normality tests were applied initially, followed by one-way analysis of variance with the Bonferroni post hoc test. All values were expressed as mean ± standard deviation (SD). P < 0.05 was considered statistically significant.
Clinical and laboratory tests were performed in MDD adolescents and HVs in the clinical laboratory. The complete blood chemistry, blood count, complete urinalysis, and thyroid function test (T3, T4, and TSH) results were within normal reference value ranges. No differences between the groups were detected. Table 1 shows their demographics and recurrence rates, while psychiatric scale scores are presented in Table 2. At 0 wk, MDD patients had an HDRS score of 19.41 ± 4.72 points, compared with 9.13 ± 3.5 at 4 wk and 6.09 ± 2.4 at 8 wk.
| Chemokine serum levels, pg/mL | HVs | Adolescents with MDD | Post hoc statistical analysis | ||||
| n = 18 | 0 wk, n = 22 | 4 wk, n = 22 | 8 wk, n = 22 | 0 wk vs HVs | 4 wk vs 0 wk | 8 wk vs 0 wk | |
| MCP-1 or CCL2 | 48.1 ± 3.7 | 107.7 ± 31.0 | 89.8 ± 19.9 | 102.9 ± 14.0 | dP < 0.0001 | aP < 0.05 | NS |
| MIP-1α or CCL3 | 5.4 ± 1.0 | 7.3 ± 1.4 | 6.7 ± 1.4 | 7.4 ± 1.0 | dP < 0.0001 | NS | NS |
| MIP-1β or CCL4 | 204.7 ± 42.2 | 324.0 ± 61.5 | 311.8 ± 48.1 | 294.8 ± 34.8 | dP < 0.0001 | NS | NS |
| IL-8 or CXCL8 | 50.2 ± 6.8 | 79.2 ± 18.0 | 66.9 ± 13.2 | 73.3 ± 9.1 | dP < 0.0001 | NS | NS |
| IP-10 or CXCL10 | 1890.0 ± 52.7 | 2309.0 ± 545.1 | 2224.0 ± 578.5 | 2710.0 ± 220.1 | aP < 0.05 | NS | aP < 0.05 |
| Eotaxin or CCL11 | 255.4 ± 50.4 | 319.8 ± 65.5 | 277.2 ± 51.8 | 328.6 ± 50.7 | bP < 0.01 | NS | NS |
| Clinical psychiatric scale | |||||||
| HDRS | N/A | 19.4 ± 4.7 | 9.1 ± 4.72 | 6.0 ± 2.4 | N/A | dP < 0.0001 | dP < 0.0001 |
The chemokine levels are shown in Table 2.
MCP-1 is produced constitutively after induction by oxidative stress, cytokines, or growth factors and secreted by several types of cells, including monocytes, smooth muscle cells, and endothelial cells[20]. We observed significant differences in serum MCP-1 levels between HVs and patients (HV vs 0 wk, 4 wk, and 8 wk) (F = 34.03, dƒ = 80.3, P < 0.0001). Before beginning pharmacological treatment (0 wk), patients showed significantly higher MCP-1 levels compared with HVs (P < 0.0001). Notably, MCP-1 declined in patients after 4 wk of fluoxetine administration (0 wk vs 4 wk, P < 0.026) but increased at 8 wk to similar levels as in 0 wk.
Normally, MIP-1α is expressed by stromal and hematopoietic stem cells and participates in cell adhesion, migration, and chemotaxis of monocytes, lymphocytes, dendritic cells, eosinophils, and natural killer (NK) cells. It is also involved in the inflammatory process, inhibits stem cell proliferation, and is a potent osteoclast activator[21]. This protein recruits and stimulates mononuclear phagocytes in the CNS, microglia, and astrocytes, which are sources of this chemokine[22]. Serum MIP-1α levels were higher at all time points in patients compared with HVs (F = 10.01, dƒ = 80.3, P < 0.0001), but not between the 8 wk clinical follow-up (4 and 8 wk) and the beginning of the pharmacological treatment (0 wk).
MIP-1β has chemoattractive properties toward several cell types including immature dendritic cells, macrophages, monocytes, and NK cells. In addition, it induces calcium mobilization in NK cells, monocytes, leukocytes, vascular smooth muscle cells, and progenitor B cells[23]. We observed significantly higher levels of MIP-1β in patients vs HVs (F = 23.55, dƒ = 80.3, P < 0.0001) but no differences throughout the clinical follow-up (0, 4, and 8 wk).
IL-8 is a chemoattractant for neutrophils and lymphocytes in inflammatory processes. We noted significant differences in serum IL-8 levels between HVs and patients during the clinical follow-up (F = 12.74, dƒ = 80.3; P < 0.0001). Patients experienced a significant decrease in IL-8 from 0 wk to 4 wk (P < 0.01), which increased at 8 wk to similar levels as those at 0 wk.
IP-10 is a T helper cell 1-related chemokine identified as an early-response gene induced by interferon (IFN)-γ in U937 monocytes[24]. The IP-10 and IFN-γ levels detected in these same patients[25] showed a correlation (r = 0.6147, P < 0.0001), while IP-10 levels were higher in patients (0, 4, and 8 wk) vs HVs (F = 12.74, dƒ = 80.3, P < 0.0001). However, IP-10 levels significantly increased in patients only at 8 vs 0 wk (P < 0.01).
Eotaxin is the most potent and selective factor that modulates the function of eosinophils and mediates the infiltration of eosinophils into the airway[26]. Eotaxin is secreted primarily from bronchial epithelial cells, eosinophils, and endothelial cells. In our patients, serum eotaxin levels were higher than HVs at 0, 4, and 8 wk (F = 8.00, dƒ = 80.3, P < 0.0001). Serum eotaxin levels did not vary in patients throughout the clinical follow-up vs 0 wk.
Disturbances in serum chemokine levels are associated with the onset of major depression[9,12-14,27,28]. These inflammatory molecules affect cell-cell interactions, neuromodulation, and synaptic transmission, all of which are altered in MDD patients[13]. Although, a relationship between serum and cerebrospinal fluid levels of chemokines has not been demonstrated, the expression of some chemokines, chemokine ligands, and receptors in developing and mature CNS has been described under both physiological and inflammatory conditions. Astrocytes, oligodendrocytes, and microglia, as well as neuronal cells, constitutively release several chemokines including MCP-1, MIP-1α, MIP-3, secondary lymphoid-tissue chemokine, IP-10, and fractalkine[29,30].
Chemokines play an important role in the regulation of neuronal development and plasticity, proliferation, migration, and neural progenitor cell differentiation. For example, fractalkine regulates microglial synaptic pruning of mature neurons, modulates several neurotransmitters, and regulates the activation state of microglia[29,30]. Therefore, these molecules influence the development and plasticity of the CNS. Exogenous application of fractalkine enhances in vivo neurogenesis in aged rats by modulating the microglia phenotype. Other chemokines such as MCP-1, CCL21, and monokine induced by IFN-γ (MIG), promote neuronal differentiation, whereas MCP-1, growth-regulated oncogene-α, and MIG favor oligodendrocyte differentiation. Also, chemokines present a unique class of neurotransmitters and neuromodulators that regulate cell survival and synaptic transmission. Fractalkine, which co-localize with serotonin in neurons of the dorsal raphe nucleus, may indirectly inhibit serotonin neurotransmission by upregulating the sensitivity of serotonin dorsal raphe nucleus neurons to gamma-aminobutyric acid inputs. Furthermore, the results from electrophysiological studies suggest that MCP-1, regulated upon activation, normal T cell expressed and presumably secreted, macrophage-derived chemokine, MCP-5, IL-8, and fractalkine chemokines can modulate electrical activity in the cortical, cerebral, hippocampal, and hypothalamic neurons[29,30]. Given the number of functions in which chemokines and their receptors are involved in the CNS, they could become novel diagnostic markers or therapeutic targets for MDD, although a more significant number of studies is required.
In this work we measured serum levels of eotaxin, IL-8, IP-10, MCP-1, MIP-1α, and MIP-1β levels in adolescents with MDD throughout 8 wk of clinical follow-up. Although other groups have reported an association between peripheral chemokine levels and the severity of depressive symptoms, there are discrepancies in their results. Furthermore, our study is the first to report the chemokine profiles in adolescents with MDD.
Levels of MCP-1 were high in adolescents with MDD without treatment vs HVs, consistent with a meta-analysis[14]. Longitudinal studies, such as those by Sutcigil et al[31], have reported similar values for MCP-1 in HVs. Their results showed MCP-1 levels of 84.54 ± 12.54 pg/mL in HVs with a mean age of 34 treated with sertraline (another SSRI) for 8 wk, after which MCP-1 levels fell by over 50%[31]. In our clinical sample, posttreatment MCP-1 levels reached pretreatment values. Piletz et al[32] reported similar findings to ours, recording high levels of MCP-1 in MDD patients (205 ± 19 pg/mL) before treatment compared with HVs (132 ± 23 pg/mL). Their patients (mean age 39) were treated with venlafaxine, a dual antidepressant, for 8 wk after which MCP-1 levels remained constant[32].
Other groups, as Simon et al[17], reported high levels of MCP-1 in MDD patients before treatment. However, their results, in subjects with a mean age of 42, had a wide SD for HVs (56.66 ± 106.19 pg/mL) and MDD patients (191.00 ± 381.69 pg/mL)[17]. Moreover, four reports failed to detect changes in MCP-1 between patients and HVs[33-36]. All of these studies generated results from patients and controls aged over 40 and were included in the meta-analysis by Eyre et al[14]. Lehto et al[13] observed lower MCP-1 levels (27.19 pg/mL) in MDD patients vs HVs (40.79 pg/mL); these data were obtained in 61 MDD patients with a mean age of 54.
An in vitro study reported that the severity of depressive symptoms is significant and positively associated with the overexpression of MCP-1 and IL-8 in lipopolysaccharide (LPS)-stimulated human blood monocytes[37], which partially explains the MCP-1 levels in our patients. Our data and those of Piletz et al[32] suggest that the administration of dual or selective antidepressants is insufficient to reduce MCP-1 levels in MDD patients after 8 wk, although Arreola et al[38] had contradictory findings. The significant decrease in MCP-1 in our patients after 4 wk with fluoxetine treatment suggests a partial effect of SSRIs on circulatory levels of this chemokine secreted by macrophages, which constitutively express serotonin transporter[38]. High levels of MCP-1 have been suggested to be early-stage indicators of endothelial damage and have been detected in coronary artery disease-associated chronic renal failure[32].
Levels of MIP-1α were high in untreated adolescents with MDD compared with HVs and remained unaltered after 8 wk of fluoxetine treatment. No other longitudinal studies exist on this chemokine. Other groups have compared serum MIP-1α levels between adult patients and HVs, similar to Simon et al[17]. However, the values had a wide SD for HVs (60.33 ± 95.91 pg/mL) and MDD patients (463.8 ± 706.88 pg/mL) while Merendino et al[22] only detected MIP-1α in two of nine patients. Therefore, more studies on this chemokine in mood disorders are needed.
Although MIP-1β has a similar response to MIP-1α, Lehto et al[13] reported significant lower levels of MIP-1β (45.41 ± 95.91 pg/mL) in 61 MDD patients with a mean age of 53 regarding HV levels (76.31 ± 9.84 pg/mL). Given the scarce information on this inflammatory protein, additional studies are necessary.
IL-8 was elevated in adolescents with MDD without treatment compared with HVs, remaining at similar values despite fluoxetine treatment for 8 wk. A 2-year study including 1600 geriatric MDD patients in the Netherlands and Australia[16,39] reported that serum IL-8 levels correlate with depressive symptoms. No subsequent longitudinal studies have been performed. Other studies have reported high circulatory levels of IL-8 in patients with MDD[17,40].
In contrast, the meta-analysis by Eyre et al[14], which included seven studies from 1998 to 2012, assessed 560 MDD patients and concluded that there was no significant difference in serum levels of IL-8 between MDD patients and HVs (P < 0.228). An in vitro study by Suarez et al[37] reported that the severity of depressive symptoms was significant and positively associated with the overexpression of MCP-1 and IL-8 in LPS-stimulated human blood monocytes, which in part explains the IL-8 levels in our patients. IL-8 has been linked to low cognitive performance, suggesting that it has several effects on the CNS[39]. Notably, IL-8 crosses the blood-brain barrier in infectious processes[41].
Our patients had significantly higher and constant levels of IP-10 than HVs during the first 4 wk of fluoxetine treatment; however, IP-10 rose significantly vs pretreatment levels by 8 wk and thus was the only molecule with this pattern. In contrast, Wong et al[42] reported differences in plasma levels of IP-10 in HVs (67.61 ± 1.16 pg/mL) and MDD patients (95.50 ± 1.16 pg/mL) before pharmacological treatment, whereas MDD patients experienced a decrease in IP-10 at 8 wk after beginning treatment with antidepressants (desipramine or fluoxetine). Further studies will be required to determine the behavior of this molecule in MDD patients.
In an in vitro study, Tsai et al[24] demonstrated that fluoxetine inhibits the secretion of IP-10 in TPH-1 cells (a cell line derived from human macrophages) that have been stimulated with LPS, in a process that is dependent on the mitogen-activated protein kinase-38 pathway. In contrast, after 8 wk on fluoxetine, IP-10 increased in our adolescents with MDD. These results are notable due to high levels of IP-10, an angiostatic factor with antifibrotic properties[9].
In a comparison between patients and HVs, we observed a significant elevation in serum eotaxin levels, which remained high throughout the clinical follow-up. There are no other longitudinal reports on this chemokine; still, there are three transversal studies showing that MDD patients had significantly higher circulatory levels of eotaxin than HVs before pharmacological treatment[17,34,43].
It has been reported that plasma eotaxin levels are related with reduced neurogenesis in aged mice and that eotaxin levels rise in blood and cerebrospinal fluid of healthy aging humans[44]. Notably, in our study, eotaxin rose significantly in adolescents with MDD despite treatment with fluoxetine. As in other cases, there are few studies on eotaxin in mood disorders. Based on its effects in the CNS, eotaxin inhibits IL-4 activity[45] and IL-4 is critical in higher functions of the normal brain, such as memory and learning[46], cognitive functions that are affected in MDD.
In this study, we observed that chemokines eotaxin, IL-8, IP-10, MCP-1, MIP-1α and MIP-1β were increased in comparison with HVs, and despite treatment with fluoxetine, they remained consistently high in the patient group. Although there were variations in serum chemokine levels throughout the 8 wk clinical follow-up, all adolescents with MDD experienced a significant decrease in clinical-psychiatric parameters after consuming fluoxetine, similar to the two other longitudinal studies[31,32]. This knowledge confirms the approach that clinical-psychiatric scales only focus on the behavioral parameters and further work is necessary to identify molecular parameters of clinical utility for psychiatrists in mood disorders.
This study had some limitations. The patient number was small, although the numbers of HVs and patients were sufficient for statistical analysis. In all cases, the discrepancies between our values and those reported by other groups can be explained by differences in the sample source such as serum or plasma, detection techniques, age of the participants, and confounding factor, such as tobacco and alcohol consumption, and body mass index. Further studies will be required to validate our findings.
In conclusion, our results showed a significant elevation in serum chemokine level in adolescents with MDD despite treatment with fluoxetine and improvement in HDRS score. This prompted us to consider the multidisciplinary management of MDD patients since high levels of MCP-1 and IP-10 are associated with endothelial damage and coronary risk. The elevation in eotaxin, IP-10, and IL-8 might explain certain features of depressed patients such as impaired cognition, memory, and learning. Further studies are necessary to examine these findings and determine their implications for future therapeutic approaches in MDD patients.
Major depressive disorder (MDD) affects 350 million people, approximately 6% of whom are adolescents; however, research on this age group is limited. The depressive symptomatology in adolescents can be confused with other psychiatric disorders, in turn affecting the proper diagnosis and normal development of patients. MDD in adolescents is associated with several negative outcomes including other psychiatric disorders later in life, educational impairment, self-injury, and suicide.
The immune system reaches maturity at about 16 years of age and upon the central nervous system second neural pruning. This makes adolescent patients with MDD have molecular characteristics that initially differ from adults and the elderly. These particularities have not been efficiently explored.
This study determined the differences in circulatory levels of eotaxin, interleukin (IL)-8, interferon gamma-induced protein (IP)-10, monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein (MIP)-1α and MIP-1β in HVs and adolescents with MDD, and assessed the changes induced by antidepressants consumed during 8 wk of clinical follow-up, which is the minimum time to observe the therapeutic efficacy of selective serotonin reuptake inhibitors, like fluoxetine.
We measured serum levels of eotaxin, IL-8, IP-10, MCP-1, MIP-1α, and MIP-1β in adolescents with MDD and performed a clinical psychiatric evaluation using the Hamilton depresión rating scale (HDRS). Eighteen HVs and twenty-two adolescents with MDD were monitored throughout 8 wk of clinical follow-up.
All evaluated chemokines decreased at 4 wk, but only MCP-1 and IL-8 differed significantly (P < 0.05) between 0 and 4 wk. In adolescents with MDD, all chemokines rose to their initial concentrations by 8 wk (vs 0 wk), but only IP-10 did so significantly (P < 0.05). All patients experienced a significant decrease in HDRS scores at 4 wk (P < 0.0001) and 8 wk (P < 0.0001) compared with 0 wk. Despite the consumption of fluoxetine, adolescents with MDD had significantly higher chemokines levels, even after considering the improvement in the HDRS score.
Our results showed a significant elevation in serum chemokine levels in adolescents with MDD despite treatment with fluoxetine and an improvement in HDRS scores. This prompted us to consider the multidisciplinary management of MDD patients since high levels of MCP-1 and IP-10 are associated with coronary risk. The elevation detected in eotaxin, IP-10, and IL-8 serum levels might explain certain features of depressed patients such as impaired cognition, memory, and learning.
Given the number of functions in which chemokines and their receptors are involved in the central nervous system, they could become novel diagnostic markers or therapeutic targets for MDD patients. However, a more significant number of studies are required, particularly in the adolescent population.
We appreciate the support of Juárez-Cruz E, Ortega EF, López-Bello G, and Hernandez-Ferreira E.
Manuscript source: Invited manuscript
Specialty type: Psychiatry
Country/Territory of origin: Mexico
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kotzalidis GD S-Editor: Yan JP L-Editor: Filipodia P-Editor: Liu JH
| 1. | WHO. Depression. 2019 [cited 5 December 2019]. Available from: https://www.who.int/en/news-room/fact-sheets/detail/depression. |
| 2. | Costello EJ, Foley DL, Angold A. 10-year research update review: the epidemiology of child and adolescent psychiatric disorders: II. Developmental epidemiology. J Am Acad Child Adolesc Psychiatry. 2006;45:8-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 225] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 3. | de Jonge-Heesen KW, van Ettekoven KM, Rasing SP, Liempd FH, Vermulst AA, Engels RC, Creemers DH. Evaluation of a school-based depression prevention program among adolescents with elevated depressive symptoms: study protocol of a randomized controlled trial. BMC Psychiatry. 2016;16:402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A, McCorkle R, Seligman DA, Schmidt K. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behav Immun. 2001;15:199-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 624] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 5. | Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctôt KL. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3039] [Cited by in RCA: 3402] [Article Influence: 226.8] [Reference Citation Analysis (0)] |
| 6. | Ching S, Zhang H, Belevych N, He L, Lai W, Pu XA, Jaeger LB, Chen Q, Quan N. Endothelial-specific knockdown of interleukin-1 (IL-1) type 1 receptor differentially alters CNS responses to IL-1 depending on its route of administration. J Neurosci. 2007;27:10476-10486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 125] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 7. | Kelley KW, Bluthé RM, Dantzer R, Zhou JH, Shen WH, Johnson RW, Broussard SR. Cytokine-induced sickness behavior. Brain Behav Immun. 2003;17 Suppl 1:S112-S118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 481] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 8. | Li Z, Wang Z, Zhang C, Chen J, Su Y, Huang J, Yi Z, Yuan C, Hong W, Wang Y, Wu Z, Hu Y, Cao L, Peng D, Guan Y, Zou Y, Yu S, Cui D, Fang Y. Reduced ENA78 levels as novel biomarker for major depressive disorder and venlafaxine efficiency: Result from a prospective longitudinal study. Psychoneuroendocrinology. 2017;81:113-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Ślusarczyk J, Trojan E, Chwastek J, Głombik K, Basta-Kaim A. A Potential Contribution of Chemokine Network Dysfunction to the Depressive Disorders. Curr Neuropharmacol. 2016;14:705-720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Sallusto F, Baggiolini M. Chemokines and leukocyte traffic. Nat Immunol. 2008;9:949-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 253] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 11. | Aloisi F, Columba-Cabezas S, Franciotta D, Rosicarelli B, Magliozzi R, Reynolds R, Ambrosini E, Coccia E, Salvetti M, Serafini B. Lymphoid chemokines in chronic neuroinflammation. J Neuroimmunol. 2008;198:106-112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Stuart MJ, Baune BT. Chemokines and chemokine receptors in mood disorders, schizophrenia, and cognitive impairment: a systematic review of biomarker studies. Neurosci Biobehav Rev. 2014;42:93-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 202] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 13. | Lehto SM, Niskanen L, Herzig KH, Tolmunen T, Huotari A, Viinamäki H, Koivumaa-Honkanen H, Honkalampi K, Ruotsalainen H, Hintikka J. Serum chemokine levels in major depressive disorder. Psychoneuroendocrinology. 2010;35:226-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Eyre HA, Air T, Pradhan A, Johnston J, Lavretsky H, Stuart MJ, Baune BT. A meta-analysis of chemokines in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2016;68:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 138] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 15. | Milenkovic VM, Sarubin N, Hilbert S, Baghai TC, Stöffler F, Lima-Ojeda JM, Manook A, Almeqbaali K, Wetzel CH, Rupprecht R, Nothdurfter C. Macrophage-Derived Chemokine: A Putative Marker of Pharmacological Therapy Response in Major Depression? Neuroimmunomodulation. 2017;24:106-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Vogelzangs N, de Jonge P, Smit JH, Bahn S, Penninx BW. Cytokine production capacity in depression and anxiety. Transl Psychiatry. 2016;6:e825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 138] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 17. | Simon NM, McNamara K, Chow CW, Maser RS, Papakostas GI, Pollack MH, Nierenberg AA, Fava M, Wong KK. A detailed examination of cytokine abnormalities in Major Depressive Disorder. Eur Neuropsychopharmacol. 2008;18:230-233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 236] [Cited by in RCA: 231] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 18. | Powell TR, McGuffin P, D'Souza UM, Cohen-Woods S, Hosang GM, Martin C, Matthews K, Day RK, Farmer AE, Tansey KE, Schalkwyk LC. Putative transcriptomic biomarkers in the inflammatory cytokine pathway differentiate major depressive disorder patients from control subjects and bipolar disorder patients. PLoS One. 2014;9:e91076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Zhou X, Cipriani A, Furukawa TA, Cuijpers P, Zhang Y, Hetrick SE, Pu J, Yuan S, Del Giovane C, Xie P. Comparative efficacy and tolerability of new-generation antidepressants for major depressive disorder in children and adolescents: protocol of an individual patient data meta-analysis. BMJ Open. 2018;8:e018357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Pae CU. The potential role of monocyte chemoattractant protein-1 for major depressive disorder. Psychiatry Investig. 2014;11:217-222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Terpos E, Politou M, Viniou N, Rahemtulla A. Significance of macrophage inflammatory protein-1 alpha (MIP-1alpha) in multiple myeloma. Leuk Lymphoma. 2005;46:1699-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Merendino RA, Di Pasquale G, De Luca F, Di Pasquale L, Ferlazzo E, Martino G, Palumbo MC, Morabito F, Gangemi S. Involvement of fractalkine and macrophage inflammatory protein-1 alpha in moderate-severe depression. Mediators Inflamm. 2004;13:205-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Chang TT, Chen JW. Emerging role of chemokine CC motif ligand 4 related mechanisms in diabetes mellitus and cardiovascular disease: friends or foes? Cardiovasc Diabetol. 2016;15:117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 24. | Tsai JH, Kuo CH, Yang P, Cheng KH, Wang PW, Chen CC, Hung CH. Effects of antidepressants on IP-10 production in LPS-activated THP-1 human monocytes. Int J Mol Sci. 2014;15:13223-13235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Pérez-Sánchez G, Becerril-Villanueva E, Arreola R, Martínez-Levy G, Hernández-Gutiérrez ME, Velasco-Velásquez MA, Alvarez-Herrera S, Cruz-Fuentes C, Palacios L, de la Peña F, Pavón L. Inflammatory Profiles in Depressed Adolescents Treated with Fluoxetine: An 8-Week Follow-up Open Study. Mediators Inflamm. 2018;2018:4074051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 26. | Wu D, Zhou J, Bi H, Li L, Gao W, Huang M, Adcock IM, Barnes PJ, Yao X. CCL11 as a potential diagnostic marker for asthma? J Asthma. 2014;51:847-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 27. | Young JJ, Bruno D, Pomara N. A review of the relationship between proinflammatory cytokines and major depressive disorder. J Affect Disord. 2014;169:15-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 293] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 28. | Stuart MJ, Singhal G, Baune BT. Systematic Review of the Neurobiological Relevance of Chemokines to Psychiatric Disorders. Front Cell Neurosci. 2015;9:357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 122] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 29. | Milenkovic VM, Stanton EH, Nothdurfter C, Rupprecht R, Wetzel CH. The Role of Chemokines in the Pathophysiology of Major Depressive Disorder. Int J Mol Sci. 2019;20:2283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 30. | Leighton SP, Nerurkar L, Krishnadas R, Johnman C, Graham GJ, Cavanagh J. Chemokines in depression in health and in inflammatory illness: a systematic review and meta-analysis. Mol Psychiatry. 2018;23:48-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 205] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 31. | Sutcigil L, Oktenli C, Musabak U, Bozkurt A, Cansever A, Uzun O, Sanisoglu SY, Yesilova Z, Ozmenler N, Ozsahin A, Sengul A. Pro- and anti-inflammatory cytokine balance in major depression: effect of sertraline therapy. Clin Dev Immunol. 2007;2007:76396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 235] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 32. | Piletz JE, Halaris A, Iqbal O, Hoppensteadt D, Fareed J, Zhu H, Sinacore J, Devane CL. Pro-inflammatory biomakers in depression: treatment with venlafaxine. World J Biol Psychiatry. 2009;10:313-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 33. | Motivala SJ, Sarfatti A, Olmos L, Irwin MR. Inflammatory markers and sleep disturbance in major depression. Psychosom Med. 2005;67:187-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 213] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 34. | Grassi-Oliveira R, Brieztke E, Teixeira A, Pezzi JC, Zanini M, Lopes RP, Bauer ME. Níveis periféricos de quimiocina em mulheres com depressão maior com ideaç̃o suicida. Rev Bras Psiquiatr. 2012;34:71-75. [RCA] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 35. | Carvalho LA, Torre JP, Papadopoulos AS, Poon L, Juruena MF, Markopoulou K, Cleare AJ, Pariante CM. Lack of clinical therapeutic benefit of antidepressants is associated overall activation of the inflammatory system. J Affect Disord. 2013;148:136-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 36. | Bai YM, Chiou WF, Su TP, Li CT, Chen MH. Pro-inflammatory cytokine associated with somatic and pain symptoms in depression. J Affect Disord. 2014;155:28-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 37. | Suarez EC, Lewis JG, Krishnan RR, Young KH. Enhanced expression of cytokines and chemokines by blood monocytes to in vitro lipopolysaccharide stimulation are associated with hostility and severity of depressive symptoms in healthy women. Psychoneuroendocrinology. 2004;29:1119-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 136] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 38. | Arreola R, Becerril-Villanueva E, Cruz-Fuentes C, Velasco-Velázquez MA, Garcés-Alvarez ME, Hurtado-Alvarado G, Quintero-Fabian S, Pavón L. Immunomodulatory effects mediated by serotonin. J Immunol Res. 2015;2015:354957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 184] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 39. | Baune BT, Smith E, Reppermund S, Air T, Samaras K, Lux O, Brodaty H, Sachdev P, Trollor JN. Inflammatory biomarkers predict depressive, but not anxiety symptoms during aging: the prospective Sydney Memory and Aging Study. Psychoneuroendocrinology. 2012;37:1521-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 138] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 40. | Song C, Lin A, Bonaccorso S, Heide C, Verkerk R, Kenis G, Bosmans E, Scharpe S, Whelan A, Cosyns P, de Jongh R, Maes M. The inflammatory response system and the availability of plasma tryptophan in patients with primary sleep disorders and major depression. J Affect Disord. 1998;49:211-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 169] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 41. | Narita M, Tanaka H, Togashi T, Abe S. Cytokines involved in CNS manifestations caused by Mycoplasma pneumoniae. Pediatr Neurol. 2005;33:105-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 42. | Wong ML, Dong C, Maestre-Mesa J, Licinio J. Polymorphisms in inflammation-related genes are associated with susceptibility to major depression and antidepressant response. Mol Psychiatry. 2008;13:800-812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 195] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 43. | Magalhaes PV, Jansen K, Stertz L, Ferrari P, Pinheiro RT, da Silva RA, Kapczinski F. Peripheral eotaxin-1 (CCL11) levels and mood disorder diagnosis in a population-based sample of young adults. J Psychiatr Res. 2014;48:13-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 44. | Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, Lucin KM, Czirr E, Park JS, Couillard-Després S, Aigner L, Li G, Peskind ER, Kaye JA, Quinn JF, Galasko DR, Xie XS, Rando TA, Wyss-Coray T. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90-94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1110] [Cited by in RCA: 1377] [Article Influence: 98.4] [Reference Citation Analysis (0)] |
| 45. | Derecki NC, Cardani AN, Yang CH, Quinnies KM, Crihfield A, Lynch KR, Kipnis J. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J Exp Med. 2010;207:1067-1080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 671] [Cited by in RCA: 630] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 46. | Gadani SP, Cronk JC, Norris GT, Kipnis J. IL-4 in the brain: a cytokine to remember. J Immunol. 2012;189:4213-4219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 423] [Article Influence: 32.5] [Reference Citation Analysis (0)] |