Published online Dec 9, 2014. doi: 10.5497/wjp.v3.i4.140
Revised: October 2, 2014
Accepted: October 31, 2014
Published online: December 9, 2014
Processing time: 168 Days and 1.9 Hours
Obesity-associated cancers, including colon cancer and breast cancer, are increasing in Asian countries with Westernized lifestyles as exemplified by reduced physical activity and increased fat/sugar consumption. An excessive accumulation of visceral adipose tissue causes insulin resistance, dyslipidemia and adipocytokine imbalance, and these factors are suggested to be involved in cancer promotion. To prevent obesity-associated cancers, researcher attention is increasing on the so-called “functional foods”. In addition, new approaches to cancer control are in high demand, and using “functional foods” as supplemental or adjuvant agents in chemotherapy is thought to be a promising approach. One of these functional ingredients is xanthophylls, which are natural fat-soluble pigments found in fruits, vegetables, algae and other plants. Xanthophylls belong to the carotenoid class and have structures containing oxygen. Some studies have revealed that xanthophylls improve the inflammation status, serum triglyceride levels, blood pressure levels and liver function test values. Furthermore, recent studies show that xanthophylls possess high anti-cancer, anti-diabetic, anti-obesity and anti-oxidant properties. In this review, we highlight the recent findings for five xanthophylls, namely astaxanthin, β-cryptoxanthin, fucoxanthin, neoxanthin and zeaxanthin/lutein, and their relevance to cancer prevention.
Core tip: Xanthophylls belong to the class of carotenoids, and are natural fat-soluble pigments found in fruits, vegetables, algae and so on. It has been shown that the versatile functions of xanthophylls have great potential for the prevention of metabolic syndrome and cancers. Xanthophylls have proved safety, and several xanthophylls provide other health benefits, including improvement of inflammation, dyslipidemia, hypertension and liver function. These findings indicate that xanthophylls could be useful to prevent obesity-associated cancer.
- Citation: Terasaki M, Mutoh M, Fujii G, Takahashi M, Ishigamori R, Masuda S. Potential ability of xanthophylls to prevent obesity-associated cancer. World J Pharmacol 2014; 3(4): 140-152
- URL: https://www.wjgnet.com/2220-3192/full/v3/i4/140.htm
- DOI: https://dx.doi.org/10.5497/wjp.v3.i4.140
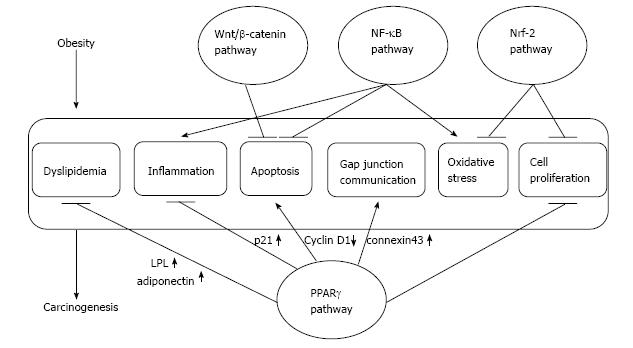
Obesity has recently attracted much interest as a risk factor for several cancers, such as breast cancer and colorectal cancer[1,2]. Both metabolic syndrome that is characterized by obesity, hyperlipidemia, type 2 diabetes and hypertension and obesity-associated cancers (Table 1) are extremely common in Western countries, and they are currently increasing in Eastern countries, including Japan. The factors linking obesity and cancer are becoming apparent, and they are insulin resistance, dyslipidemia and a subsequent adipocytokine imbalance (Figure 1)[1,2]. Carotenoid intake is reported to be inversely associated with obesity and with the risk of many cancers[3-6].
| Type of cancer |
| Breast (postmenopausal) |
| Colorectum |
| Endometrium |
| Esophagus |
| Gallbladder |
| Kidney |
| Pancreas |
| Thyroid |
Carotenoids are fat-soluble pigments found in fruits, vegetables, algae and other plants. Humans cannot synthesize carotenoids, and we should therefore consume them as part of our diet. Carotenoids belong to the tetraterpenoid category, and they can be divided into xanthophylls and carotenes according to whether the structure contains oxygen or not. Carotenoids with structures containing oxygen are xanthophylls. As the name indicates, the color of xanthophylls is usually yellow, and they are usually lipophilic because of the long unsaturated aliphatic chain in their structure.
Because conventional chemotherapy has failed to reduce the mortality rates of common cancers, including obesity-associated cancers, new approaches to controlling the development of cancer are in great demand[7]. One approach is the use of functional foods/plant-derived agents as supplemental or adjuvant agents in chemotherapy[8,9]. Another approach is chemoprevention for the control of cancer development[8,9]. In both methods, using xanthophylls seems to be an attractive approach. As shown in this review, xanthophylls provided health benefits, such as improvements in inflammation, dyslipidemia, hypertension and liver function. Moreover, the biological significance of xanthophylls as important candidates for the chemoprevention of cancer is becoming clearer, and the safety of xanthophylls has been affirmed, as described in this review. Another candidate called - carotene is the most abundant dietary carotenoid, and long-term supplementation with this compound has been shown to be ineffective for cancer chemoprevention in several recent large-scale intervention trials[10-12].
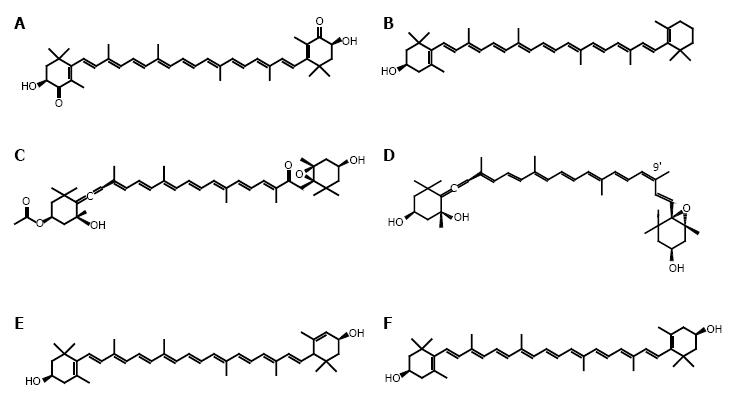
In this review, we focus on recent findings for five xanthophylls as follows: astaxanthin, β-cryptoxanthin, fucoxanthin, neoxanthin and zeaxanthin/lutein, and their relevance to cancer prevention (Figure 2).
Astaxanthin (AX) is a natural fat-soluble red pigment and belongs to the xanthophyll subclass of carotenoids. Dietary sources of AX are eggs of salmon and trout, skin of red sea bream, crabs, shrimps and lobsters. AX is synthesized in microalgae (Chlorella zofingiensis, Chlorococcum and Haematococcus pluvialis). Krills (Euphausia superba) feed on the microalgae and in turn are fed upon by fishes. The microalga, H. pluvialis, is the main source of natural AX and is able to accumulate up to 4%AX on dry weight basis[13-15]. AX extracted from H. pluviali is used as a food dye in many countries. AX exists in stereoisomers and geometric isomers. H. pluvialis biosynthesizes the (3S, 3’S)-isomer, meanwhile P. rhodozyma biosynthesizes the (3R, 3’R)-isomer. AX has two hydroxyl groups and is able to react with fatty acids and proteins. AX is found as free, mono- and di-ester forms in organisms[13]. AX can take to transverse cell membrane orientation, and shows strong antioxidative activity[13,15]. After oral administration of AX, AX changes to all-E, 9Z-, 13Z-geometrical isomers and 3R,3’R-, 3R,3’S meso-, 3S,3’S-optical isomers, all of which can be detected in human blood[16].
Many experimental and clinical studies have demonstrated the safety of AX[13,17]. In a subchronic toxicity study in rats, feeding AX-rich microalgae biomass corresponding to doses of 465 and 557 mg AX/kg per day for 90 d in male and female rats, respectively, revealed no adverse events[18]. A randomized, double-blind, placebo-controlled study has demonstrated that it is safe to administrate 6 mg/d AX in healthy adults for 8 wk[19], and a significant decrease of triglycerides and increase of adiponectin and high density lipoprotein cholesterol in participants with mild dyslipidemia by administration of AX at doses of 12 mg/d and 18 mg/d for 12 wk[20].
Oxidative stress and inflammation are closely related to carcinogenesis (Figure 1), and many antioxidants, including carotenoids have been demonstrated to decrease cancer development in experimental animal models[14]. There are papers on preventive effects of AX on urinary bladder[21], oral[22,23], and colorectal[24,25] carcinogenesis. In mouse urinary bladder cancer induced by N-butyl-N(4-hydroxybutyl)nitrosamine (OH-BBN), AX administration at a dose of 50 ppm in water for 20 wk after OH-BBN exposure for 20 wk resulted in a decrease in the incidences of precancerous lesions and bladder cancer[21]. In rat oral carcinogenesis induced by 4-nitroquinoline 1-oxide (4-NQO), the incidence of oral precancerous lesions in rats treated with 20 ppm 4-NQO and 100 ppm AX was smaller compared to those of the non-treatment group, and oral neoplasms did not observed in rats fed AX among the 4-NQO exposure[22]. In these studies, AX decreased cell growth activity in the non-cancerous epithelial tissues of 4-NQO-exposed animals[21,22]. AX has also been demonstrated to show preventive effects in 7,12-dimethylbenz[a]anthracene (DMBA)-induced hamster buccal pouch carcinogenesis via nuclear factor-erythroid 2 related factor 2 (Nrf2) activation[23]. Moreover, AX has been shown to inhibit nuclear factor kappa B (NF-κB) and Wnt/β-catenin signaling pathways[26]. Related to colorectal carcinogenesis, AX at 500 ppm in diet significantly decreased the development of aberrant crypt foci (ACF) and the incidence and multiplicity of colorectal tumors induced by azoxymethane (AOM)[24]. AX at 200 ppm in diet also suppressed mucosal ulcers induced by dextran sulfate sodium (DSS), and development of dysplastic ACF and colonic adenocarcinoma induced by both treatment of DSS and AOM[25]. In addition, AX reduced the number and size of aflatoxin B1-induced liver preneoplastic foci in rats[27]. Growth of WAZ-2T cells, mammary tumor cells, inoculated into the mice mammary fat pad was also inhibited by AX at 100 ppm or 400 ppm in diet[28]. Lipid peroxidation activity in tumors was reduced in tumors treated with 400 ppm[28]. AX markedly attenuated the promotion of hepatic metastasis of P815 mastocytoma cells in a syngenic graft model under restraint stress[29]. In in vitro cell culture systems, AX suppressed invasion of rat ascites hepatoma AH109A cells[30]. AX inhibited cell proliferation and decreased cell viability of leukemia K562 cells via induction of apoptosis along with up-regulation of peroxisome proliferator-activated receptor (PPAR)γ and p21, and down-regulation of cyclin D1 [31]. Induction of connexin 43, gap junction protein, through activation of PPARγ is suggested to be one of the anti-tumor mechanisms of AX[32,33]. Up-regulation of the Nrf2 pathway is also involved in antioxidant activity of AX[23,31,34], and may improve mitochondrial function[35]. However, the role of Nrf2 activation in anti-tumorigenesis is controversial. The oncogenic K-ras gene induces Nrf2 expression, and detoxification of reactive oxygen species promotes tumor growth[36]. Deficiency of Nrf2 has been reported to increase induction of tumors in urethane-induced mouse lung carcinogenesis, but reduce the number of malignant tumors harboring activated mutation in the K-ras gene, indicating that Nrf2 prevents initiation but accelerates progression under the activation of the K-ras signaling pathway[37]. Indeed, there is a report that effects of AX differ at the stages of initiation and the stage of promotion in mammary tumors. AX fed before tumor initiation delayed mammary tumor growth and modulated immune response, but AX supplementation after tumor initiation resulted in more rapid tumor growth[38]. Thus, use of antioxidants for cancer prevention is considered to be useful at the time before tumor initiation, but more caution is required in using them after the stage accompanied with activated K-ras signaling.
A randomized, double-blind, placebo-controlled study has demonstrated that AX reduces oxidation of fatty acids[39], decreases oxidative stress markers[40] and inflammation[41], and improves dyslipidemia[20] and age-related dysfunction of eyes[42,43] and brain[44]. However, human cancer prevention studies using AX have not yet been reported.
Distribution and nature ofβ-cryptoxanthin
β-cryptoxanthin (β-CRX) is one of the naturally occurring carotenoid pigments, and is also classified as a xanthophyll. Its unique character is that it is found in specific fruits and vegetables such as mango, papaya, loquat, Japanese persimmon, peach, sweet red peppers and citrus fruits of the mandarin family[45,46]. Satsuma mandarin, Citrus unshiu, is one of the most β-CRX rich fruits in Japan. The content of β-CRX in C. unshiu reaches several mg/100g wet weight. The level of β-CRX in Valencia orange is very low and grapefruit has been found to be devoid of it.
In the human body, β-CRX is easily converted to vitamin A (retinol) and is therefore considered as a pro-vitamin A. It is also known that β-CRX might be easily absorbed[47], and is accumulated in various organs[48]. Moreover, it can be stored for several months in the human body[49]. Serum β-CRX concentration could be around 96 μg/dL[50]. It is also reported that β-CRX concentration in Japanese mother’s milk and serum are nearly parallel with their intake of the Satsuma mandarin, and are higher than other countries[51,52].
Many epidemiological studies showed the intake of β-CRX was significantly associated with reduced risks of type 2 diabetes [relative risk (RR) = 0.58][53] and rheumatoid arthritis (RR = 0.59)[54]. β-CRX supplementation significantly decreased cigarette smoke-induced lung squamous metaplasia and inflammation[55]. Regarding cancer risk, several observational epidemiologic studies suggest that β-CRX could potentially prevent cancer development. The demonstrated cancer risks for lung, esophageal and bladder were 0.76 (RR), 0.16 [odds ratio (OR)] and 0.74 (RR), respectively, comparing the highest to lowest quintile of intake[56-58]. A greater intake of β-CRX was also inversely associated with developing undetermined cervical atypical squamous cells (OR = 0.4)[59]. Interestingly, the serum level of β-CRX is lower in the patients of liver cancer than that in healthy counterparts[60]. These results suggest that a high serum β-CRX concentration or intake of β-CRX is beneficial to human health.
The scientific panel on additives and products or substances used in animal feed (FEEDAP) panel members considered β-CRX to appear not to be mutagenic and show no clastogenic activity[61]. In subchronic studies, The FEEDAP panel could not find any adverse effects[61]. Also an acceptable daily intake has not been determined[61]. Previously, we have reported the chemoprevention effect of β-CRX against chemically-induced bladder carcinogenesis in ICR mice[62]. Mice were fed with 1, 5 and 25 ppm of β-CRX for 24 wk, and no clinical signs of toxicity and poor condition, low survival or histopathological changes were found[62]. Many epidemiological studies[53-60,63-68] indicated that administration of β-CRX is safe for human health.
Various functions of β-CRX have been reported recently. β-CRX is an antioxidant phytochemical and may help prevent oxidative damage[69]. Thus, it is believed that β-CRX has health benefits for people with risk of chronic diseases.
Numerous possible mechanisms for the anti-carcinogenic potential of β-CRX have been proposed. These include the antioxidant function that is associated with the enhancement of DNA repair[55,69], suppression of efficacy of key proinflammatory cytokine expression, such as tumor necrosis factor-α[55] and an apoptotic induction effect[70]. Also, β-CRX is known to stimulate the expression of the RB gene (a tumor-suppressor gene) and p73 gene (a p53-related gene)[71] and reduce the expression of NF-κB and activator protein-1 (AP-1), that induces numerous genes including inflammation, cell proliferation, and apoptosis[55]. These mechanisms indicate that β-CRX may be a promising chemopreventive agent against cancer. Indeed, β-CRX exerts an anti-tumor promoter action in vitro[72] and inhibits chemically induced carcinogenesis in vivo[62,71,73,74]. Previously, we investigated the effects of β-CRX extracted from C. unshiu oranges on OH-BBN-induced urinary bladder carcinogenesis in male ICR mice[62]. OH-BBN-exposed mice were fed with 1, 5 and 25 ppm of β-CRX for 24 wk starting 1 wk after the cessation of OH-BBN exposure. Feeding with β-CRX decreased the incidence and multiplicity of precancerous and cancerous urinary bladder lesions. Especially, 25 ppm β-CRX markedly reduced the occurrence of bladder cancer. Meanwhile, β-CRX is also reported to reduce mouse skin[71], mouse lung[74] and rat colon[71] carcinogenesis. In our report, β-CRX lowered ratios of cyclin D1-positive cell in various urinary bladder lesions, meaning that reduction in the incidence of precancerous and cancerous urinary bladder lesions is due to reduced cell cycle progression[62].
The efficacy of β-CRX supplementation on obesity have been investigated[75]. Seventeen postmenopausal obese women were provided 200 mL of a beverage containing β-CRX (1.56 mg/serving and 4.7 mg/d) for 3 wk[75]. As a result, the levels of serum β-CRX were significantly elevated from 0.28 (initial period) to 1.15 mg/mL, and high molecular weight-adiponectin was also elevated from 9.8 to 11.1 mg/mL[75]. At the end of the study, the levels of serum triglyceride (P = 0.057) and total plasminogen activator inhibitor-1 (PAI-1) (P = 0.052) tended to decrease. Nishino et al[60] reported an intervention study where β-CRX-rich mandarin orange juice (3 mg β-CRX in 80 mL) was provided for 12 wk to obese men or obese men with elevated serum γ-glutamyl transpeptidase (γGTP) levels[60]. After drinking β-CRX for 12 wk, body weight (P < 0.001), BMI (P < 0.001) and β-GTP levels (P < 0.005) were decreased.
An intervention trial regarding prevention of liver cancer has also been reported[60]. Viral hepatitis with cirrhosis patients were randomly assigned into two groups in the trial. The treatment group was administered mandarin orange juice enriched with β-CRX and with the carotenoids mixture (lycopene, β-carotene and α-carotene). Patients in the control group were administered a carotenoids mixture alone. At year 2.5, cumulative incidence of liver cancer/hepatocellular carcinoma development in the mandarin orange juice group was lower than that of the carotenoids mixture alone group (P = 0.05). The combinational use of natural carotenoids containing β-CRX might be valuable for the prevention of liver cancer in hepatitis virus infected patients with cirrhosis.
Brown seaweeds include Undaria pinnatifida (wakame), Hizikia fusiforme (hijiki), Laminaria japonica (ma-kombu) and Sargassum fulvellum. The Japanese have been estimated to intake wakame at 1 g/d[76]. Brown seaweeds are known to contain many bioactive components, i.e., fucoxanthin (FX), fucoidan, vitamins, minerals, dietary fibers, proteins, ω-3 polyunsaturated fatty acids (PUFAs), polysaccharides, other carotenoids and various functional polyphenols. Fucoidan is a sulfated polysaccharide that is one of the major bioactive components in seaweed[77,78], but we would like to focus on FX in this review. FX is a xanthophyll belonging to non-provitamin A carotenoids, constructed with an unusual allenic bond, an epoxide group, and a conjugated carbonyl group in a polyene chain[79]. Some reports demonstrated that the FX content of U. pinnatifida is approximately 1.0-3.0 mg/g dry weight through one life cycle[80,81]. It has been proven that mice convert FX into keto-carotenoids by oxidation of the secondary hydroxyl groups (FX + H2O → FuOH; FuOH + NAD+→ amarouciaxanthin A + NADH)[79]. On the other hand, oral administration of kombu extract containing FX in humans revealed that the FuOH and the cis-isomer of FuOH could be found in the serum, detected by HPLC[82].
FX has been proved to be safe with no side effects by single (1000 or 2000 mg/kg BW) and repeated (500 or 1000 mg/kg BW for 30 d) oral dose toxicity studies in male and female mice[83]. In the repeated doses study, histological examination of the gonadal tissues, kidneys, liver and spleen revealed no abnormal changes[83]. In rats, 13-wk oral subchronic toxicity studies suggested that more than 2000 mg/kg BW of microalgal FX oil induce the 50% lethal[84].
Many studies suggested FX possesses anti-cancer potential, especially shown in colon cancer cell lines (Caco-2, DLD-1 and HT-29), liver cell lines (HepG2), prostate cancer cell lines (DU 145, LNCaP and PC-3) and urinary bladder[85-88]. The main biomolecules involved in anti-cancer mechanism is assumed to be the biomolecules related to apoptosis and cell cycle[89,90] and those may associate with antioxidant activity through their free radical scavenging action[91]. Moreover, inhibition of PI3K/Akt and NF-κB signals were reported in human cervical and breast cancer cells, respectively[92,93].
Its metabolite fucoxanthinol (FuOH) also has inhibitory effects on cancer cell growth[94,95], and 1,2-dimethylhydrazine-induced formation of colonic ACF in mice and AOM/DSS-induced colon carcinogenesis[25,96]. To find new cancer prevention approaches, we investigated the combination effect of FuOH and 1α,25-dihydroxyvitamin D3 (1α,25(OH)2D3), and found inhibition of cell viability and induction of apoptosis in DLD-1 and HT-29 cells[97]. Down-regulation of PPARγ and NF-κB p52 were suggested to be involved in the inhibition of cell viability due to the combination of FuOH and 1α,25(OH)2D3 . It has been shown that activation of PPARγ suppresses intestinal polyp development in Apc-mutant mice and AOM-induced colonic ACF development in obese KK-Ay mice[98,99].
FX has been reported to provide health benefits in humans, such as improvement of obesity, reduction of inflammation, healthy triglyceride levels, and improvements in blood pressure levels[100,101].
After daily intake of U. pinnatifida, FuOH is detectable in human plasma[82]. Although metabolites of FX could be measured as a marker of exposure, effects of FX or FuOH in human carcinogenesis have not been reported to date. From the aspect of obesity-associated cancer, we here introduce one study that has been conducted to assess the effects of FX supplementation on weight loss. FX supplementation on obese patients with nonalcoholic fatty liver disease results in the improvement of liver inflammatory markers, such as alanine aminotransferase, aspartate aminotransferase, C-reactive protein, γ-glutamyltransferase (γGT, GGT)[101]. Of note, it has been demonstrated that increased of GGT plasma levels are associated with an increased risk of pancreatic cancer[102,103], nevertheless GGT has no causative role itself.
It is also interesting to mention that intake of 5 g/d U. pinnatifida stimulated a significant 50% reduction in urinary urokinase-like plasminogen activator receptor (uPAR) proteins in postmenopausal women. uPAR, is the membrane receptor for uPA, responsible for extracellular membrane proteins degradation and PAI-1, responsible for the inhibition of plasminogen activation[104]. Generally, uPAR is known to be higher in postmenopausal women as well as in breast cancer patients[105]. Moreover, it has been reported that uPA and/or PAI-1 is positively correlated with poor prognosis in patients with breast cancer, i.e., correlation with cancer metastatic potential[106,107]. Thus, uPA, PAI-1and uPAR might be used as prognostic markers for breast cancer[108], and FX may reduce such a tumor marker.
Neoxanthin (NX), a non-provitamin A carotenoid, has an unusual allenic bond and a 5,6-monoepoxide as well as FX. NX is widely present in terrestrial and marine biota and the occurrence of two geometric cis/trans isomers is known to be species dependent[109-111]. The 9’-cis form of NX (9’-cis NX) is mainly localized and used in the photosynthetic organs of spinach leaves and marine algae such as Euglenophyta. It is also used as a precursor of abscisic acid, a plant hormone[112,113]. Whereas the all-trans form of NX is predominant in the petals of globeflower and yellow rose, this xanthophyll is not involved in the photosynthetic system[111,114]. We mainly obtain the 9’-cis NX from leafy green vegetables. Fresh spinach contains 9’-cis NX around 5 mg/100 g in fresh leaf[110]. It has been estimated that 9’-cis NX exists at 0.95 μmol/L in digested fluid (9 L/d), when we ingest 100 g/d spinach.
The 5,6-monoepoxide moiety in 9’-cis NX is easily isomerized to 5,8-epoxide under the acidic conditions of the stomach and generates almost equal amounts of (8’-R/S)-neochrome[115,116]. After a 1-wk spinach intervention (3 mg 9’-cis NX/day), highly hydrophilic xanthophylls of 9’-cis NX and (8’-R and 8’-S)-neochromes appeared at a very low level in human plasma (about 1 nmol/L)[117]. It is known that the uptake of various carotenoids by human colon cancer cells (Caco-2 cells) positively correlates with the lipophilicity of the carotenoids[118]. The highly hydrophilic xanthophylls such as NX, FX and violaxanthin could be detected slightly in human plasma, when we intake purified forms and food matrices[79,101,117,119-121]. Because of the poor intestinal absorption of NX, a considerable amount of ingested 9’-cis NX and (8’-R and 8’-S)-neochrome would be delivered to the colon, and even if absorbed in the small intestine, they would be metabolized easily.
It has been reported that both 9’-cis NX and all-trans NX possess strong potential of cell growth inhibition and apoptosis induction in human prostate cancer cells[87,94,115,122], human colon cancer[122-124], mouse melanoma[122] and mouse embryonic mesenchymal cells[125]. In addition, several researchers have reported that 9’-cis NX, all-trans NX and (8’R/S)-neochrome have cancer preventive effects[126], and also anti-tumor promoter functions[70]. Moreover, induction of cell cycle arrest[115], anti-oxidant properties[127] and anti-obesity properties[128] have been reported. Recently, we additionally demonstrated that 9’-cis NX rapidly accumulated in the mitochondria, caused mitochondria ΔΨ loss and thereafter the release of cytochrome c and production of apoptosis-inducing factor in human colon cancer cells[123]. It is regrettable that there is little information about the anti-cancer mechanisms of dietary NX in mammals, except for that described above.
No safety profile and clinical studies have been reported on 9’-cisNX, any -trans NX and (8’R/S)-neochromes. However, epidemiological data show that higher intake of fruits and vegetables, rich in highly hydrophilic epoxyxanthophylls such as NX, is associated with a lower risk of colorectal cancer[129,130]. Further studies are required to elucidate the clinical beneficial properties of NX.
Zeaxanthin (ZX) and lutein also belong to the xanthophyll family. Their unique character is that they are the only carotenoid among more than 600 species of carotenoid existing in eye tissue, especially in the retina[131]. Lutein can be photochemically transformed to meso-ZX. They are stereoisomer of each other, differ by the location of a double bond. Lutein is abundant in egg yolk, and in dark-green leafy vegetables, such as broccoli, brussels sprouts, kale and spinach[132]. In the human body, lutein is distributed at the skin, breasts, cervix uteri, and also found in serum in high amounts. Serum lutein and ZX levels are reported to be around 180 and 20 ng/mL, respectively[133]. They are assumed to play a critical role in ocular health because they act as strong anti-oxidants and filtered out high-energy blue light[134]. Of note, no correlation between plasma concentrations of lutein/zeaxanthin and BMI or insulin resistance has been reported[135].
In many papers, target organs for lutein are reported to be the eyeballs, the skin and the heart. Regarding ocular conditions, age-related macular degeneration, cataracts, and retina pigmentosa have been reported to have some correlation with lutein. Lutein also possesses a preventive function of cardiovascular diseases/stroke[131,134,136,137].
Regarding lung cancer, some epidemiologic studies state lutein has an important cancer preventive function[4,14]. A ten-year study of 120000 United States people revealed that lung cancer incidence was significantly reduced in those who ingested a high amount of total carotenoids, including lutein and ZX[138]. Similar relationships were found in Fijians, when compared to the other South Pacific islands’ people. Fijians intake 25 mg lutein daily on an average (200 g dark greens), whereas other 20 South Pacific countries intake less lutein in diets[139]. Thus, there was a clear inverse association with lutein intake and lung cancer incidence.
Regarding colorectal cancer, inverse associations with dietary lutein intake have been reported[124], and serum ZX concentration by Okuyama et al[140]. However, no association has been detected between the levels of plasma lutein and the risk of gastric cancer[141].
Regarding skin cancer risk, the specific effects of lutein are not fully known. The only reported data is that a combination of carotenoids may protect erythema development in human skin[142], and that may be correlated with the presence of skin cancer or precancerous lesions[124].
Regarding breast cancer, there is some possibility for protective effects of lutein[6,14,143]. Intake of lutein-rich foods significantly lowered the risk of premenopausal breast cancer. The Nurse’s Health Study demonstrated a weak inverse association, but significant, between lutein and ZX intake and the breast cancer risk among premenopausal women[6]. Of note, the protective effect of lutein and ZX was strongest in patients have a family history of breast cancer. Also there is a report that increasing serum levels of lutein and ZX were associated with a reduced breast cancer risk, but the trend was only marginally significant in a case-control study[143]. There is a report comparing biopsy samples from breast cancer tissue and benign mammary tissue. In this report, increasing lutein and ZX concentrations tends to decrease the risk of breast cancer[144]. Meanwhile, Other studies have shown that there are no differences of lutein and ZX concentrations in mammary adipose tissue between benign breast tumors and breast cancer[145]. New York University Women’s Health Study, a nested case-control prospective study, demonstrated an inverse relationship between plasma levels of lutein, but not ZX, and risk of breast cancer[146].
Regarding other cancers, significant inverse relations were observed for lutein and ZX in oral cavity and pharyngeal cancer[147].
No toxicities or adverse reactions for intake of lutein/ZX have been reported at doses up to 40 mg/d for 2 mo[131,148]. High doses of β-carotene supplements (> 30 mg/d) are well known to be associated with carotenodermia[149], and the same could happen when we consume high doses of lutein and ZX. Also it has been demonstrated that lutein has no mutagenic effect in the Ames test[150].
Lutein/ZX is thought to have a superior anti-oxidant ability to scavenge free radicals than other carotenoids. An in vitro study showed that lutein could quench peroxy radicals and play a guarding role against oxidative injury[151,152]. In this experiment, a synergistic antioxidant effect was obtained with a combination of lutein and lycopene[153]. Carotenoids also show a superb function for immune response[154].
Lutein could also function as an anti-carcinogenic reagent, such as a modulator of cell growth and apoptosis signaling. Lutein induces cell cycle arrest in human prostate and esophageal cancer cell[155,156]. Lutein induces apoptosis in transformed cancer cells but do not induce apoptosis in normal human mammary cells through modulating the ratio of Bcl-xL/Bax protein expression[157]. Meanwhile, ZX, structural isomer of lutein, induced cell cycle arrest in human beast cancer cells[158]. Lutein stimulates some genes involved in T-cell transformations activated by antigens, cytokines and mitogens[159]. Lutein interacts with carcinogens such as 1-nitropyrene and aflatoxin B1, and lowered its carcinogenetic activity[150,160]. In a recent report, female BALB/c mice were fed a diet containing lutein for 14 d, and then inoculated with 0 to 2.5 × 103 mammary tumor cells. The results demonstrated that 0.002% and 0.02% lutein lowered both mammary tumor incidence and tumor growth[161].
The versatile functions of xanthophylls have shown great potential for the prevention of metabolic syndrome and cancers, both in vitro and in vivo. Xanthophylls have been verified as safe with no side events, and several xanthophylls provide other health benefits, including improvements in inflammation, dyslipidemia, hypertension and liver function, as shown in this review. The accumulated evidence indicates the functionality of xanthophylls as anti-obesity and anti-insulin-resistance functional foods, implying that xanthophylls could be useful in preventing obesity-associated cancer.
The chemical synthesis of each xanthophyll is not impossible, but it may be very expensive. However, the promising results obtained from in vivo studies encourage researchers to undertake more clinical studies in humans. We have some information about xanthophylls trials, and we should further promote human clinical studies to obtain information about the adequate dosage of xanthophylls needed to prevent cancers.
P- Reviewer: Gagliardi G, Muscarella P, Schweiger U S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Fujii G, Yamamoto M, Takahashi M, Mutoh M. Role of adipocytokines in colorectal carcinogenesis. Curr Res in Cancer. 2011;5:39-48. |
| 2. | Ishino K, Mutoh M, Totsuka Y, Nakagama H. Metabolic syndrome: A novel high-risk state for colorectal cancer. Cancer Lett. 2013;334:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Eastwood MA. Interaction of dietary antioxidants in vivo: how fruit and vegetables prevent disease? QJM. 1999;92:527-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 127] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Holick CN, Michaud DS, Stolzenberg-Solomon R, Mayne ST, Pietinen P, Taylor PR, Virtamo J, Albanes D. Dietary carotenoids, serum beta-carotene, and retinol and risk of lung cancer in the alpha-tocopherol, beta-carotene cohort study. Am J Epidemiol. 2002;156:536-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 135] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 5. | Rock CL. Carotenoid update. J Am Diet Assoc. 2003;103:423-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Zhang S, Hunter DJ, Forman MR, Rosner BA, Speizer FE, Colditz GA, Manson JE, Hankinson SE, Willett WC. Dietary carotenoids and vitamins A, C, and E and risk of breast cancer. J Natl Cancer Inst. 1999;91:547-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 226] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 7. | Sporn MB, Suh N. Chemoprevention: an essential approach to controlling cancer. Nat Rev Cancer. 2002;2:537-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 243] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 8. | Ferguson LR, Schlothauer RC. The potential role of nutritional genomics tools in validating high health foods for cancer control: broccoli as example. Mol Nutr Food Res. 2012;56:126-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Temraz S, Mukherji D, Shamseddine A. Potential targets for colorectal cancer prevention. Int J Mol Sci. 2013;14:17279-17303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Heinonen OP, Albanes D. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med. 1994;330:1029-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3454] [Cited by in RCA: 2942] [Article Influence: 94.9] [Reference Citation Analysis (0)] |
| 11. | Hennekens CH, Buring JE, Manson JE, Stampfer M, Rosner B, Cook NR, Belanger C, LaMotte F, Gaziano JM, Ridker PM. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med. 1996;334:1145-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1708] [Cited by in RCA: 1431] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 12. | Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Valanis B, Williams JH. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2565] [Cited by in RCA: 2171] [Article Influence: 74.9] [Reference Citation Analysis (0)] |
| 13. | Ambati RR, Phang SM, Ravi S, Aswathanarayana RG. Astaxanthin: sources, extraction, stability, biological activities and its commercial applications--a review. Mar Drugs. 2014;12:128-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1298] [Cited by in RCA: 1075] [Article Influence: 97.7] [Reference Citation Analysis (0)] |
| 14. | Tanaka T, Shnimizu M, Moriwaki H. Cancer chemoprevention by carotenoids. Molecules. 2012;17:3202-3242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 359] [Cited by in RCA: 319] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 15. | Kidd P. Astaxanthin, cell membrane nutrient with diverse clinical benefits and anti-aging potential. Altern Med Rev. 2011;16:355-364. [PubMed] |
| 16. | Coral-Hinostroza GN, Ytrestøyl T, Ruyter B, Bjerkeng B. Plasma appearance of unesterified astaxanthin geometrical E/Z and optical R/S isomers in men given single doses of a mixture of optical 3 and 3’R/S isomers of astaxanthin fatty acyl diesters. Comp Biochem Physiol C Toxicol Pharmacol. 2004;139:99-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Fassett RG, Coombes JS. Astaxanthin in cardiovascular health and disease. Molecules. 2012;17:2030-2048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 18. | Stewart JS, Lignell A, Pettersson A, Elfving E, Soni MG. Safety assessment of astaxanthin-rich microalgae biomass: Acute and subchronic toxicity studies in rats. Food Chem Toxicol. 2008;46:3030-3036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | Spiller GA, Dewell A. Safety of an astaxanthin-rich Haematococcus pluvialis algal extract: a randomized clinical trial. J Med Food. 2003;6:51-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 115] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 20. | Yoshida H, Yanai H, Ito K, Tomono Y, Koikeda T, Tsukahara H, Tada N. Administration of natural astaxanthin increases serum HDL-cholesterol and adiponectin in subjects with mild hyperlipidemia. Atherosclerosis. 2010;209:520-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 166] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 21. | Tanaka T, Morishita Y, Suzui M, Kojima T, Okumura A, Mori H. Chemoprevention of mouse urinary bladder carcinogenesis by the naturally occurring carotenoid astaxanthin. Carcinogenesis. 1994;15:15-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 120] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Tanaka T, Makita H, Ohnishi M, Mori H, Satoh K, Hara A. Chemoprevention of rat oral carcinogenesis by naturally occurring xanthophylls, astaxanthin and canthaxanthin. Cancer Res. 1995;55:4059-4064. [PubMed] |
| 23. | Kavitha K, Thiyagarajan P, Rathna Nandhini J, Mishra R, Nagini S. Chemopreventive effects of diverse dietary phytochemicals against DMBA-induced hamster buccal pouch carcinogenesis via the induction of Nrf2-mediated cytoprotective antioxidant, detoxification, and DNA repair enzymes. Biochimie. 2013;95:1629-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Tanaka T, Kawamori T, Ohnishi M, Makita H, Mori H, Satoh K, Hara A. Suppression of azoxymethane-induced rat colon carcinogenesis by dietary administration of naturally occurring xanthophylls astaxanthin and canthaxanthin during the postinitiation phase. Carcinogenesis. 1995;16:2957-2963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 78] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Yasui Y, Hosokawa M, Mikami N, Miyashita K, Tanaka T. Dietary astaxanthin inhibits colitis and colitis-associated colon carcinogenesis in mice via modulation of the inflammatory cytokines. Chem Biol Interact. 2011;193:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 26. | Kavitha K, Kowshik J, Kishore TK, Baba AB, Nagini S. Astaxanthin inhibits NF-κB and Wnt/β-catenin signaling pathways via inactivation of Erk/MAPK and PI3K/Akt to induce intrinsic apoptosis in a hamster model of oral cancer. Biochim Biophys Acta. 2013;1830:4433-4444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 147] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 27. | Gradelet S, Le Bon AM, Bergès R, Suschetet M, Astorg P. Dietary carotenoids inhibit aflatoxin B1-induced liver preneoplastic foci and DNA damage in the rat: role of the modulation of aflatoxin B1 metabolism. Carcinogenesis. 1998;19:403-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 72] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Chew BP, Park JS, Wong MW, Wong TS. A comparison of the anticancer activities of dietary beta-carotene, canthaxanthin and astaxanthin in mice in vivo. Anticancer Res. 1999;19:1849-1853. [PubMed] |
| 29. | Kurihara H, Koda H, Asami S, Kiso Y, Tanaka T. Contribution of the antioxidative property of astaxanthin to its protective effect on the promotion of cancer metastasis in mice treated with restraint stress. Life Sci. 2002;70:2509-2520. [PubMed] |
| 30. | Kozuki Y, Miura Y, Yagasaki K. Inhibitory effects of carotenoids on the invasion of rat ascites hepatoma cells in culture. Cancer Lett. 2000;151:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 73] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Zhang X, Zhao WE, Hu L, Zhao L, Huang J. Carotenoids inhibit proliferation and regulate expression of peroxisome proliferators-activated receptor gamma (PPARγ) in K562 cancer cells. Arch Biochem Biophys. 2011;512:96-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 32. | Hix LM, Lockwood SF, Bertram JS. Bioactive carotenoids: potent antioxidants and regulators of gene expression. Redox Rep. 2004;9:181-191. [PubMed] |
| 33. | Vine AL, Bertram JS. Upregulation of connexin 43 by retinoids but not by non-provitamin A carotenoids requires RARs. Nutr Cancer. 2005;52:105-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Saw CL, Yang AY, Guo Y, Kong AN. Astaxanthin and omega-3 fatty acids individually and in combination protect against oxidative stress via the Nrf2-ARE pathway. Food Chem Toxicol. 2013;62:869-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 35. | Wolf AM, Asoh S, Hiranuma H, Ohsawa I, Iio K, Satou A, Ishikura M, Ohta S. Astaxanthin protects mitochondrial redox state and functional integrity against oxidative stress. J Nutr Biochem. 2010;21:381-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 132] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 36. | DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K, Mangal D, Yu KH, Yeo CJ, Calhoun ES. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1879] [Cited by in RCA: 1784] [Article Influence: 127.4] [Reference Citation Analysis (0)] |
| 37. | Satoh H, Moriguchi T, Takai J, Ebina M, Yamamoto M. Nrf2 prevents initiation but accelerates progression through the Kras signaling pathway during lung carcinogenesis. Cancer Res. 2013;73:4158-4168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 197] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 38. | Nakao R, Nelson OL, Park JS, Mathison BD, Thompson PA, Chew BP. Effect of dietary astaxanthin at different stages of mammary tumor initiation in BALB/c mice. Anticancer Res. 2010;30:2171-2175. [PubMed] |
| 39. | Karppi J, Rissanen TH, Nyyssönen K, Kaikkonen J, Olsson AG, Voutilainen S, Salonen JT. Effects of astaxanthin supplementation on lipid peroxidation. Int J Vitam Nutr Res. 2007;77:3-11. [PubMed] |
| 40. | Choi HD, Kim JH, Chang MJ, Kyu-Youn Y, Shin WG. Effects of astaxanthin on oxidative stress in overweight and obese adults. Phytother Res. 2011;25:1813-1818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 41. | Park JS, Chyun JH, Kim YK, Line LL, Chew BP. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr Metab (Lond). 2010;7:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 288] [Cited by in RCA: 328] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 42. | Parisi V, Tedeschi M, Gallinaro G, Varano M, Saviano S, Piermarocchi S. Carotenoids and antioxidants in age-related maculopathy italian study: multifocal electroretinogram modifications after 1 year. Ophthalmology. 2008;115:324-333.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 43. | Piermarocchi S, Saviano S, Parisi V, Tedeschi M, Panozzo G, Scarpa G, Boschi G, Lo Giudice G. Carotenoids in Age-related Maculopathy Italian Study (CARMIS): two-year results of a randomized study. Eur J Ophthalmol. 2012;22:216-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 44. | Katagiri M, Satoh A, Tsuji S, Shirasawa T. Effects of astaxanthin-rich Haematococcus pluvialis extract on cognitive function: a randomised, double-blind, placebo-controlled study. J Clin Biochem Nutr. 2012;51:102-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 45. | Yano M, Kato M, Ikoma Y, Kawasaki A, Fukazawa Y, Sugiura M, Matsumoto H, Oohara Y, Nagao A, Ogawa K. Quantitation of carotenoids in raw and processed fruits in Japan. Food Sci Technol Res. 2005;11:13-18. [RCA] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 46. | Holden JM, Eldridge AL, Beecher GR, Buzzard IM, Bhagwat S, Davis CS, Douglass LW, Gebhardt S, Haytowitz D, Schakel S. Carotenoid content of US foods: An update of the database. J Food Comp Anal. 1999;12:169-196. [RCA] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 370] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 47. | Wahlqvist ML, Wattanapenpaiboon N, Macrae FA, Lambert JR, MacLennan R, Hsu-Hage BH. Changes in serum carotenoids in subjects with colorectal adenomas after 24 mo of beta-carotene supplementation. Australian Polyp Prevention Project Investigators. Am J Clin Nutr. 1994;60:936-943. [PubMed] |
| 48. | Sugiura M, Ogawa K, Yano M. Absorption, storage and distribution of β-cryptoxanthin in rat after chronic administration of Satsuma mandarin (Citrus unshiu MARC.) juice. Biol Pharm Bull. 2013;36:147-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 49. | Sugiura M, Kato M, Matsumoto H, Nagao A, Yano M. Serum concentration of beta-cryptoxanthin in Japan reflects the frequency of Satsuma mandarin (Citrus unshiu Marc.) consumption. J Health Sci. 2002;48:350-353. [RCA] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 50. | Sugiura M, Matsumoto H, Kato M, Ikoma Y, Yano M, Nagao A. Seasonal changes in the relationship between serum concentration of beta-cryptoxanthin and serum lipid levels. J Nutr Sci Vitaminol (Tokyo). 2004;50:410-415. [PubMed] |
| 51. | Sugiura M, Matsumoto H, Kato M, Ikoma Y, Yano M, Nagao A. Multiple linear regression analysis of the seasonal changes in the serum concentration of beta-cryptoxanthin. J Nutr Sci Vitaminol (Tokyo). 2004;50:196-202. [PubMed] |
| 52. | Canfield LM, Clandinin MT, Davies DP, Fernandez MC, Jackson J, Hawkes J, Goldman WJ, Pramuk K, Reyes H, Sablan B. Multinational study of major breast milk carotenoids of healthy mothers. Eur J Nutr. 2003;42:133-141. [PubMed] |
| 53. | Montonen J, Knekt P, Järvinen R, Reunanen A. Dietary antioxidant intake and risk of type 2 diabetes. Diabetes Care. 2004;27:362-366. [PubMed] |
| 54. | Cerhan JR, Saag KG, Merlino LA, Mikuls TR, Criswell LA. Antioxidant micronutrients and risk of rheumatoid arthritis in a cohort of older women. Am J Epidemiol. 2003;157:345-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 165] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 55. | Liu C, Bronson RT, Russell RM, Wang XD. β-Cryptoxanthin supplementation prevents cigarette smoke-induced lung inflammation, oxidative damage, and squamous metaplasia in ferrets. Cancer Prev Res (Phila). 2011;4:1255-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 56. | Männistö S, Smith-Warner SA, Spiegelman D, Albanes D, Anderson K, van den Brandt PA, Cerhan JR, Colditz G, Feskanich D, Freudenheim JL. Dietary carotenoids and risk of lung cancer in a pooled analysis of seven cohort studies. Cancer Epidemiol Biomarkers Prev. 2004;13:40-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 120] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 57. | De Stefani E, Brennan P, Boffetta P, Ronco AL, Mendilaharsu M, Deneo-Pellegrini H. Vegetables, fruits, related dietary antioxidants, and risk of squamous cell carcinoma of the esophagus: a case-control study in Uruguay. Nutr Cancer. 2000;38:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 58. | Zeegers MP, Goldbohm RA, van den Brandt PA. Are retinol, vitamin C, vitamin E, folate and carotenoids intake associated with bladder cancer risk? Results from the Netherlands Cohort Study. Br J Cancer. 2001;85:977-983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 74] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 59. | Goodman MT, McDuffie K, Hernandez B, Hankin JH, Wilkens LR, Franke AA, Kolonel LN, Kuypers J, Kiviat N, Bertram CC. The Association of Plasma Micronutrients with the Risk of Cervical Atypical Squamous Cells of Undetermined Significance (ASCUS). Asian Pac J Cancer Prev. 2000;1:337-345. [PubMed] |
| 60. | Nishino H, Murakoshi M, Satomi Y. Health promotion by antioxidants. Functional Foods in Health and Disease. 2011;1:574-581 Available from: http://www.functionalfoodscenter.net/files/48097641.pdf. |
| 61. | Opinion of the scientific panel on additives and products or substances used in animal feed on the request from the commission on the safety of use of colouring agents in animal nutrition. The EFSA Journal. 2006;386:1-40 Available from: http: //www.efsa.europa.eu/en/efsajournal/doc/320.pdf. |
| 62. | Miyazawa K, Miyamoto S, Suzuki R, Yasui Y, Ikeda R, Kohno H, Yano M, Tanaka T, Hata K, Suzuki K. Dietary beta-cryptoxanthin inhibits N-butyl-N-(4-hydroxybutyl)nitrosamine-induced urinary bladder carcinogenesis in male ICR mice. Oncol Rep. 2007;17:297-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 63. | Sugiura M, Nakamura M, Ikoma Y, Yano M, Ogawa K, Matsumoto H, Kato M, Ohshima M, Nagao A. Serum carotenoid concentrations are inversely associated with serum aminotransferases in hyperglycemic subjects. Diabetes Res Clin Pract. 2006;71:82-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 64. | Sugiura M, Nakamura M, Ikoma Y, Yano M, Ogawa K, Matsumoto H, Kato M, Ohshima M, Nagao A. High serum carotenoids are inversely associated with serum gamma-glutamyltransferase in alcohol drinkers within normal liver function. J Epidemiol. 2005;15:180-186. [PubMed] |
| 65. | Nakamura M, Sugiura M, Aoki N. High beta-carotene and beta-cryptoxanthin are associated with low pulse wave velocity. Atherosclerosis. 2006;184:363-369. [PubMed] |
| 66. | Sugiura M, Nakamura M, Ikoma Y, Yano M, Ogawa K, Matsumoto H, Kato M, Ohshima M, Nagao A. The homeostasis model assessment-insulin resistance index is inversely associated with serum carotenoids in non-diabetic subjects. J Epidemiol. 2006;16:71-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 67. | Sugiura M, Nakamura M, Ogawa K, Ikoma Y, Matsumoto H, Ando F, Shimokata H, Yano M. Associations of serum carotenoid concentrations with the metabolic syndrome: interaction with smoking. Br J Nutr. 2008;100:1297-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 68. | Sugiura M, Nakamura M, Ogawa K, Ikoma Y, Ando F, Shimokata H, Yano M. Dietary patterns of antioxidant vitamin and carotenoid intake associated with bone mineral density: findings from post-menopausal Japanese female subjects. Osteoporos Int. 2011;22:143-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 69. | Lorenzo Y, Azqueta A, Luna L, Bonilla F, Domínguez G, Collins AR. The carotenoid beta-cryptoxanthin stimulates the repair of DNA oxidation damage in addition to acting as an antioxidant in human cells. Carcinogenesis. 2009;30:308-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 70. | Uchiyama S, Yamaguchi M. Beta-cryptoxanthin stimulates apoptotic cell death and suppresses cell function in osteoclastic cells: change in their related gene expression. J Cell Biochem. 2006;98:1185-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 71. | Nishino H, Tokuda H, Murakoshi M, Satomi Y, Masuda M, Onozuka M, Yamaguchi S, Takayasu J, Tsuruta J, Okuda M. Cancer prevention by natural carotenoids. Biofactors. 2000;13:89-94. [PubMed] |
| 72. | Tsushima M, Maoka T, Katsuyama M, Kozuka M, Matsuno T, Tokuda H, Nishino H, Iwashima A. Inhibitory effect of natural carotenoids on Epstein-Barr virus activation activity of a tumor promoter in Raji cells. A screening study for anti-tumor promoters. Biol Pharm Bull. 1995;18:227-233. [PubMed] |
| 73. | Narisawa T, Fukaura Y, Oshima S, Inakuma T, Yano M, Nishino H. Chemoprevention by the oxygenated carotenoid beta-cryptoxanthin of N-methylnitrosourea-induced colon carcinogenesis in F344 rats. Jpn J Cancer Res. 1999;90:1061-1065. [PubMed] |
| 74. | Iskandar AR, Liu C, Smith DE, Hu KQ, Choi SW, Ausman LM, Wang XD. β-cryptoxanthin restores nicotine-reduced lung SIRT1 to normal levels and inhibits nicotine-promoted lung tumorigenesis and emphysema in A/J mice. Cancer Prev Res (Phila). 2013;6:309-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 75. | Iwamoto M, Imai K, Ohta H, Shirouchi B, Sato M. Supplementation of highly concentrated β-cryptoxanthin in a satsuma mandarin beverage improves adipocytokine profiles in obese Japanese women. Lipids Health Dis. 2012;11:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 76. | Ministry of Health. Labour and Welfare Japan (2004) The National Nutrition Survey in Japan [in Japanese]. Tokyo, Japan: Dai-ichi shuppan 2002; . |
| 77. | Cumashi A, Ushakova NA, Preobrazhenskaya ME, D’Incecco A, Piccoli A, Totani L, Tinari N, Morozevich GE, Berman AE, Bilan MI. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology. 2007;17:541-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 765] [Cited by in RCA: 651] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 78. | Shibata H, Iimuro M, Uchiya N, Kawamori T, Nagaoka M, Ueyama S, Hashimoto S, Yokokura T, Sugimura T, Wakabayashi K. Preventive effects of Cladosiphon fucoidan against Helicobacter pylori infection in Mongolian gerbils. Helicobacter. 2003;8:59-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 85] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 79. | Yonekura L, Kobayashi M, Terasaki M, Nagao A. Keto-carotenoids are the major metabolites of dietary lutein and fucoxanthin in mouse tissues. J Nutr. 2010;140:1824-1831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 80. | Campbell SJ, Bité JS, Burridge TR. Seasonal patterns in the photosynthetic capacity, tissue pigment and nutrient content of different developmental stages of Undaria pinnatifida (Phaeophyta: Laminariales) in port phillip bay, south-eastern Australia. Bot Mar. 1999;42:231-242. [DOI] [Full Text] |
| 81. | Terasaki M, Narayan B, Kamogawa H, Nomura M, Stephen NM, Kawagoe C, Hosokowa M, Miyashita K Carotenoid profile of edible Japanese seaweeds: An improved HPLC method for separation of major carotenoids. J Aquatic Food Prod Tech. 2012;21:468-479. [RCA] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 82. | Hashimoto T, Ozaki Y, Mizuno M, Yoshida M, Nishitani Y, Azuma T, Komoto A, Maoka T, Tanino Y, Kanazawa K. Pharmacokinetics of fucoxanthinol in human plasma after the oral administration of kombu extract. Br J Nutr. 2012;107:1566-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 83. | Beppu F, Niwano Y, Tsukui T, Hosokawa M, Miyashita K. Single and repeated oral dose toxicity study of fucoxanthin (FX), a marine carotenoid, in mice. J Toxicol Sci. 2009;34:501-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 84. | Iio K, Okada Y, Ishikura M. [Single and 13-week oral toxicity study of fucoxanthin oil from microalgae in rats]. Shokuhin Eiseigaku Zasshi. 2011;52:183-189. [PubMed] [DOI] [Full Text] |
| 85. | Hosokawa M, Kudo M, Maeda H, Kohno H, Tanaka T, Miyashita K. Fucoxanthin induces apoptosis and enhances the antiproliferative effect of the PPARgamma ligand, troglitazone, on colon cancer cells. Biochim Biophys Acta. 2004;1675:113-119. [PubMed] |
| 86. | Das SK, Hashimoto T, Kanazawa K. Growth inhibition of human hepatic carcinoma HepG2 cells by fucoxanthin is associated with down-regulation of cyclin D. Biochim Biophys Acta. 2008;1780:743-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 87. | Kotake-Nara E, Kushiro M, Zhang H, Sugawara T, Miyashita K, Nagao A. Carotenoids affect proliferation of human prostate cancer cells. J Nutr. 2001;131:3303-3306. [PubMed] |
| 88. | Zhang Z, Zhang P, Hamada M, Takahashi S, Xing G, Liu J, Sugiura N. Potential chemoprevention effect of dietary fucoxanthin on urinary bladder cancer EJ-1 cell line. Oncol Rep. 2008;20:1099-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 89. | Miyashita K, Nishikawa S, Beppu F, Tsukui T, Abe M, Hosokawa M. The allenic carotenoid fucoxanthin, a novel marine nutraceutical from brown seaweeds. J Sci Food Agric. 2011;91:1166-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 90. | Liu CL, Huang YS, Hosokawa M, Miyashita K, Hu ML. Inhibition of proliferation of a hepatoma cell line by fucoxanthin in relation to cell cycle arrest and enhanced gap junctional intercellular communication. Chem Biol Interact. 2009;182:165-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 91. | Liu CL, Chiu YT, Hu ML. Fucoxanthin enhances HO-1 and NQO1 expression in murine hepatic BNL CL.2 cells through activation of the Nrf2/ARE system partially by its pro-oxidant activity. J Agric Food Chem. 2011;59:11344-11351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 92. | Ye G, Lu Q, Zhao W, Du D, Jin L, Liu Y. Fucoxanthin induces apoptosis in human cervical cancer cell line HeLa via PI3K/Akt pathway. Tumour Biol. 2014;35:11261-11267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 93. | Rwigemera A, Mamelona J, Martin LJ. Inhibitory effects of fucoxanthinol on the viability of human breast cancer cell lines MCF-7 and MDA-MB-231 are correlated with modulation of the NF-kappaB pathway. Cell Biol Toxicol. 2014;30:157-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 94. | Kotake-Nara E, Asai A, Nagao A. Neoxanthin and fucoxanthin induce apoptosis in PC-3 human prostate cancer cells. Cancer Lett. 2005;220:75-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 95. | Tafuku S, Ishikawa C, Yasumoto T, Mori N. Anti-neoplastic effects of fucoxanthin and its deacetylated product, fucoxanthinol, on Burkitt’s and Hodgkin’s lymphoma cells. Oncol Rep. 2012;28:1512-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 96. | Kim JM, Araki S, Kim DJ, Park CB, Takasuka N, Baba-Toriyama H, Ota T, Nir Z, Khachik F, Shimidzu N. Chemopreventive effects of carotenoids and curcumins on mouse colon carcinogenesis after 1,2-dimethylhydrazine initiation. Carcinogenesis. 1998;19:81-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 204] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 97. | Terasaki M, Nagao A, Maeda H, Miyashita K, Masuda S. Combined antiproliferative effect of dietary PPARγ suppressing lipids fucoxanthinol and 1α,25-dihydroxyvitamin D3 in human colon cancer cells. (Proceeding of The Japanese Society for Carotenoid Research). Carotenoid Science. 2012;17:40-43. |
| 98. | Mutoh M, Niho N, Wakabayashi K. Concomitant suppression of hyperlipidemia and intestinal polyp formation by increasing lipoprotein lipase activity in Apc-deficient mice. Biol Chem. 2006;387:381-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 99. | Ueno T, Teraoka N, Takasu S, Nakano K, Takahashi M, Yamamoto M, Fujii G, Komiya M, Yanaka A, Wakabayashi K. Suppressive effect of pioglitazone, a PPAR gamma ligand, on azoxymethane-induced colon aberrant crypt foci in KK-Ay mice. Asian Pac J Cancer Prev. 2012;13:4067-4073. [PubMed] |
| 100. | Maeda H, Hosokawa M, Sashima T, Funayama K, Miyashita K. Effect of medium-chain triacylglycerols on anti-obesity effect of fucoxanthin. J Oleo Sci. 2007;56:615-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 101. | Abidov M, Ramazanov Z, Seifulla R, Grachev S. The effects of Xanthigen in the weight management of obese premenopausal women with non-alcoholic fatty liver disease and normal liver fat. Diabetes Obes Metab. 2010;12:72-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 173] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 102. | Penn R, Worthington DJ. Is serum gamma-glutamyltransferase a misleading test? Br Med J (Clin Res Ed). 1983;286:531-535. [PubMed] |
| 103. | Hori M, Takahashi M, Hiraoka N, Yamaji T, Mutoh M, Ishigamori R, Furuta K, Okusaka T, Shimada K, Kosuge T. Association of pancreatic Fatty infiltration with pancreatic ductal adenocarcinoma. Clin Transl Gastroenterol. 2014;5:e53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 130] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 104. | Eden G, Archinti M, Furlan F, Murphy R, Degryse B. The urokinase receptor interactome. Curr Pharm Des. 2011;17:1874-1889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 105. | Teas J, Vena S, Cone DL, Irhimeh M. The consumption of seaweed as a protective factor in the etiology of breast cancer: proof of principle. J Appl Phycol. 2013;25:771-779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 106. | Jänicke F, Prechtl A, Thomssen C, Harbeck N, Meisner C, Untch M, Sweep CG, Selbmann HK, Graeff H, Schmitt M. Randomized adjuvant chemotherapy trial in high-risk, lymph node-negative breast cancer patients identified by urokinase-type plasminogen activator and plasminogen activator inhibitor type 1. J Natl Cancer Inst. 2001;93:913-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 274] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 107. | Look MP, van Putten WL, Duffy MJ, Harbeck N, Christensen IJ, Thomssen C, Kates R, Spyratos F, Fernö M, Eppenberger-Castori S. Pooled analysis of prognostic impact of urokinase-type plasminogen activator and its inhibitor PAI-1 in 8377 breast cancer patients. J Natl Cancer Inst. 2002;94:116-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 377] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 108. | Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, Somerfield MR, Hayes DF, Bast RC. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287-5312. [PubMed] |
| 109. | Goodwin TW. The biochemistry of the carotenoids, Vol. I. Plants. 2nd ed. New York, NY: Chapman and Hall 1980; . |
| 110. | Khachik F, Beecher GR, Whittaker NF. Separation, identification, and quantification of the major carotenoid and chlorophyll constituents in extracts of several green vegetables by liquid chromatography. J Agric Food Chem. 1986;34:603-616. [DOI] [Full Text] |
| 111. | Takaichi S, Mimuro M. Distribution and geometric isomerism of neoxanthin in oxygenic phototrophs: 9’-cis, a sole molecular form. Plant Cell Physiol. 1998;39:968-977. [DOI] [Full Text] |
| 112. | Ruban AV, Lee PJ, Wentworth M, Young AJ, Horton P. Determination of the stoichiometry and strength of binding of xanthophylls to the photosystem II light harvesting complexes. J Biol Chem. 1999;274:10458-10465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 198] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 113. | Seo M, Koshiba T. Complex regulation of ABA biosynthesis in plants. Trends Plant Sci. 2002;7:41-48. [PubMed] |
| 114. | Marki-Fischer E, Eugster CH Neoflor and 6-epineoflor from flowers of Trollius europaeus; highfield 1H-NMR spectra of (all-E)-neoxanthin and (9’Z)-neoxanthin. Helv Chim Acta. 1990;73:1637-1643. [RCA] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 115. | Asai A, Terasaki M, Nagao A. An epoxide-furanoid rearrangement of spinach neoxanthin occurs in the gastrointestinal tract of mice and in vitro: formation and cytostatic activity of neochrome stereoisomers. J Nutr. 2004;134:2237-2243. [PubMed] |
| 116. | Eugster CH (1995) Chemical derivatization: microscale tests for the presence of common functional groups in carotenoids. In: Carotenoids Vol. 1A: Isolation and Analysis. Britton G, Liaaen-Jensen S, Pfander H, editors. Birkhäuser Verlag, Basel, Switzerland 1995; 71-80. |
| 117. | Asai A, Yonekura L, Nagao A. Low bioavailability of dietary epoxyxanthophylls in humans. Br J Nutr. 2008;100:273-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 118. | Sugawara T, Kushiro M, Zhang H, Nara E, Ono H, Nagao A. Lysophosphatidylcholine enhances carotenoid uptake from mixed micelles by Caco-2 human intestinal cells. J Nutr. 2001;131:2921-2927. [PubMed] |
| 119. | Barua AB, Olson JA. Xanthophyll epoxides, unlike beta-carotene monoepoxides, are not detectibly absorbed by humans. J Nutr. 2001;131:3212-3215. [PubMed] |
| 120. | Hashimoto T, Ozaki Y, Taminato M, Das SK, Mizuno M, Yoshimura K, Maoka T, Kanazawa K. The distribution and accumulation of fucoxanthin and its metabolites after oral administration in mice. Br J Nutr. 2009;102:242-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 123] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 121. | Pérez-Gálvez A, Martin HD, Sies H, Stahl W. Incorporation of carotenoids from paprika oleoresin into human chylomicrons. Br J Nutr. 2003;89:787-793. [PubMed] |
| 122. | Kotake-Nara E, Sugawara T, Nagao A. Antiproliferative effect of neoxanthin and fucoxanthin on culture cells. Fish Sci. 2005;71:459-461 Available from: http: //link.springer.com/article/10.1111/j.1444-2906.2005.00986.x#page-1. |
| 123. | Terasaki M, Asai A, Zhang H, Nagao A. A highly polar xanthophyll of 9’-cis-neoxanthin induces apoptosis in HCT116 human colon cancer cells through mitochondrial dysfunction. Mol Cell Biochem. 2007;300:227-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 124. | Ugocsai K, Varga A, Molnár P, Antus S, Molnár J. Effects of selected flavonoids and carotenoids on drug accumulation and apoptosis induction in multidrug-resistant colon cancer cells expressing MDR1/LRP. In Vivo. 2005;19:433-438. [PubMed] |
| 125. | Chang JM, Lin JK. Isolation of neoxanthin from spinach and its prevention on lipid peroxidation. J Chin Med. 1993;4:235-245 Available from: http: //tao.wordpedia.com/show_pdf.ashx?sess=m3o4bi2vfuk2zou5qjretbmz&file_name=JO00000295_4-3_235-245&file_type=r. |
| 126. | Chang JM, CHen WC, Hong D, Lin JK. The inhibition of DMBA-induced carcinogenesis by neoxanthin in hamster buccal pouch. Nutr Cancer. 1995;24:325-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 127. | Murakami A, Nakashima M, Koshiba T, Maoka T, Nishino H, Yano M, Sumida T, Kim OK, Koshimizu K, Ohigashi H. Modifying effects of carotenoids on superoxide and nitric oxide generation from stimulated leukocytes. Cancer Lett. 2000;149:115-123. [PubMed] |
| 128. | Okada T, Nakai M, Maeda H, Hosokawa M, Sashima T, Miyashita K. Suppressive effect of neoxanthin on the differentiation of 3T3-L1 adipose cells. J Oleo Sci. 2008;57:345-351. [PubMed] |
| 129. | Mayne ST. Beta-carotene, carotenoids, and disease prevention in humans. FASEB J. 1996;10:690-701. [PubMed] |
| 130. | Slattery ML, Benson J, Curtin K, Ma KN, Schaeffer D, Potter JD. Carotenoids and colon cancer. Am J Clin Nutr. 2000;71:575-582. [PubMed] |
| 131. | Lutein and zeaxanthin. Monograph. Altern Med Rev. 2005;10:128-135. [PubMed] |
| 132. | Sommerburg O, Keunen JE, Bird AC, van Kuijk FJ. Fruits and vegetables that are sources for lutein and zeaxanthin: the macular pigment in human eyes. Br J Ophthalmol. 1998;82:907-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 293] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 133. | Kelly ER, Plat J, Haenen GR, Kijlstra A, Berendschot TT. The effect of modified eggs and an egg-yolk based beverage on serum lutein and zeaxanthin concentrations and macular pigment optical density: results from a randomized trial. PLoS One. 2014;9:e92659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 134. | Landrum JT, Bone RA. Lutein, zeaxanthin, and the macular pigment. Arch Biochem Biophys. 2001;385:28-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 485] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 135. | Ben Amara N, Tourniaire F, Maraninchi M, Attla N, Amiot-Carlin MJ, Raccah D, Valéro R, Landrier JF, Darmon P. Independent positive association of plasma β-carotene concentrations with adiponectin among non-diabetic obese subjects. Eur J Nutr. 2014;. [PubMed] |
| 136. | Kritchevsky SB. beta-Carotene, carotenoids and the prevention of coronary heart disease. J Nutr. 1999;129:5-8. [PubMed] |
| 137. | Ascherio A, Rimm EB, Hernán MA, Giovannucci E, Kawachi I, Stampfer MJ, Willett WC. Relation of consumption of vitamin E, vitamin C, and carotenoids to risk for stroke among men in the United States. Ann Intern Med. 1999;130:963-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 140] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 138. | Michaud DS, Feskanich D, Rimm EB, Colditz GA, Speizer FE, Willett WC, Giovannucci E. Intake of specific carotenoids and risk of lung cancer in 2 prospective U.S. cohorts. Am J Clin Nutr. 2000;72:990-997. [PubMed] |
| 139. | Le Marchand L, Hankin JH, Bach F, Kolonel LN, Wilkens LR, Stacewicz-Sapuntzakis M, Bowen PE, Beecher GR, Laudon F, Baque P. An ecological study of diet and lung cancer in the South Pacific. Int J Cancer. 1995;63:18-23. [PubMed] |
| 140. | Okuyama Y, Ozasa K, Oki K, Nishino H, Fujimoto S, Watanabe Y. Inverse associations between serum concentrations of zeaxanthin and other carotenoids and colorectal neoplasm in Japanese. Int J Clin Oncol. 2014;19:87-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 141. | Tsubono Y, Tsugane S, Gey KF. Plasma antioxidant vitamins and carotenoids in five Japanese populations with varied mortality from gastric cancer. Nutr Cancer. 1999;34:56-61. [PubMed] |
| 142. | Stahl W, Heinrich U, Jungmann H, Sies H, Tronnier H. Carotenoids and carotenoids plus vitamin E protect against ultraviolet light-induced erythema in humans. Am J Clin Nutr. 2000;71:795-798. [PubMed] |
| 143. | Dorgan JF, Sowell A, Swanson CA, Potischman N, Miller R, Schussler N, Stephenson HE. Relationships of serum carotenoids, retinol, alpha-tocopherol, and selenium with breast cancer risk: results from a prospective study in Columbia, Missouri (United States). Cancer Causes Control. 1998;9:89-97. [PubMed] |
| 144. | Zhang S, Tang G, Russell RM, Mayzel KA, Stampfer MJ, Willett WC, Hunter DJ. Measurement of retinoids and carotenoids in breast adipose tissue and a comparison of concentrations in breast cancer cases and control subjects. Am J Clin Nutr. 1997;66:626-632. [PubMed] |
| 145. | Yeum KJ, Ahn SH, Rupp de Paiva SA, Lee-Kim YC, Krinsky NI, Russell RM. Correlation between carotenoid concentrations in serum and normal breast adipose tissue of women with benign breast tumor or breast cancer. J Nutr. 1998;128:1920-1926. [PubMed] |
| 146. | Toniolo P, Van Kappel AL, Akhmedkhanov A, Ferrari P, Kato I, Shore RE, Riboli E. Serum carotenoids and breast cancer. Am J Epidemiol. 2001;153:1142-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 121] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 147. | Bravi F, Bosetti C, Filomeno M, Levi F, Garavello W, Galimberti S, Negri E, La Vecchia C. Foods, nutrients and the risk of oral and pharyngeal cancer. Br J Cancer. 2013;109:2904-2910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 148. | Dagnelie G, Zorge IS, McDonald TM. Lutein improves visual function in some patients with retinal degeneration: a pilot study via the Internet. Optometry. 2000;71:147-164. [PubMed] |
| 149. | Granado F, Olmedilla B, Gil-Martínez E, Blanco I. Lutein ester in serum after lutein supplementation in human subjects. Br J Nutr. 1998;80:445-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 150. | González de Mejía E, Ramos-Gómez M, Loarca-Piña G. Antimutagenic activity of natural xanthophylls against aflatoxin B1 in Salmonella typhimurium. Environ Mol Mutagen. 1997;30:346-353. [PubMed] [DOI] [Full Text] |
| 151. | Kawashima T. A marine carotenoid, fucoxanthin, induces regulatory T cells and inhibits Th17 cell differentiation in vitro. Biosci Biotechnol Biochem. 2011;75:2066-2069. [PubMed] |
| 152. | Iannone A, Rota C, Bergamini S, Tomasi A, Canfield LM. Antioxidant activity of carotenoids: An electron-spin resonance study on β-carotene and lutein interaction with free radicals generated in a chemical system. J Biochem Mol Toxicol. 1998;1:299-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 153. | Sujak A, Gabrielska J, Grudzinski W, Borc R, Mazurek P, Gruszecki WI. Lutein and zeaxanthin as protectors of lipid membranes against oxidative damage: The structural aspects. Arch Biochem Biophys. 1999;371:301-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 219] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 154. | Stahl W, Junghans A, de Boer B, Driomina ES, Briviba K, Sies H. Carotenoid mixtures protect multilamellar liposomes against oxidative damage: Synergistic effects of lycopene and lutein. FEBS Lett. 1998;427:305-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 209] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 155. | Rafi MM, Kanakasabai S, Gokam SV, Krueger EG, Bright JJ. Dietary Lutein Modulates Growth and Survival Genes in Prostate Cancer Cells. J Med Food. 2014;Aug 27; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 156. | Pei YX, Heng ZC, Duan GC, Wang MC. [The mechanisms and effects of lutein on inducing the cell differentiation of human esophagus cancer EC9706]. Sichuan Daxue Xuebao Yixueban. 2007;38:629-632. [PubMed] |
| 157. | Sumantran VN, Zhang R, Lee DS, Wicha MS. Differential regulation of apoptosis in normal versus transformed mammary epithelium by lutein and retinoic acid. Cancer Epidemiol Biomarkers Prev. 2000;9:257-263. [PubMed] |
| 158. | Li Z, Wang Y, Mo B. [The effects of carotenoids on the proliferation of human breast cancer cell and gene expression of bcl-2]. Zhonghua Yufangyixue Zazhi. 2002;36:254-257. [PubMed] |
| 159. | Park JS, Chew BP, Wong TS, Zhang JX, Magnuson NS. Dietary lutein but not astaxanthin or beta-carotene increases pim-1 gene expression in murine lymphocytes. Nutr Cancer. 1999;33:206-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 160. | Gonzalez de Mejia E, Loarca-Pina G, Ramos-Gomez M. Antimutagenicity of xanthophylls present in Aztec Marigold (Tagetes erecta) against 1-nitropyrene. Mutat Res. 1997;389:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |