Revised: March 3, 2014
Accepted: March 6, 2014
Published online: March 9, 2014
Processing time: 176 Days and 8.1 Hours
Implantable electrochemical microsensors are characterized by high sensitivity, while amperometric biosensors are very selective in virtue of the biological detecting element. Each sensor, specific for every neurochemical species, is a miniaturized high-technology device resulting from the combination of several factors: electrode material, shielding polymers, applied electrochemical technique, and in the case of biosensors, biological sensing material, stabilizers, and entrapping chemical nets. In this paper, we summarize the available technology for the in vivo electrochemical monitoring of neurotransmitters (dopamine, norepinephrine, serotonin, acetylcholine, and glutamate), bioenergetic substrates (glucose, lactate, and oxygen), neuromodulators (ascorbic acid and nitric oxide), and exogenous molecules such as ethanol. We also describe the most represented biotelemetric technologies in order to wirelessly transmit the signals of the above-listed neurochemicals. Implantable (Bio)sensors, integrated into miniaturized telemetry systems, represent a new generation of analytical tools that could be used for studying the brain’s physiology and pathophysiology and the effects of different drugs (or toxic chemicals such as ethanol) on neurochemical systems.
Core tip: Electrochemical microsensors and amperometric biosensors arouse enormous scientific interest because of their low-cost technology and because they guarantee real-time monitoring of changes of the most important brain compounds. In conjunction with miniaturized telemetric devices, the electrochemical sensors, allow the neurochemical monitoring of extracellular space of discrete brain regions in awake, untethered animals for days or weeks. This new scientific approach opens new frontiers for studying the physiological and physiopathological pathways in wild-type animals and in genetic models of the most widespread neurodegenerative diseases.
- Citation: Farina D, Alvau MD, Puggioni G, Calia G, Bazzu G, Migheli R, Sechi O, Rocchitta G, Desole MS, Serra PA. Implantable (Bio)sensors as new tools for wireless monitoring of brain neurochemistry in real time. World J Pharmacol 2014; 3(1): 1-17
- URL: https://www.wjgnet.com/2220-3192/full/v3/i1/1.htm
- DOI: https://dx.doi.org/10.5497/wjp.v3.i1.1
The identification, observation, and quantification of extracellular biomolecules in the central nervous system (CNS) is a field of growing interest for studying the brain in physiological conditions and for identifying neurochemical changes during neurological diseases. The study of neurochemistry in real time is very important in preclinical (and recently also in clinical) research and for developing new therapeutic strategies for many neuropsychiatric diseases, such as schizophrenia, depression, epilepsy, multiple sclerosis, and neurodegenerative diseases (i.e., Parkinson’s and Alzheimer’s diseases), and also for neural conditions that deeply influence individual and social behavior such as addiction.
For decades, the extracellular neurochemistry of the CNS has been studied using in vivo microdialysis. Microdialysis is a minimally invasive technique suitable for measuring low-molecular-weight compounds in the extracellular compartment of several organs, tissues, or specific brain regions[1]. The microdialysis idea originated in the 1970s with the aim of implanting a hollow dialysis fiber (microdialysis probe) into a tissue for simulating the role of a blood capillary and recovering molecules from the extracellular compartment to highlight their regional changes in concentration[2,3]. When implanted in the brain, the microdialysis probe is perfused with an appropriate Ringer solution (that mimics the composition of the extracellular space fluid) so that neurochemicals are able to diffuse down their concentration gradients out of the probe. The recovered microdialysis samples are analyzed using different analytical methods. The poor temporal resolution and the need to have an available expensive analytical laboratory (for analyzing microdialysis samples) represent the major limitations of this technique.
In recent decades, implantable electrochemical sensors and biosensors have been emerging because of their versatility, their multiple applications, and most of all, their high spatial and temporal resolution[4-6]. In particular, implantable amperometric sensors have been proven to be very sensitive so as to allow the detection of very low concentrations of the studied analytes[5]. The basic idea of implantable electrochemical sensors is to “concentrate” an entire analytical laboratory “on the tip of a pin” without the need of an expensive analytical apparatus or of a dedicated laboratory.
In the past years, despite their high sensitivity, the main limitation for the use of electrochemical sensors was related to their poor selectivity. Recently, the development of new sensing materials and new shielding polymers and, mainly, the introduction of biological elements such as molecular recognition sites have allowed overcoming this limitation in a large part.
Today, each sensor, specific for every neurochemical species, is a miniaturized high-technology device resulting from the combination of several factors: electrode material, shielding polymers, applied electrochemical technique, and in the case of biosensors, biological sensing material, stabilizers, and entrapping chemical nets.
The dimensions of implantable electrochemical sensors vary from a few micrometers (5-10) up to 125 μm (always lower than those of a microdialysis probe, around 220 μm), and their sensing surface can be increased without increasing their invasiveness using new nanomaterials (i.e., carbon nanotubes); this process is often indicated as “nanostructuration” or simply “nano-on-micro”. But one of the most exciting perspectives, for future development and applications, is to combine implantable sensors with miniaturized electronic devices in order to transmit neurochemical signals at a distance so that awake animals are allowed to be totally free to move[4-6].
In this study, we highlight the state-of-art of electrochemical microsensors and biosensors, already used in preclinical research for recording neurochemical changes, suitable to be integrated in biotelemetry systems for the wireless monitoring of brain neurochemistry.
We have chosen to describe the available technology for the in vivo electrochemical monitoring of neurotransmitters (dopamine, norepinephrine, serotonin, acetylcholine, and glutamate), bioenergetic substrates (glucose, lactate, and oxygen), neuromodulators (ascorbic acid and nitric oxide), and exogenous molecules such as ethanol. In the next section, we also describe the most represented biotelemetric technologies to combine with the sensors in order to wirelessly transmit the signals of the above-listed neurochemicals.
Brain neurotransmitters such as the tyrosine derivatives dopamine, norepinephrine and the neuroactive tryptophan derivative serotonin have been implicated in the neurochemistry and physiology of mental diseases and neurological disorders.
Catecholamine biosynthesis is a common pathway from tyrosine[7], where the hydroxylation of tyrosine to L-3,4-dihydroxyphenylalanine by tyrosine hydroxylase is the rate-limiting step. Dopamine, a catechol-like neurotransmitter derived by L-3,4-dihydroxyphenylalanine decarboxylation, is actively involved in reward pathways[8,9] and in cognitive functions[10]. Its metabolism mainly occurs by reaction with monoamine oxidase and catechol-O-methyltransferase with the formation of dihydroxyphenylacetic acid , homovanillic acid, and 3-methoxytyramine. Neuronal death of catecholaminergic cells in the substantia nigra, with a consequent significant reduction of dopamine levels[11] as well as dihydroxyphenylacetic acid, homovanillic acid[12] and 3-methoxytyramine[13] in the striatum is a hallmark in Parkinson’s disease[1]. On the other hand, an increase in dopaminergic levels is involved in the etiopathogenesis of schizophrenia[14,15].
Formed by β-hydroxylation of dopamine, norepinephrine plays multiple roles as a hormone and a neurotransmitter. Norepinephrine is involved in directly increasing heart rate, suppressing neuroinflammation[16], and triggering the glycogenolysis and the release of glucose from energy stores[17], and along with serotonin, it is implicated in depression and anxiety disorders[18]. Moreover, the serotonergic system is also implicated in several neuroregulatory processes such as stress, aggression, pain, sleep, appetite, reproduction, circadian rhythm, and cardiovascular and respiratory functions[19].
All of these compounds are electrochemically active, show a similar 2-electron oxidation reaction with similar peak potentials at physiological pH, and can be directly detected by electrochemical oxidation of the molecule[20].
DA (Ox) → DA-QUINONE + 2e- + 2H+ (1)
NE (Ox) → NE-QUINONE + 2e- + 2H+ (2)
SEROTONIN (Ox) → SEROTONIN-
QUINONE + 2e- + 2H+ (3)
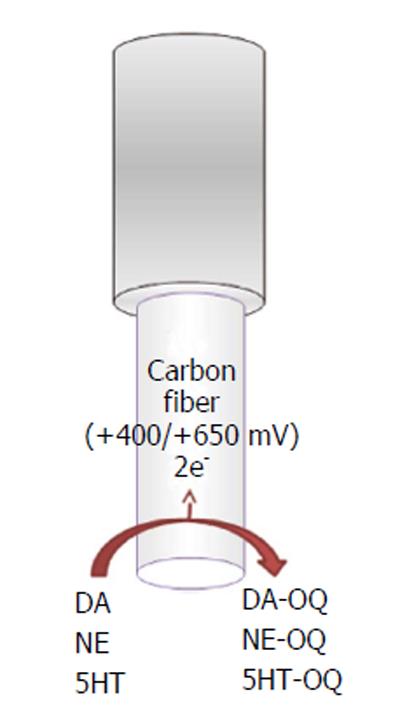
The electroactive neurotransmitters can be directly detected in vitro and in vivo using different electrochemical techniques (Figure 1) such as constant potential amperometry (CPA)[21], chronoamperometry[22,23], differential pulse voltammetry (DPV)[24], and fast-scan cyclic voltammetry (FSCV)[8,25-27]. Different microelectrodes for voltammetric recordings in the CNS are available, such as carbon paste microelectrodes, where carbon powder is mixed with silicon oil[10]; epoxy carbon microelectrodes, where epoxy resin is mixed with carbon paste; and carbon fiber, gold, and platinum (Pt) microelectrodes[20].
Along carbon-fiber microelectrodes, FSCV is the most common technique used for dopamine, norepinephrine and serotonin in vivo monitoring.
Carbon-fiber microelectrodes (Figure 1) are made by inserting a carbon fiber (outer diameter ranging between 5 and 30 μm, most commonly about 7 μm) into a glass capillary, which is pulled with a pipette puller and sealed by epoxy resin with 25 to 100 μm of the fiber protruding from the glass. The final geometry of the electrode, cylindrical[28] or disk shaped[29], is obtained by cutting or polishing the protruding carbon fiber[30]. Because of their dimension, carbon-fiber microelectrodes minimize distortion caused by ohmic drop, and then, coupled with a minimal tissue damages when implanted into the brain, they are suitable for high-temporal-resolution measurements[28]. In addition, a 7 μm carbon fiber does not stimulate glial reaction[25], in agreement with the evidence that probes that are less than 12 μm in diameter are not encapsulated as demonstrated by previous studies[31]. FSCV is a technique with high resolution and selectivity, where the potential applied to the microsensor is cycled between the reduction and the oxidation peaks of the analyte of interest[20]. For dopamine and norepinephrine recordings, a scan rate in a triangle fashion at 400 V/s is applied. The potential of the carbon-fiber microelectrode is ramped linearly from -400 mV vs Ag/AgCl to +1.3 V and back and held at -400 mV between scans[32]. To obtain the 5HT recording, an N-waveform scan rate is used, in which the applied potential is scanned first from 0 mV to +1200 mV then to -600 mV and back to 0 vs Ag/AgCl[27]. Typically, the waveform is applied for 10 ms, and voltammetric scans are repeated at 100 ms intervals. During the anodic sweep, the catecholamine (dopamine and/or norepinephrine) and serotonin present at the electrode surface are oxidized into corresponding orthoquinone and then reduced back at the original form during the cathodic sweep. The number of molecules that undergo electrolysis is directly proportional to the measured current[21]. The peak positions during oxidation and the reduction sweep as well as the peak shape can be used to distinguish different analytes[33].
Using fast-scan cyclic voltammetry, dopamine, norepinephrine, and serotonin have been shown a similar oxidation peak at approximately +650 mV vs Ag/AgCl[33-35] and a single reduction peak around -200 mV for dopamine and norepinephrine or Wdouble reduction peaks around 0 and -500 mV vs Ag/AgCl for serotonin[27].
Because they are virtually identical, voltammograms alone cannot be used to distinguish dopamine and norepinephrine[36], but histology and pharmacology, such as the use of dopamine drugs (raclopride, GBR 12909), can aid in this distinction even in simultaneous measurements with FSCV[37]. Ascorbic acid is the main electroactive interference molecule in the extracellular fluid (ECF) of the brain for electrochemical measurements. Ascorbic acid is 104-106 times higher than the concentrations of catecholamines in the ECF of the brain, and its concentration is approximately 0.5 mmol/L[37,38]. The carbon-fiber microsensor selectivity for catecholamines can be enhanced by applying on fibers a negatively charged resin (Nafion) able to concentrate cations such as dopamine on the active surface of the sensor and, at the same time, to repel anions such as ascorbic acid and dihydroxyphenylacetic acid[22,39].
Although carbon-fiber microelectrodes are the most used sensors for dopamine and norepinephrine for in vivo recording, new strategies are developed to monitor catecholamines real time in the brain.
As recently suggested by Njagi et al[40], an amperometric biosensor can be fabricated deposing an enzyme, such as tyrosinase, onto the surface of a carbon-fiber electrode. The enzyme immobilized in a biocompatible matrix and with a final diameter of about 100 μm provides an alternative to FSCV for in vivo monitoring of dopamine[40].
The neurotransmitter acetylcholine and its metabolite choline play a critical role in various functions of the CNS[41]. The concentration of acetylcholine in the ECF of the brain is 0.1-6 nmol/L[42]; the abnormalities in their concentrations are related to several neural diseases[43]. In particular, it is involved in learning and memory formation[44], in the development and maintenance of addiction[45], and in neurodegenerative disorders such as Alzheimer’s disease[46] and Parkinson’s disease[47,48]; dysregulation of cholinergic transmission is correlated to cognitive alterations such as those manifested in Alzheimer’s disease[49]. Furthermore, organophosphorus (OP) and carbamate pesticides and neurotoxic compounds are capable to inhibit the acetylcholinesterase enzyme (AChE), which is responsible of the hydrolysis of acetylcholine[50].
Therefore, the in vivo determination of acetylcholine and choline is important because a rapid and an effective method for simultaneous determination of levels of acetylcholine and choline is needed for the characterization of cholinergic transmission in normal and pathological physiology[51,52]. The most common methods developed for the simultaneous determination of acetylcholine and choline require a conversion into more easily detectable compounds[52].
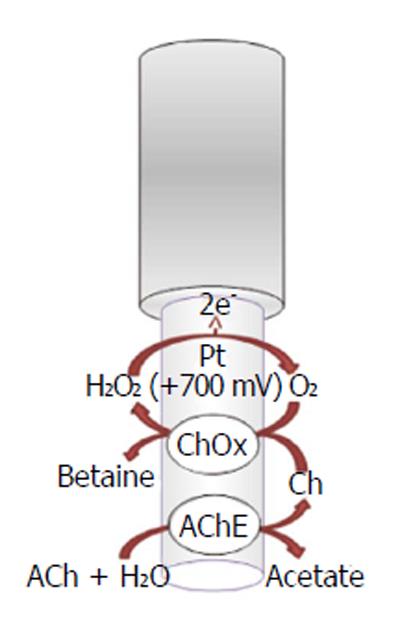
A lot of strategies have been used to obtain selective detection for acetylcholine and choline with biosensors. Among all acetylcholinesterase-based biosensors, amperometric acetylcholinesterase/choline oxidase (ChOx) biosensor is especially performing because of its potential high sensitivity, reproducibility, and excellent selectivity for in vivo simultaneous determination of neurotransmitters; these devices are usable for in situ determination of choline and acetylcholine and have been implanted in rat brain[51]. The working mechanism of acetylcholinesterase (Figure 2) is based on the following biochemical reaction[53]:
ACh + H2O → Ch + acetate (4)
While the choline, in the presence of oxygen, is oxidized by choline oxidase, forming hydrogen peroxide (H2O2), which can be easily oxidized onto electrode surface:
Ch + H2O + 2O2→ betaine + 2H2O2 (5)
The oxidation current of hydrogen peroxide can be used for the evaluation of acetylcholine, choline, and acetylcholinesterase activity. Acetylcholine signal is attenuated by acetylcholinesterase inhibitors such as neostigmine or physostigmine[54,55]. The enzymes acetylcholinesterase and choline oxidase are immobilized on the solid electrode surface such as platinum-iridium (Pt/Ir)[51,56] (Figure 2) or carbon fibers[57]. In order to prevent signal of interferents, different shielding strategies are currently used different. For example, ascorbate oxidase (AAO) is used to minimize interference from ascorbic acid, which is present in relatively high concentrations in the brain ECF[58]; polymeric films are also used onto the sensor surface that limit the access of potential interferences due to electrostatic repulsion (e.g., Nafion) and nonconducting polymers [e.g., poly-(phenylenediamines) (PPD)] that restrict the permeability of small organic molecules (e.g., major interferences ascorbate and urate) while retaining a high permeability to small species such as hydrogen peroxide[59]. The acetylcholinesterase/choline oxidase layer is trapped onto the surface electrode by the cross-linking of amino groups of the enzymes with glutaraldehyde[51]. Moreover, the enzyme layer also includes bovine serum albumin (BSA) that provides stabilization of the enzyme activity in the immobilized state[51].
Hence, the amperometric sensors for acetylcholine and choline are successfully applied and provide a useful tool to analyze basic mechanisms of cholinergic physiology in normal and pathological conditions and those involved in the activity of pharmacological cholinergic drugs.
Even if glutamate is a nonessential amino acid, it has been shown to be the most abundant in the brain. As fully described, glutamate represents the most important excitatory neurotransmitter. In plasma, glutamate concentrations reach 50-100 μmol/L while in the whole brain, they are 10-12 mmol/L, but we must take into account that glutamate reaches only 0.5-2.0 μmol/L in ECFs[60]. Glutamate is well known to be involved in most phases of normal brain functions such as memory and learning, cognition, cell migration, differentiation, and death; but at the same time, it is known to play important roles as a highly toxic endogenous excitotoxin[61]. Recently, some authors have highlighted its involvement not only in the development of the CNS, particularly related to neuronal survival, growth, and differentiation, but also in the development of several circuits[62]. In this regard, for example, it has been widely shown that low glutamate levels during neurogenesis may have a key role in the development of schizophrenia[63], and high glutamate levels can also interfere with astroglial proliferation and neuronal differentiation[61]. Glutamate has been of particular importance because of its possible involvement in neurodegenerative diseases such as amyotrophic lateral sclerosis, multiple sclerosis, Parkinson’s disease, and others. In fact, the chronic overexcitation of neurons, stimulated by glutamate, is a newer concept that has linked glutamate excitotoxicity to neurodegeneration in amyotrophic lateral sclerosis, Huntington’s disease, Parkinson’s disease, and Alzheimer’s dementia[64].
The importance of glutamate has generated a strong interest in the development of several tools for the detection of this amino acid. Different methods have been developed to determine glutamate, including optical methods, patch clamp, and microdialysis[65], but also including fluorometric, chromatographic, or spectrophotometric techniques, which, however, have some intrinsic limitations, such as being time-consuming, requiring pretreatment of the sample, being labor intensive, and requiring skilled handling. Nowadays, electrochemical methods are considered as one of the most promising approaches because of easiness, high spatial resolution, high sensitivity, and specificity[66]. From the neurochemical point of view, a wide range of amperometric biosensor designs, based mainly on glutamate oxidase enzyme loading [GluOx; molecular weight, 140 kDa; solution Michaelis constant (KM), 0.21 mmol/L in neutral buffer; pI, 6.2], have been developed[67-75].
The aim of monitoring brain glutamate using amperometric biosensors, however, is very challenging, mainly because the baseline ECF concentration of glutamate is estimated to be ≤ 5 μmol/L[76-103].
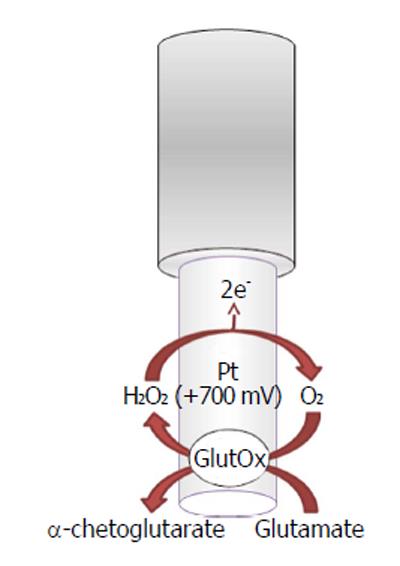
Glutamate oxidase-based biosensors (Figure 3) exploit the capability of the oxidase to selectively convert L-glutamate as follows:
L-glutamate + H2O + GluOx/FAD →
α-ketoglutarate + NH3 + GluOx/FADH2 (6)
GluOx/FADH2 + O2→ GluOx/FAD + H2O2 (7)
H2O2→O2 + 2H++ 2e- (8)
The byproduct hydrogen peroxide is then oxidized, on the transducer surface, by applying a positive potential generating a current flow directly proportional to the glutamate concentrations.
Pt generally is the electrode material of choice for electrooxidation of hydrogen peroxide[77,78]. Various strategies are as well realized in order to shield the biosensor from electroactive interfering substances that usually occur in ECF: first of all, ascorbic acid, through the electrochemical deposition of polymers[68-74]; the use of anionic substances such as Nafion[68,70,79]; or the coimmobilization of the ascorbate oxidase enzyme[75].
The amperometric biosensors have been proven to be interesting devices for in vivo measurement of glutamate concentrations and also for their response time, which has been estimated to be about a few seconds[73,74], making these biosensors suitable for the study of the rapid changes in the concentrations of glutamate both in physiological conditions or during pharmacological treatments.
Ascorbic acid is a water-soluble vitamin. It is widely known for its role as an antioxidant, but it is as much recognized as a cofactor in several enzymatic reactions, including those concerning the synthesis of catecholamines, carnitine, or cholesterol[80].
Because humans are lacking the enzyme L-gulono-1,4-lactone oxidase, they cannot synthesize ascorbate, so they, therefore, have efficient machineries for both absorption and recycling of this vitamin[81]. Among them is the transporter sodium-dependent vitamin C transporter-1 (SVCT1) involved in the body homeostasis of ascorbic acid, and the transporter SVCT2 that is necessary for the defense of active cells against oxidative stress[82]. Even the ubiquitous GLUT-type glutamate transporters play a key role in the homeostasis of this vitamin inasmuch as they are involved in the uptake of dehydroascorbate, the oxidized form of ascorbate, in order to be recycled to ascorbate[83].
In the CNS, ascorbic acid is an essential micronutrient, and although the entire brain concentrations are between 1 and 2 mmol/L, the neuronal concentrations have been evaluated to be as high as 10 mmol/L, whereas concentrations in glial cells are about 1 mmol/L[84,85]. At the same time, the ascorbate concentrations present in brain ECF have been estimated to comprise between 200 and 400 μmol/L[81].
Those findings suggest not only that ascorbate has a significant role in normal neuronal physiology but also that, given the structural characteristics as an electron donor and free-radical scavenger, it has assumed its role as a neuroprotective molecule and as an important component of the neuronal antioxidant pool[81].
Neurons and glia are able to interact with each other in order to conserve CNS ascorbate, using the mechanism of heteroexchange in which ascorbate release is related principally to glutamate uptake[86,87].
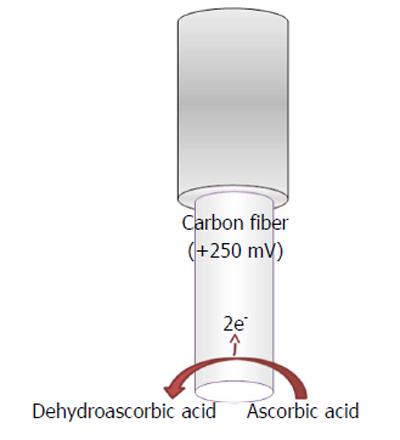
Ascorbic acid is easily oxidized in the following manner (Figure 4)
L-ascorbic acid → Dehydroascorbic acid
+ 2e- + 2H+ (9)
by applying a mild anodic potential[4] at the transducer surface (Figure 4), when a constant potential is applied, and generating a current flow directly proportional to the ascorbate concentrations.
For ascorbate in vivo monitoring, the transducer is typically made of composite materials of carbon such as carbon paste[87,88] or fibers[89] and multiwalled carbon nanotube (MWNT)-modified carbon fibers[90].
The transducer surface is sometimes modified for excluding electroactive interfering species such as positive catecholamines, so the electrode modification is carried out by the deposition of overoxidized poly (1,2-phenylenediamine)[89].
Cyclic voltammetry (CV)[89,90], square-wave voltammetry[89], and differential pulse voltammetry[91] have been used for in vivo measurements of ascorbic acid in the brain of animal models. The latter methods have been proven to be the most sensitive for sensing and biosensing because they change the potential pulsing from one potential to another in a relatively short range of time, different to what happens for the CV where the potential is constantly modified in a linear way[92].
Constant potential voltammetric techniques have also been used for in vivo monitoring of ascorbic acid in the brain by applying mild positive potentials such as +120 mV vs Ag/AgCl, when this is the implanted reference electrode (RE)[4], or +250 mV when the implanted RE is Ag+[93].
All the applied techniques have confirmed what was found with other methods that the ascorbate concentrations present in neuronal extracellular spaces are close to 500 μmol/L, emphasizing the reliability and specificity of the reading of the ascorbic acid sensors.
Glucose, a main nutrient in the brain[94], is the most important factor for its energetic metabolism[95-98] and is actively involved in ATP synthesis; it is an important modulator of memory in multiple tasks and improves memory in patients with Alzheimer’s disease and Down’s syndrome[99,100].
Lactate is another important molecule involved in brain energetic metabolism as energetic substrate for neurons[96] or product of glycolysis under anaerobic condition[94,97].
For a long time, lactate production in the brain was viewed as a lack of oxygen, as the lack of an aerobic oxidation process, or as a mismatch between glycolytic and oxidative rates, but it has recently been identified as an alternative food to glucose[97,100,101].
Contemporary studies in the amount of glucose and lactate in the brain are significant both in physiological conditions and in the presence of disease[102-104].
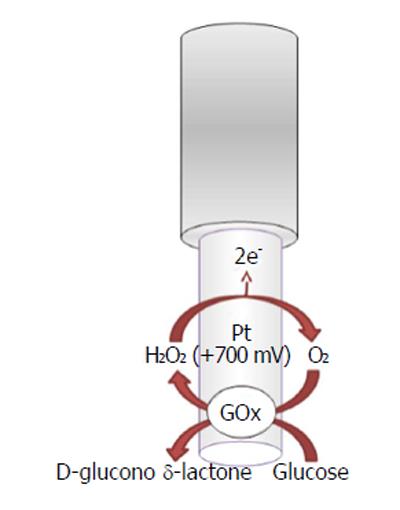
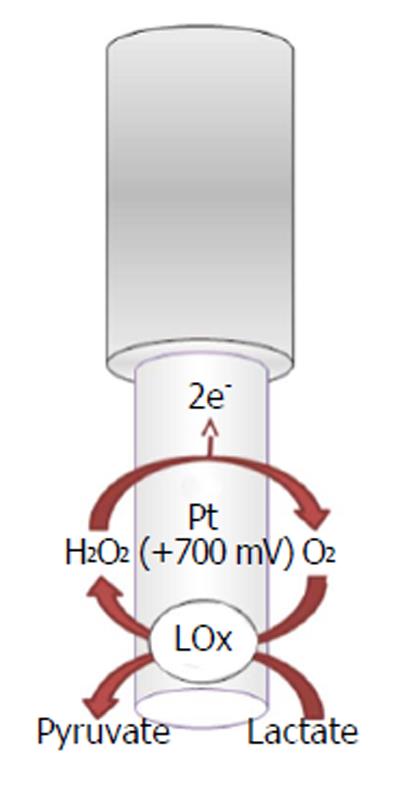
The recognition and quantification levels of glucose and lactate are possible by using innovative devices such as biosensors constituted by an electric transducer and a biological component such as enzymes; for example, glucose oxidase (GOx), L-lactate oxidase (LOx), or L-lactate dehydrogenase (LDH) is commonly used in the design, respectively, of glucose and lactate amperometric biosensors and their exploiting simple enzymatic reactions and relatively easy sensor design configuration[105]. In particular, amperometric methods have been widely used in glucose and lactate sensing. The biochemical reactions, in presence of oxygen, occurring at glucose and lactate biosensors are as follows[5,106,107]:
β-D-Glucose + FAD+-GOx →
D-Glucono-δ-Lactone + FADH2-GOx (10)
FADH2-GOx + O2→ FAD+-GOx + H2O2
L-Lactate + FAD+-LOx → Pyruvate + FADH2-LOx (11)
LOx + O2→ FAD+-LOx + H2O2
L-Lactate + NAD+-LDH →
Pyruvate + NADH-LDH (12)
In the electrochemical biosensor (Figures 5 and 6), the hydrogen peroxide byproduct from oxidase enzymes is directly proportional to the quantity of substrate glucose or lactate transformed by the enzymes as shown below in equation (8)[4].
Many studies of neuronal applying biosensors in experimental models in vivo are present in literature[108]. These studies show different types of biosensor designs, made with several transducer materials. Biosensors are mainly composed of noble metals, such as gold and/or Pt, although recently, other systems use conductive carbon based materials.
A new approach for the simultaneous detection of brain glucose and lactate in real time is reached by the use of a biotelemetric device fixed on the head of the animal[109-111].
In a previous study[6], O-phenylenediamine (OPD) monomers were electrodeposited onto a Pt/Ir cylinder electrode (diameter, 125 μm) surface. The next step was to immobilize GOx, stabilized with polyethylenimine (PEI), by immersing the transducer in the BSA solution and after in the glutaraldehyde solution (GTA). The lactate biosensor was initially made in the same way by changing the oxidase enzyme, but substituting the BSA/GTA with a final layer of polyurethane (PU)[6] for increasing the linear region. CPA was used, fixing the applied potential for hydrogen peroxide oxidation at +700 mV vs Ag/AgCl RE.
There are numerous problems with this approach because it is necessary to apply a high potential to detect hydrogen peroxide (+700 mV)[112,113] and the concentration of oxygen can change in the region in which the biosensor is implanted and the resulting current is not directly correlated with the extracellular concentrations of lactate[113-115].
Furthermore, the presence of interfering electroactive species in the tissues and the reactions of biopolymerization are needed to be considered[116,117]. In the nineties, to solve these problems, Karyakin proposed to modify the transduction element using carbon compounds coated with a thin film of Prussian blue (PB), Fe4 [Fe(CN)6]3[113,114,118-121].
After the introduction of PB in the field of biosensors were formulated different materials as supports and methodologies of deposition to improve its electrocatalytic properties and stability[122]. In recent years, some research groups have worked on glucose and lactate microbiosensors based on PB electrodes made of carbon fiber (CFE) modified to detect enzyme-generated hydrogen peroxide low applied potential (0 mV).
Afterward, the enzyme stabilizer PEI was added to improve the performance of the enzyme[122], and GOx and LOx were subsequently immobilized. In order to avoid signal of interferents, OPD was electrodeposited[122]. For the first time, a glucose and lactate microbiosensor, based on PB-modified CFE, is able to detect physiological changes in molecular levels at a low applied potential in the CNS[123].
Moreover, the ultrasmall biosensor size is apposite for in vivo neuroscience studies. In contrast, the first generation of microbiosensor transducers based on noble metals have high dimensions (diameter, approximately 100 μm) even if they have been used successfully over the last few decades for the monitoring of neurochemical species[116]. Consequently, the use of carbon-fiber microbiosensors (diameter, approximately 10 μm), modified with PB, seems to be more suitable for use in these studies because it reduces brain damage during insertion[124] and provides an even higher temporal resolution, allowing the real-time correlation with animal behavior[125].
Oxygen and endogenous nitric oxide are gaseous molecules playing a pivotal role in mediating important biological processes yet are involved in very distinct aspects of organism physiology. Oxygen is indispensable for animal life; an adequate tissue oxygen content, delivered by hemoglobin through the bloodstream, is fundamental to supply cellular metabolic demands, as oxygen is involved in energy production as well as in aerobic cellular metabolism[126].
In contrast, an insufficient oxygen concentration in tissues leads to hypoxia, a severe altered condition in which low oxygen availability prevents aerobic metabolism and oxidative phosphorylation in the cell, yielding to impoverishment of high-energy compounds such as ATP and, lastly, inducing cellular dysfunction and death[127,128].
Though oxygen is a crucial substrate for cellular functions, it also provokes damage because of the toxicity of oxygen-derived reactive species (ROS), such as hydrogen peroxide, singlet oxygen, hydroxyl radicals, and superoxide anion[129]. ROS free radicals attack lipids, proteins, DNA, and RNA and expose cells to oxidative stress, which has been demonstrated to be involved in the pathogenesis of several neurodegenerative diseases[129,130].
Endogenous nitric oxide is a gaseous signaling molecule released in low concentration (tens of nanomoles to low micromoles), characterized by possessing a lifetime of a few seconds[131], as nitric oxide is a highly reactive free-radical species. Nitric oxide production mainly involves the enzymes NO-synthases, which catalyze nitric oxide formation as a byproduct of the reduction of the amino acid L-arginine into l-citrulline[132,133]. Nitric oxide acts as a transitory paracrine and autocrine signaling molecule, by activating the soluble guanylyl cyclase, increasing cellular cyclic guanosine monophosphate (c)[134].
Since its discovery in 1987[135-137], when first nitric oxide was recognized as being involved in the physiological actions of endothelium-derived relaxing factor, mediating vasodilatation, the knowledge of the important role that nitric oxide plays in physiopathology and pharmacology exponentially increased. In fact, further studies revealed how nitric oxide actions are implicated in the cardiovascular system, in the immune response[138], as well as in the nervous systems, mediating neurotransmission[131,139]. Furthermore, nitric oxide is a mediator of both antitumor and antimicrobial activities[140].
Otherwise, the disruption of nitric oxide production seems to be involved in diseases such as atherosclerosis[141], hypertension, cerebral and coronary vasospasm, and ischemia-reperfusion injury. In fact, nitric oxide is attacked by ROS, specifically by superoxide anion, forming peroxynitrite, which generates further reactive nitrogen species (RNS) such as nitrogen dioxide and dinitrogen trioxide. Like ROS, RNS damage lipids, proteins, and other macromolecules, thus also contributing to the onset of diabetes and neurodegenerative diseases[141-143].
The detection of oxygen and nitric oxide tension in the brain has been studied in vivo, providing critical information about the physiopathology and pharmacological implications of these molecules.
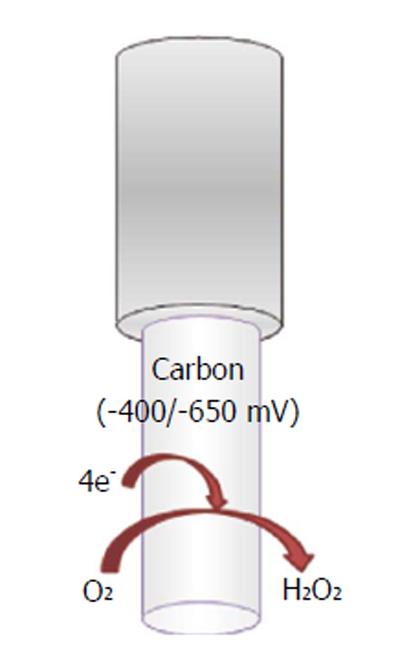
A wide variety of O2-sensitive microsensors have been developed. Electrochemical devices exploiting amperometric techniques of detection, such as CPA, differential-pulse amperometry (DPA), CV, and fast-scan voltammetry (FCV), allow the reliable direct reduction of oxygen. Carbon paste and noble metal transducers are the most commonly diffused. Reactions involved in the electrochemical reduction of oxygen at the electrode’s surface can occur via two mechanisms: a single-step reaction yields to detectable intermediates (Figure 7):
oxygen + 4H+ + 4e-→ 2H2O (13)
In the second mechanism, two-step O2 reduction forms H2O2 as measurable intermediate:
oxygen + 2H++ 2e-→H2O2 (14)
H2O2 + 2H++ 2e-→ 2H2O (15)
Changes after physiological stimulations or pharmacological treatments were recorded in the extracellular space of the striatum, by using optic microfibers, assessing that oxygen concentration is about 50 μmol/L[143].
Electrochemical oxygen microelectrodes using CPA at a noble metal transducer bare, such as gold or Pt, allowed the long-term monitoring of oxygen subcutaneous and venous dynamics[144,145].
Nevertheless, several groups preferred to use carbon-paste electrodes (CPEs) because of their longer in vivo stability, less surface fouling[146], and quite easy manufacture[147] (Figure 7). Venton et al[148] used the FCV technique in a study in which dissolved oxygen was measured in the rat caudate-putamen, by using 5 μm Nafion-coated carbon fibers with a subsecond time resolution. FCV was used also in a study that targeted oxygen levels in the striatum of primates during reward delivery. In this case, the diameter of the carbon fibers ranged from 12 to 33 μm[149].
Lowry et al[101,150,151] largely used carbon paste-based miniaturized electrodes in an experimental session in which the effects of anesthesia were studied in vivo, as well as the effects of hypoxia and hyperoxia on brain energy metabolism in the striatum[147-149]. Changes in oxygen at CPEs were usually monitored by using the DPA technique[151,152]. Two equally sized cathodic pulses were applied: the first from a resting potential at -150 to -350 mV, corresponding to the foot of the reduction wave for oxygen, and the second, which corresponds to the peak of the reduction wave, from -350 to -550 mV.
In addition, oxygen microsensors were used by Finnerty et al[153] in real-time monitoring of oxygen levels in an animal model of schizophrenia, coupled with the use of a glucose biosensor and an nitric oxide microsensor. Oxygen reduction at CPEs has been widely detected also via CPA[152]. For example, by applying a constant cathodic potential of -650 mV vs a saturated calomel RE, oxygen reduction was recorded in real time in the hippocampus of freely moving rats[115].
Furthermore, CPEs of 200 μm in diameter were implanted in the dorsal and the ventral hippocampus of rats to investigate spatial processing and anxiety. Even in this case, the applied potential was -650 mV vs a silver wire REF[154]. The CPA technique was also used by Bazzu et al[110] to monitor striatal oxygen levels in a telemetric in vivo study. Working electrodes, consisting of miniaturized conical-shaped epoxy-carbon electrodes (180 μm), allowed oxygen detection by fixing the reduction potential at -400 mV vs Ag/AgCl REF.
Recently, oxygen amperometry was applied to a behavioral study of reward processing in the rat nucleus accumbens. CPEs (200 μm in diameter) were used by applying a constant potential of -650 mV vs a silver wire REF to reduce oxygen. Data showed similar results to those obtained in human fMRI studies, confirming how oxygen amperometry is a powerful technique for the measurement of brain function[155].
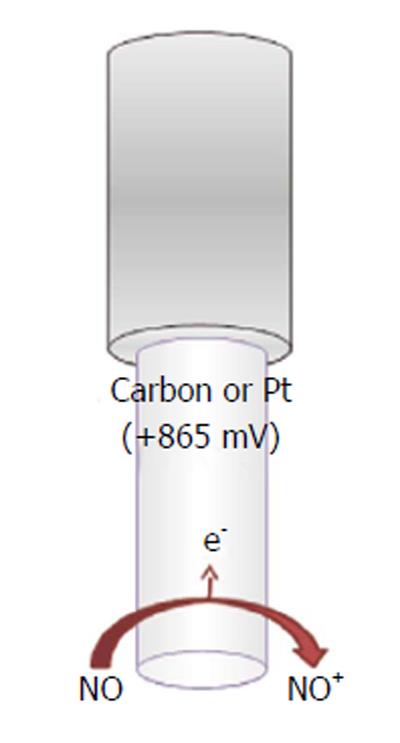
In the attempts of monitoring the concentration of the unstable nitric oxide molecule in vivo and to test nitric oxide donor drugs, several microsensors have been developed since the 1990s[156]. The majority exploits electrochemical amperometric techniques to directly detect nitric oxide. Commonly, an oxidant potential is applied (higher than +850 mV vs Ag/AgCl), in view of the fact that nitric oxide and oxygen reduction potential are very close, so oxygen interferes with nitric oxide measurement (at nitric oxide-reducing potentials) (Figure 8).
Basically, a double reaction occurs at the transducer’s face, usually carbon fiber or noble metals[157-161], involving the formation of NO+, which is further converted into nitrite (Figure 8):
NO - e-→ NO+ (16)
NO+ + OH-→ HNO2→ H+ + NO2- (17)
Otherwise, metalloporphyrin-modified sensors[162-164] are also largely used:
Fe(II) + NO → Fe(II) - NO (18)
Fe(II) - NO + H+ + e-→ Fe(II) - HNO (19)
Fe(II) - HNO + 2H+ + 2 e-→ Fe(II) + H2NOH (20)
Because of the enormous interest kindled by the wide range of actions of nitric oxide, several in vivo experiments were conducted to monitor nitric oxide release on different tissues[165-168]. Friedemann et al[169] developed an electrochemical electrode using carbon fiber as a transducer, coated with Nafion and further electropolymerized with OPD. Nitric oxide was quantified amperometrically using differential pulse voltammetry[169].
Wu et al[170,171] research group conducted several experiments in which physiological nitric oxide actions on a cat’s brain were investigated. Nitric oxide concentration was measured in real-time using voltammetry techniques, implanting Nafion-/porphyrin-/OPD-coated carbon-fiber electrodes. A highly sensitive and selective NO electrode was used to measure the nitric oxide concentration in a rat hippocampus[172]. In addition, an electrochemical nitric oxide microbiosensor based on cytochrome C, immobilized onto a functionalized conducting polymer layer, was implanted in the striatum. Nafion was used for its shielding properties toward interference electroactive molecules present in the brain, chiefly ascorbic acid[173]. Brown et al[174] and Finnerty et al[175] obtained a simple and useful design by modifying a Pt sensor with multicoated Nafion layers. This electrochemical sensor was successfully implanted in the striatum of freely moving rats, allowing the real-time nitric oxide at Nafion-coated Pt. Santos et al[176] recently developed an electrochemical biomimetic sensor based on nanocomposite hemin-based microelectrode, measuring exogenous NO in the rat hippocampus in vivo using CV.
In the last decades, ethanol has become the most widespread psychotropic toxic substance in Western countries because it is widely legally accepted and also because it is available at a low cost. Acute, subacute and chronic exposure to ethanol may have important effects on the CNS, therefore it becomes significative to monitor ethanol kinetic and its effects on the brain using the most appropriate techniques[177]. The main effects of ethanol consumption cause significant effects on the CNS, principally enhancing the action of the neurotransmitter GABA and generating disinhibition, ataxia, and sedation[178]. Subchronic exposure to ethanol enhances the dopamine neurotransmission in the mesolimbic system[179,180] and increases dopamine levels in the nucleus accumbens[181], playing an important role as a “rewarding” molecule[182-184].
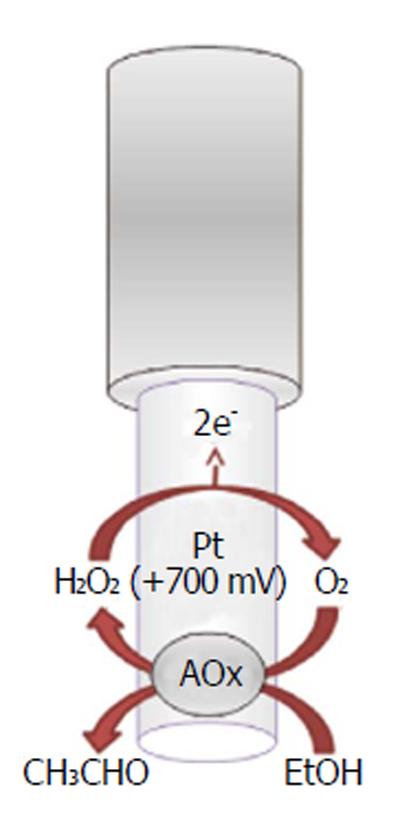
Recently, implantable electrochemical biosensors have been developed for monitoring the real-time changes of ethanol concentrations in the brain ECFs of freely moving animals (Figure 9). As previously described for other implantable biosensors, the ethanol biosensor exploits the presence of an enzyme, the alcohol oxidase, to selectively quantify ethanol using the production of a directly oxidizable byproduct (hydrogen peroxide), electrochemically detectable on the surface of a Pt transducer[185,186]. The main characteristic of this biosensor is its capability of monitoring ethanol changes second by second and over a period of two weeks. This neurochemical tool has been proven to be successful, especially when associated with a miniaturized telemetric system (see next paragraph). According to the results of previous studies[177,185,186], the ethanol biosensor has been demonstrated to be a reliable device for the short-time monitoring of exogenous ethanol in the CNS, and it could be used for studying ethanol pharmacokinetics during addiction and the real-time effect of drugs on ethanol levels in the CNS.
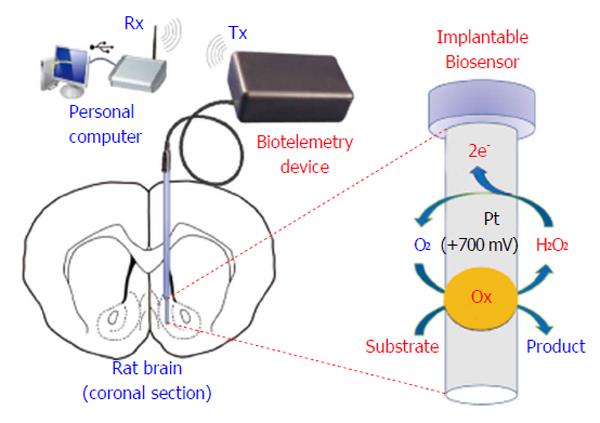
Biotelemetry has be defined as the recording of physiological parameters by uni- or bidirectional electromagnetic signals[6,187], or more simply, it represents a variety of techniques intended for real-time monitoring of physiological parameters. Innovative biotelemetry systems (Figure 10) have been developed for studying brain neurochemistry[188], in particular for monitoring CNS dopamine in freely moving animals[189-191] and, more recently, in humans[192]. The wireless detection of dopamine requires complex waveform generation and high-resolution synchronization; indeed, as previously shown, FSCV allows the redox detection of dopamine up to ten times per second[189-191]. Also chronoamperometry and differential pulse voltammetry techniques have been demonstrated to work in conjunction with telemetric devices[158,193-196]; the resulting systems are very complex, not easily miniaturizable, and difficult to use in small rodents. On the contrary, non-pulsed techniques, such as CPA, free the microcontroller unit (MCU) from high-density calculations, allowing an increase in the number of implantable sensors and facilitating the miniaturization of the electronics[109,197]. The battery-powered biotelemetric device (Figure 10), composed of an amperometric module, an MCU, and a transmitter, polarizes the sensors and sends sensor data to a receiving unit connected to a PC. The system electronics exhibits low power consumption, high stability, and good linear response[3]. A CPA-based biotelemetry device may be easily interfaced with amperometric microsensors and biosensors[6,109,197] and leave enough MCU computing power available for other tasks such as motion detection using inertial physical sensors. Indeed, in a previous study, we described this new approach with the simultaneous detection of brain glucose, lactate, and movements in real time using a biotelemetric device fixed to the head of a freely moving rat[6].
Although voltammetric techniques have been widely used in last decades, microdialysis still remains the “gold standard” for in vivo neurochemical study of the brain extracellular compartment. The advantages in using this technique include the possibility of measuring several neurochemicals at the same time with high sensitivity and very high selectivity, providing a more complete picture of the ECF. Its invasiveness, associated with low temporal resolution, and the necessity of using connecting tubes to carry out the experiments do not make it particularly suitable for monitoring fast neurochemical changes and do not allow the application of wireless techniques. As an alternative, electrochemical sensors are increasingly-used tools to study the neurochemical modifications in the ECF. The main characteristics of these devices are represented by very low invasiveness (carbon fibers in particular), when compared with microdialysis probes, and, most of all, their capability of monitoring variations of analytes in seconds or fractions. Furthermore, some electrochemical sensors have been demonstrated to be effective for weeks or months when implanted in the brain and, as described in this review, they are the optimal candidates for wireless detection. The Table 1 summarizes the principal characteristics of the main techniques indicated in this review.
| Characteristics of | Technique | ||||
| the technique | Electrochemical techniques (voltammetry) | Microdialysis | |||
| CPA | CA | DPV | FSCV | ||
| Brain invasiveness | + | + | + | + | ++ |
| Selectivity | + | + | ++ | ++ | +++ |
| Sensitivity | ++ | ++ | + | + | +++ |
| Concentration range | nmol/L-mmol/L | nmol/L-mmol/L | nmol/L-mmol/L | nmol/L-mmol/L | fmol/L-mmol/L |
| Temporal resolution | ++++ | +++ | ++ | +++ | + |
| Spatial resolution | ++ | +++ | ++ | +++ | + |
| Monitoring period | d/wk | d/wk | d/wk | d/wk | h/d |
| Untethered detection | ++ | + | + | + | - |
Implantable (Bio)sensors, integrated into miniaturized telemetry systems, represent a new generation of analytical tools for studying brain neurochemistry of awake, freely moving animals in real time. This approach, based on simple and inexpensive components, could be used as a rapid and reliable model for studying the physiology, the pathophysiology, and the effects of different drugs (or toxic compounds such as ethanol) on brain neurochemical systems.
P- Reviewers: Chawla M, Fang Y, Ju HX, Panagis G, Trohman R S- Editor: Song XX L- Editor: A E- Editor: Liu SQ
| 1. | Delgado JM, DeFeudis FV, Roth RH, Ryugo DK, Mitruka BM. Dialytrode for long term intracerebral perfusion in awake monkeys. Arch Int Pharmacodyn Ther. 1972;198:9-21. [PubMed] |
| 2. | Ungerstedt U. In: Robinson TE and Justice JB (eds.). Microdialysis in the Neurosciences. Netherlands: Elsevier Science BV 1991; 3-18. |
| 3. | Calia G, Rocchitta G, Migheli R, Puggioni G, Spissu Y, Bazzu G, Mazzarello V, Lowry JP, O’Neill RD, Desole MS. Biotelemetric monitoring of brain neurochemistry in conscious rats using microsensors and biosensors. Sensors (Basel). 2009;9:2511-2523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Bazzu G, Biosa A, Farina D, Spissu Y, Dedola S, Calia G, Puggioni G, Rocchitta G, Migheli R, Desole MS. Dual asymmetric-flow microdialysis for in vivo monitoring of brain neurochemicals. Talanta. 2011;85:1933-1940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Serra PA, Puggioni G, Bazzu G, Calia G, Migheli R, Rocchitta G. Design and construction of a distributed sensor NET for biotelemetric monitoring of brain energetic metabolism using microsensors and biosensors. Croatia: Intech 2010; 241-260. [DOI] [Full Text] |
| 6. | Rocchitta G, Secchi O, Alvau MD, Farina D, Bazzu G, Calia G, Migheli R, Desole MS, O’Neill RD, Serra PA. Simultaneous telemetric monitoring of brain glucose and lactate and motion in freely moving rats. Anal Chem. 2013;85:10282-10288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Cooper JR, Bloom FE, Roth RH. The Biochemical Basis of Neuropharmacology. 2003;. |
| 8. | Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PE, Akil H. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 799] [Cited by in RCA: 696] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 9. | Wightman RM, Robinson DL. Transient changes in mesolimbic dopamine and their association with ‘reward’. J Neurochem. 2002;82:721-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 195] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 10. | O’Neill RD. Long-term monitoring of brain dopamine metabolism in vivo with carbon paste electrodes. Sensors. 2005;5:317-342. [RCA] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Bazzu G, Rocchitta G, Migheli R, Alvau MD, Zinellu M, Puggioni G, Calia G, Mercanti G, Giusti P, Desole MS. Effects of the neurotoxin MPTP and pargyline protection on extracellular energy metabolites and dopamine levels in the striatum of freely moving rats. Brain Res. 2013;1538:159-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Serra PA, Sciola L, Delogu MR, Spano A, Monaco G, Miele E, Rocchitta G, Miele M, Migheli R, Desole MS. The neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine induces apoptosis in mouse nigrostriatal glia. Relevance to nigral neuronal death and striatal neurochemical changes. J Biol Chem. 2002;277:34451-34461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Serra PA, Pluchino S, Marchetti B, Desole MS, Miele E. The MPTP mouse model: cues on DA release and neural stem cell restorative role. Parkinsonism Relat Disord. 2008;14 Suppl 2:S189-S193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Howes OD, Murray RM. Schizophrenia: an integrated sociodevelopmental-cognitive model. Lancet. 2014;383:1677-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 590] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 15. | Howes OD, Kambeitz J, Kim E, Stahl D, Slifstein M, Abi-Dargham A, Kapur S. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012;69:776-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 693] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 16. | Heneka MT, Nadrigny F, Regen T, Martinez-Hernandez A, Dumitrescu-Ozimek L, Terwel D, Jardanhazi-Kurutz D, Walter J, Kirchhoff F, Hanisch UK. Locus ceruleus controls Alzheimer’s disease pathology by modulating microglial functions through norepinephrine. Proc Natl Acad Sci USA. 2010;107:6058-6063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 382] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 17. | Fillenz M. In vivo neurochemical monitoring and the study of behaviour. Neurosci Biobehav Rev. 2005;29:949-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety. 2000;12 Suppl 1:2-19. [PubMed] |
| 19. | Kermorgant M, Lancien F, Mimassi N, Le Mével JC. Central ventilatory and cardiovascular actions of serotonin in trout. Respir Physiol Neurobiol. 2014;192:55-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Robinson DL, Hermans A, Seipel AT, Wightman RM. Monitoring rapid chemical communication in the brain. Chem Rev. 2008;108:2554-2584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 597] [Cited by in RCA: 474] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 21. | Migheli R, Puggioni G, Dedola S, Rocchitta G, Calia G, Bazzu G, Esposito G, Lowry JP, O’Neill RD, Desole MS. Novel integrated microdialysis-amperometric system for in vitro detection of dopamine secreted from PC12 cells: design, construction, and validation. Anal Biochem. 2008;380:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Nevalainen N, Af Bjerkén S, Lundblad M, Gerhardt GA, Strömberg I. Dopamine release from serotonergic nerve fibers is reduced in L-DOPA-induced dyskinesia. J Neurochem. 2011;118:12-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Gratton A, Hoffer BJ, Gerhardt GA. In vivo electrochemical studies of monoamine release in the medial prefrontal cortex of the rat. Neuroscience. 1989;29:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 91] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Ozel RE, Wallace KN, Andreescu S. Chitosan coated carbon fiber microelectrode for selective in vivo detection of neurotransmitters in live zebrafish embryos. Anal Chim Acta. 2011;695:89-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Clark JJ, Sandberg SG, Wanat MJ, Gan JO, Horne EA, Hart AS, Akers CA, Parker JG, Willuhn I, Martinez V. Chronic microsensors for longitudinal, subsecond dopamine detection in behaving animals. Nat Methods. 2010;7:126-129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 283] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 26. | Willuhn I, Burgeno LM, Everitt BJ, Phillips PE. Hierarchical recruitment of phasic dopamine signaling in the striatum during the progression of cocaine use. Proc Natl Acad Sci USA. 2012;109:20703-20708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 183] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 27. | John CE, Jones SR. Fast scan cyclic voltammetry of dopamine and serotonin in mouse brain slices. ), Electrochemical Methods for Neuroscience. Boca Raton (FL): CRC Press 2006; 49-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 28. | Peters JL, Miner LH, Michael AC, Sesack SR. Ultrastructure at carbon fiber microelectrode implantation sites after acute voltammetric measurements in the striatum of anesthetized rats. J Neurosci Methods. 2004;137:9-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Dressman SF, Peters JL, Michael AC. Carbon fiber microelectrodes with multiple sensing elements for in vivo voltammetry. J Neurosci Methods. 2002;119:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Kawagoe KT, Zimmerman JB, Wightman RM. Principles of voltammetry and microelectrode surface states. J Neurosci Methods. 1993;48:225-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 284] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 31. | Seymour JP, Kipke DR. Neural probe design for reduced tissue encapsulation in CNS. Biomaterials. 2007;28:3594-3607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 299] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 32. | Park J, Takmakov P, Wightman RM. In vivo comparison of norepinephrine and dopamine release in rat brain by simultaneous measurements with fast-scan cyclic voltammetry. J Neurochem. 2011;119:932-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 33. | Heien ML, Johnson MA, Wightman RM. Resolving neurotransmitters detected by fast-scan cyclic voltammetry. Anal Chem. 2004;76:5697-5704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 271] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 34. | Park J, Kile BM, Wightman RM. In vivo voltammetric monitoring of norepinephrine release in the rat ventral bed nucleus of the stria terminalis and anteroventral thalamic nucleus. Eur J Neurosci. 2009;30:2121-2133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 35. | Hashemi P, Dankoski EC, Wood KM, Ambrose RE, Wightman RM. In vivo electrochemical evidence for simultaneous 5-HT and histamine release in the rat substantia nigra pars reticulata following medial forebrain bundle stimulation. J Neurochem. 2011;118:749-759. [PubMed] |
| 36. | Herr NR, Park J, McElligott ZA, Belle AM, Carelli RM, Wightman RM. In vivo voltammetry monitoring of electrically evoked extracellular norepinephrine in subregions of the bed nucleus of the stria terminalis. J Neurophysiol. 2012;107:1731-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Mefford IN, Oke AF, Adams RN. Regional distribution of ascorbate in human brain. Brain Res. 1981;212:223-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 84] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 38. | Nagy G, Rice ME, Adams RN. A new type of enzyme electrode: the ascorbic acid eliminator electrode. Life Sci. 1982;31:2611-2616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 69] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | Willuhn I, Wanat MJ, Clark JJ, Phillips PE. Dopamine signaling in the nucleus accumbens of animals self-administering drugs of abuse. Curr Top Behav Neurosci. 2010;3:29-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 40. | Njagi J, Chernov MM, Leiter JC, Andreescu S. Amperometric detection of dopamine in vivo with an enzyme based carbon fiber microbiosensor. Anal Chem. 2010;82:989-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 164] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 41. | Sarter M, Bruno JP, Givens B. Attentional functions of cortical cholinergic inputs: what does it mean for learning and memory? Neurobiol Learn Mem. 2003;80:245-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 211] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 42. | Uutela P, Reinilä R, Piepponen P, Ketola RA, Kostiainen R. Analysis of acetylcholine and choline in microdialysis samples by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2005;19:2950-2956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 43. | Watanabe T, Yamagata N, Takasaki K, Sano K, Hayakawa K, Katsurabayashi S, Egashira N, Mishima K, Iwasaki K, Fujiwara M. Decreased acetylcholine release is correlated to memory impairment in the Tg2576 transgenic mouse model of Alzheimer’s disease. Brain Res. 2009;1249:222-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 44. | Anagnostaras SG, Murphy GG, Hamilton SE, Mitchell SL, Rahnama NP, Nathanson NM, Silva AJ. Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nat Neurosci. 2003;6:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 412] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 45. | Dani JA, Ji D, Zhou FM. Synaptic plasticity and nicotine addiction. Neuron. 2001;31:349-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 197] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 46. | Klucken J, McLean PJ, Gomez-Tortosa E, Ingelsson M, Hyman BT. Neuritic alterations and neural system dysfunction in Alzheimer’s disease and dementia with Lewy bodies. Neurochem Res. 2003;28:1683-1691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 47. | Calabresi P, Picconi B, Parnetti L, Di Filippo M. A convergent model for cognitive dysfunctions in Parkinson’s disease: the critical dopamine-acetylcholine synaptic balance. Lancet Neurol. 2006;5:974-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 250] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 48. | Zhu W, Wang D, Zheng J, An Y, Wang Q, Zhang W, Jin L, Gao H, Lin L. Effect of (R)-salsolinol and N-methyl-(R)-salsolinol on the balance impairment between dopamine and acetylcholine in rat brain: involvement in pathogenesis of Parkinson disease. Clin Chem. 2008;54:705-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 49. | Kuo MF, Grosch J, Fregni F, Paulus W, Nitsche MA. Focusing effect of acetylcholine on neuroplasticity in the human motor cortex. J Neurosci. 2007;27:14442-14447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 145] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 50. | Hildebrandt A, Brago’s R, Lacorte S, Marty JL. Performance of a portable biosensor for the analysis of organophosphorus and carbamate insecticides in water and food. Sens Actuators B Chem. 2008;133:195-201. [DOI] [Full Text] |
| 51. | Mitchell KM. Acetylcholine and choline amperometric enzyme sensors characterized in vitro and in vivo. Anal Chem. 2004;76:1098-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 52. | Khan A, Ab Ghani S. Multienzyme microbiosensor based on electropolymerized o-phenylenediamine for simultaneous in vitro determination of acetylcholine and choline. Biosens Bioelectron. 2012;31:433-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 53. | Chen Q, Kobayashi Y, Takeshita H, Hoshi T, Anzai J. Avidin biotin system-based enzyme multilayer membranes for biosensor application: Optimization of loading of choline esterase and choline oxidase in the bienzyme membrane for acetylcholine biosensors. Electroanalysis. 1998;10:94-97. |
| 54. | Sarter M, Parikh V. Choline transporters, cholinergic transmission and cognition. Nat Rev Neurosci. 2005;6:48-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 298] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 55. | Zamfir LG, Rotariu L, Bala C. Acetylcholinesterase biosensor for carbamate drugs based on tetrathiafulvalene-tetracyanoquinodimethane/ionic liquid conductive gels. Biosens Bioelectron. 2013;46:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 56. | Wu BY, Hou SH, Yin F, Zhao ZX, Wang YY, Wang XS, Chen Q. Amperometric glucose biosensor based on multilayer films via layer-by-layer self-assembly of multi-wall carbon nanotubes, gold nanoparticles and glucose oxidase on the Pt electrode. Biosens Bioelectron. 2007;22:2854-2860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 134] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 57. | Garguilo MG, Michael AC. Amperometric microsensors for monitoring choline in the extracellular fluid of brain. J Neurosci Methods. 1996;70:73-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 58. | Cammack J, Ghasemzadeh B, Adams RN. Electrochemical monitoring of brain ascorbic acid changes associated with hypoxia, spreading depression, and seizure activity. Neurochem Res. 1992;17:23-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 59. | Curulli A, Dragulescu S, Cremisini C, Palleschi G. Bienzyme amperometric probes for choline and choline esters assembled with nonconducting electrosynthesized polymers. Electroanalysis. 2001;13:236-242. |
| 60. | Hawkins RA. The blood-brain barrier and glutamate. Am J Clin Nutr. 2009;90:867S-874S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 208] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 61. | Sundaram RS, Gowtham L, Nayak BS. The role of excitatory neurotransmitter glutamate in brain physiology and pathology. Asian. J Pharm Clin Res. 2012;5:1-7. |
| 62. | Suzuki K, Martin PM. Neurotoxicants and developing brain. Developmental Neurotoxicology. Boca Raton: CRC Press 1994; 9-32. |
| 63. | Hirsch SR, Das I, Garey LJ, de Belleroche J. A pivotal role for glutamate in the pathogenesis of schizophrenia, and its cognitive dysfunction. Pharmacol Biochem Behav. 1997;56:797-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 79] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 64. | Lau A, Tymianski M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch. 2010;460:525-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 730] [Cited by in RCA: 844] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 65. | McLamore ES, Mohanty S, Shi J, Claussen J, Jedlicka SS, Rickus JL, Porterfield DM. A self-referencing glutamate biosensor for measuring real time neuronal glutamate flux. J Neurosci Methods. 2010;189:14-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 66. | Batra B, Pundir CS. An amperometric glutamate biosensor based on immobilization of glutamate oxidase onto carboxylated multiwalled carbon nanotubes/gold nanoparticles/chitosan composite film modified Au electrode. Biosens Bioelectron. 2013;47:496-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 67. | Tolosa VM, Wassum KM, Maidment NT, Monbouquette HG. Electrochemically deposited iridium oxide reference electrode integrated with an electroenzymatic glutamate sensor on a multi-electrode array microprobe. Biosens Bioelectron. 2013;42:256-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 68. | Wassum KM, Tolosa VM, Tseng TC, Balleine BW, Monbouquette HG, Maidment NT. Transient extracellular glutamate events in the basolateral amygdala track reward-seeking actions. J Neurosci. 2012;32:2734-2746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 69. | Frey O, Holtzman T, McNamara RM, Theobald DE, van der Wal PD, de Rooij NF, Dalley JW, Koudelka-Hep M. Enzyme-based choline and L-glutamate biosensor electrodes on silicon microprobe arrays. Biosens Bioelectron. 2010;26:477-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 70. | Wahono N, Qin S, Oomen P, Cremers TI, de Vries MG, Westerink BH. Evaluation of permselective membranes for optimization of intracerebral amperometric glutamate biosensors. Biosens Bioelectron. 2012;33:260-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 71. | Rothwell SA, Kinsella ME, Zain ZM, Serra PA, Rocchitta G, Lowry JP, O’Neill RD. Contributions by a novel edge effect to the permselectivity of an electrosynthesized polymer for microbiosensor applications. Anal Chem. 2009;81:3911-3918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 72. | Tian F, Gourine AV, Huckstepp RT, Dale N. A microelectrode biosensor for real time monitoring of L-glutamate release. Anal Chim Acta. 2009;645:86-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 73. | McMahon CP, Rocchitta G, Kirwan SM, Killoran SJ, Serra PA, Lowry JP, O’Neill RD. Oxygen tolerance of an implantable polymer/enzyme composite glutamate biosensor displaying polycation-enhanced substrate sensitivity. Biosens Bioelectron. 2007;22:1466-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 74. | McMahon CP, Rocchitta G, Serra PA, Kirwan SM, Lowry JP, O’Neill RD. Control of the oxygen dependence of an implantable polymer/enzyme composite biosensor for glutamate. Anal Chem. 2006;78:2352-2359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 75. | Rahman MA, Kwon NH, Won MS, Choe ES, Shim YB. Functionalized conducting polymer as an enzyme-immobilizing substrate: an amperometric glutamate microbiosensor for in vivo measurements. Anal Chem. 2005;77:4854-4860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 76. | Miele M, Boutelle MG, Fillenz M. The source of physiologically stimulated glutamate efflux from the striatum of conscious rats. J Physiol. 1996;497:745-751. [PubMed] |
| 77. | Hamdi N, Wang J. Monbouquette HG. Polymer films as permselective coatings for H2O2-sensing electrodes. J Electroanal Chem. 2005;581:258-264. [RCA] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 78. | O’Neill RD, Chang SC, Lowry JP, McNeil CJ. Comparisons of platinum, gold, palladium and glassy carbon as electrode materials in the design of biosensors for glutamate. Biosens Bioelectron. 2004;19:1521-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 79. | Yao T, Okano G. Simultaneous determination of L-glutamate, acetylcholine and dopamine in rat brain by a flow-injection biosensor system with microdialysis sampling. Anal Sci. 2008;24:1469-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 80. | Harrison FE, May JM. Vitamin C function in the brain: vital role of the ascorbate transporter SVCT2. Free Radic Biol Med. 2009;46:719-730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 503] [Cited by in RCA: 446] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 81. | May JM. Vitamin C transport and its role in the central nervous system. Subcell Biochem. 2012;56:85-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 82. | Savini I, Rossi A, Pierro C, Avigliano L, Catani MV. SVCT1 and SVCT2: key proteins for vitamin C uptake. Amino Acids. 2008;34:347-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 263] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 83. | Vera JC, Rivas CI, Fischbarg J, Golde DW. Mammalian facilitative hexose transporters mediate the transport of dehydroascorbic acid. Nature. 1993;364:79-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 382] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 84. | Rice ME, Russo-Menna I. Differential compartmentalization of brain ascorbate and glutathione between neurons and glia. Neuroscience. 1998;82:1213-1223. [PubMed] |
| 85. | Rice ME. Ascorbate regulation and its neuroprotective role in the brain. Trends Neurosci. 2000;23:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 603] [Cited by in RCA: 625] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 86. | O’Neill RD. The measurement of brain ascorbate in vivo and its link with excitatory amino acid neurotransmission. 221-268. |
| 87. | Miele M, Boutelle MG, Fillenz M. The physiologically induced release of ascorbate in rat brain is dependent on impulse traffic, calcium influx and glutamate uptake. Neuroscience. 1994;62:87-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 85] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 88. | O’Neill RD, Fillenz M, Sundstrom L, Rawlins JN. Voltammetrically monitored brain ascorbate as an index of excitatory amino acid release in the unrestrained rat. Neurosci Lett. 1984;52:227-233. [PubMed] |
| 89. | Hocevar SB, Zivin M, Milutinovic A, Hawlina M, Hutton E, Ogorevc B. Simultaneous in vivo measurement of dopamine, serotonin and ascorbate in the striatum of experimental rats using voltammetric microprobe. Front Biosci. 2006;11:2782-2789. [PubMed] |
| 90. | Gonon F, Buda M, Cespuglio R, Jouvet M, Pujol JF. Voltammetry in the striatum of chronic freely moving rats: detection of catechols and ascorbic acid. Brain Res. 1981;223:69-80. [PubMed] |
| 91. | Zhang M, Liu K, Xiang L, Lin Y, Su L, Mao L. Carbon nanotube-modified carbon fiber microelectrodes for in vivo voltammetric measurement of ascorbic acid in rat brain. Anal Chem. 2007;79:6559-6565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 181] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 92. | Chen A, Shah B. Electrochemical sensing and biosensing based on square wave voltammetry. Anal. Methods. 2013;5:2158-2173. [DOI] [Full Text] |
| 93. | Miele M, Fillenz M. In vivo determination of extracellular brain ascorbate. J Neurosci Methods. 1996;70:15-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 99] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 94. | Yao T, Yano T, Nanjyo Y, Nishino H. Simultaneous determination of glucose and L-lactate in rat brain by an electrochemical in vivo flow-injection system with an on-line microdialysis sampling. Anal Sci. 2003;19:61-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 95. | Lowry JP, Miele M, O’Neill RD, Boutelle MG, Fillenz M. An amperometric glucose-oxidase/poly(o-phenylenediamine) biosensor for monitoring brain extracellular glucose: in vivo characterisation in the striatum of freely-moving rats. J Neurosci Methods. 1998;79:65-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 68] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 96. | Magistretti PJ, Pellerin L. Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging. Philos Trans R Soc Lond B Biol Sci. 1999;354:1155-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 509] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 97. | Fillenz M. The role of lactate in brain metabolism. Neurochem Int. 2005;47:413-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 98. | Chen C, Xie Q, Yang D, Xiao H, Fu Y, Tan Y, Yao S. Recent advances in electrochemical glucose biosensors: a review. RSC Adv. 2013;3:4473-4491. [RCA] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 578] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 99. | Manning CA, Honn VJ, Stone WS, Jane JS, Gold PE. Glucose effects on cognition in adults with Down’s syndrome. Neuropsychology. 1998;12:479-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 100. | Manning CA, Ragozzino ME, Gold PE. Glucose enhancement of memory in patients with probable senile dementia of the Alzheimer’s type. Neurobiol Aging. 1993;14:523-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 144] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 101. | Lowry JP, Fillenz M. Real-time monitoring of brain energy metabolism in vivo using microelectrochemical sensors: the effects of anesthesia. Bioelectrochemistry. 2001;54:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 102. | Ahmad F, Yusof AP, Bainbridge M, Ab Ghani S. The application of glucose biosensor in studying the effects of insulin and anti-hypertensive drugs towards glucose level in brain striatum. Biosens Bioelectron. 2008;23:1862-1868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 103. | Baker DA, Gough DA. A continuous, implantable lactate sensor. Anal Chem. 1995;67:1536-1540. [RCA] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 104. | Parra A, Casero E, V´azquez L, Pariente F, E . Lorenzo E. Design and characterization of a lactate biosensor based on immobilized lactate oxidase onto gold surfaces. Anal Chim Acta. 2006;555:308-315. [RCA] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 105. | Rassaei L, Olthuis W, Tsujimura S, Sudhölter EJ, van den Berg A. Lactate biosensors: current status and outlook. Anal Bioanal Chem. 2014;406:123-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 106. | Dixon BM, Lowry JP, O’Neill RD. Characterization in vitro and in vivo of the oxygen dependence of an enzyme/polymer biosensor for monitoring brain glucose. J Neurosci Methods. 2002;119:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 107. | Nesakumar N, Sethuraman S, Krishnan UM, Rayappan JB. Fabrication of lactate biosensor based on lactate dehydrogenase immobilized on cerium oxide nanoparticles. J Colloid Interface Sci. 2013;410:158-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 108. | Jaffari SA, Turner AP. Recent advances in amperometric glucose biosensors for in vivo monitoring. Physiol Meas. 1995;16:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 109. | Serra PA, Rocchitta G, Bazzu G, Manca A, Puggioni GM, Lowry JP, O’Neill RD. Design and construction of a low cost single-supply embedded telemetry system for amperometric biosensor applications. Sens Actuat B. 2007;122:118-126. [DOI] [Full Text] |
| 110. | Bazzu G, Puggioni GG, Dedola S, Calia G, Rocchitta G, Migheli R, Desole MS, Lowry JP, O’Neill RD, Serra PA. Real-time monitoring of brain tissue oxygen using a miniaturized biotelemetric device implanted in freely moving rats. Anal Chem. 2009;81:2235-2241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 111. | Van Gompel JJ, Chang SY, Goerss SJ, Kim IY, Kimble C, Bennet KE, Lee KH. Development of intraoperative electrochemical detection: wireless instantaneous neurochemical concentration sensor for deep brain stimulation feedback. Neurosurg Focus. 2010;29:E6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 112. | Liu J, Wang J. A novel improved design for the first-generation glucose biosensor. Food Technol Biotechnol. 2001;39:55-58. |
| 113. | Stoytcheva M, Zlatev R, Velkova Z, Valdez B, Ovalle M. Analytical characteristics of electrochemical biosensors. Portugaliae Electrochim Acta. 2009;27:353-362. [RCA] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 114. | McMahon CP, Killoran SJ, O’Neill RD. Design variations of a polymer-enzyme composite biosensor for glucose: enhanced analyte sensitivity without increased oxygen dependence. J Electroanal Chem. 2005;580:193-202. [RCA] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 115. | Kealy J, Bennett R, Lowry JP. Simultaneous recording of hippocampal oxygen and glucose in real time using constant potential amperometry in the freely-moving rat. J Neurosci Methods. 2013;215:110-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 116. | O’Neill RD, Lowry JP, Rocchitta G, McMahon CP, Serra PA. Designing sensitive and selective polymer/enzyme composite biosensors for brain monitoring in vivo. Trends Anal Chem. 2008;27:78-88. [RCA] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 117. | Palmisano F, Zambonin PG. Ascorbic acid interferences in hydrogen peroxide detecting biosensors based on electrochemically immobilized enzymes. Anal Chem. 1993;65:2690-2692. [RCA] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 118. | Karyakin AA, Gitelmacher OV, Karyakina EE. A high sensitive glucose amperometric biosensor based on Prussian Blue modified electrodes. Anal Lett. 1994;27:2861-2869. [RCA] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 110] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 119. | Karyakin AA, Karyakina EE, Gorton L. Prussian-Blue-based amperometric biosensors in flow-injection analysis. Talanta. 1996;43:1597-1606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 120. | Huang J, Song Z, Li J, Yang Y, Shi H, Wu B, Anzai J, Osa T, Chen Q. A highly-sensitive L-lactate biosensor based on sol-gel film combined with multi-walled carbon nanotubes (MWCNTs) modified electrode. Material Science and Engineering: C. 2007;27:29-34. [RCA] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 121. | Salazar P, O’Neill RD, Martín M, Rochea R, González-Mora JL. Amperometric glucose microbiosensor based on a Prussian Blue modified carbon fiber electrode for physiological applications. Sensors and Actuators B. 2011;152:137-143. [DOI] [Full Text] |
| 122. | Salazar P, Martín M, O’Neill RD, Roche R, González-Mora JL. Biosensors based on Prussian blue modified carbon fibers electrodes for monitoring lactate in the extracellular space of brain tissue. Int J Electrochem Sci. 2012;7:5910-5926. |
| 123. | Salazar P, Martín M, Roche R, González-Mora JL, O’Neill RD. Microbiosensors for glucose based on Prussian Blue modified carbon fiber electrodes for in vivo monitoring in the central nervous system. Biosens Bioelectron. 2010;26:748-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 124. | Plaxco KW, Soh HT. Switch-based biosensors: a new approach towards real-time, in vivo molecular detection. Trends Biotechnol. 2011;29:1-5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 125. | Wilson GS, Gifford R. Biosensors for real-time in vivo measurements. Biosens Bioelectron. 2005;20:2388-2403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 380] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 126. | Schultz JE. Brain Energy Metabolism. Von B. K. Siesjö, John Wiley and Sons, Chichester, New York, Brisbane Toronto, 1978, 607 S., DM 78,65. Pharmazie in unserer Zeit. 1978;7:192. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 127. | Bardt TF, Unterberg AW, Härtl R, Kiening KL, Schneider GH, Lanksch WR. Monitoring of brain tissue PO2 in traumatic brain injury: effect of cerebral hypoxia on outcome. Acta Neurochir Suppl. 1998;71:153-156. [PubMed] |
| 128. | Beck T, Krieglstein J. Cerebral circulation, metabolism, and blood-brain barrier of rats in hypocapnic hypoxia. Am J Physiol. 1987;252:H504-H512. |
| 129. | Carraway MS, Piantadosi CA. Oxygen toxicity. Respir Care Clin N Am. 1999;5:265-295. [PubMed] |
| 130. | Zuo L, Motherwell MS. The impact of reactive oxygen species and genetic mitochondrial mutations in Parkinson’s disease. Gene. 2013;532:18-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 131. | Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109-142. [PubMed] |
| 132. | Palmer RM. The L-arginine: nitric oxide pathway. Curr Opin Nephrol Hypertens. 1993;2:122-128. [PubMed] |
| 133. | Moncada S, Palmer RM, Higgs EA. Biosynthesis of nitric oxide from L-arginine. A pathway for the regulation of cell function and communication. Biochem Pharmacol. 1989;38:1709-1715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 943] [Cited by in RCA: 917] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 134. | Ignarro LJ. Nitric oxide. A novel signal transduction mechanism for transcellular communication. Hypertension. 1990;16:477-483. [PubMed] |
| 135. | Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci USA. 1987;84:9265-9269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3371] [Cited by in RCA: 3211] [Article Influence: 84.5] [Reference Citation Analysis (0)] |
| 136. | Ignarro LJ, Byrns RE, Buga GM, Wood KS. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ Res. 1987;61:866-879. [PubMed] |
| 137. | Moncada S, Radomski MW, Palmer RM. Endothelium-derived relaxing factor. Identification as nitric oxide and role in the control of vascular tone and platelet function. Biochem Pharmacol. 1988;37:2495-2501. [PubMed] |
| 138. | Holán V, Krulová M, Zajícová A, Pindjáková J. Nitric oxide as a regulatory and effector molecule in the immune system. Mol Immunol. 2002;38:989-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 139. | Solomonson LP. Nitric oxide. New discoveries, biomedical implications. J Fla Med Assoc. 1991;78:107-109. [PubMed] |
| 140. | Lowenstein CJ, Hill SL, Lafond-Walker A, Wu J, Allen G, Landavere M, Rose NR, Herskowitz A. Nitric oxide inhibits viral replication in murine myocarditis. J Clin Invest. 1996;97:1837-1843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 192] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 141. | Ignarro LJ, Napoli C. Novel features of nitric oxide, endothelial nitric oxide synthase, and atherosclerosis. Curr Diab Rep. 2005;5:17-23. [PubMed] |
| 142. | Schmidt HH, Warner TD, Ishii K, Sheng H, Murad F. Insulin secretion from pancreatic B cells caused by L-arginine-derived nitrogen oxides. Science. 1992;255:721-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 312] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 143. | Vincent SR. Nitric oxide and arginine-evoked insulin secretion. Science. 1992;258:1376-1378. [PubMed] |
| 144. | Ward WK, Wood MD, Slobodzian EP. Continuous amperometric monitoring of subcutaneous oxygen in rabbit by telemetry. J Med Eng Technol. 2002;26:158-167. [PubMed] |
| 145. | Holmström N, Nilsson P, Carlsten J, Bowald S. Long-term in vivo experience of an electrochemical sensor using the potential step technique for measurement of mixed venous oxygen pressure. Biosens Bioelectron. 1998;13:1287-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 146. | Bolger FC, Lowry JP. Brain tissue oxygen: In vivo monitoring with carbon paste electrodes. Sensors. 2005;5:473-487. [RCA] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 147. | O’Neill RD, Grünewald RA, Fillenz M, Albery WJ. Linear sweep voltammetry with carbon paste electrodes in the rat striatum. Neuroscience. 1982;7:1945-1954. [PubMed] |
| 148. | Venton BJ, Michael DJ, Wightman RM. Correlation of local changes in extracellular oxygen and pH that accompany dopaminergic terminal activity in the rat caudate-putamen. J Neurochem. 2003;84:373-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 111] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 149. | Ariansen JL, Heien ML, Hermans A, Phillips PE, Hernadi I, Bermudez MA, Schultz W, Wightman RM. Monitoring extracellular pH, oxygen, and dopamine during reward delivery in the striatum of primates. Front Behav Neurosci. 2012;6:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 150. | Lowry JP, Demestre M, Fillenz M. Relation between cerebral blood flow and extracellular glucose in rat striatum during mild hypoxia and hyperoxia. Dev Neurosci. 1998;20:52-58. [PubMed] |
| 151. | Lowry JP, Boutelle MG, O’Neill RD, Fillenz M. Characterization of carbon paste electrodes in vitro for simultaneous amperometric measurement of changes in oxygen and ascorbic acid concentrations in vivo. Analyst. 1996;121:761-766. [PubMed] |
| 152. | Bolger FB, McHugh SB, Bennett R, Li J, Ishiwari K, Francois J, Conway MW, Gilmour G, Bannerman DM, Fillenz M. Characterisation of carbon paste electrodes for real-time amperometric monitoring of brain tissue oxygen. J Neurosci Methods. 2011;195:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 153. | Finnerty NJ, Bolger FB, Pålsson E, Lowry JP. An investigation of hypofrontality in an animal model of schizophrenia using real-time microelectrochemical sensors for glucose, oxygen, and nitric oxide. ACS Chem Neurosci. 2013;4:825-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 154. | McHugh SB, Fillenz M, Lowry JP, Rawlins JN, Bannerman DM. Brain tissue oxygen amperometry in behaving rats demonstrates functional dissociation of dorsal and ventral hippocampus during spatial processing and anxiety. Eur J Neurosci. 2011;33:322-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 155. | Francois J, Conway MW, Lowry JP, Tricklebank MD, Gilmour G. Changes in reward-related signals in the rat nucleus accumbens measured by in vivo oxygen amperometry are consistent with fMRI BOLD responses in man. Neuroimage. 2012;60:2169-2181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 156. | Shibuki K. An electrochemical microprobe for detecting nitric oxide release in brain tissue. Neurosci Res. 1990;9:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 294] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 157. | Kashevskii AV, Lei J, Safronov AY, Ikeda O. Electrocatalytic properties of meso-tetraphenylporphyrin cobalt for nitric oxide oxidation in methanolic solution and in Nafion® film. J Electroanal Chem. 2002;531:71-79. [RCA] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 158. | Kashevskii AV, Safronov A. Y, Ikeda O. Behaviors of H2TPP and CoTPPCl in Nafion® film and the catalytic activity for nitric oxide oxidation. J Electroanal Chem. 2001;510:86-95. [RCA] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 159. | Mao L, Shi G, Tian Y, Liu H, Jin L, Yamamoto K, Tao S, Jin J. A novel thin-layer amperometric detector based on chemically modified ring-disc electrode and its application for simultaneous measurements of nitric oxide and nitrite in rat brain combined with in vivo microdialysis. Talanta. 1998;46:1547-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 160. | Bedioui F, Trevin S, Devynck J, Lantoine F, Brunet A, Devynck MA. Elaboration and use of nickel planar macrocyclic complex-based sensors for the direct electrochemical measurement of nitric oxide in biological media. Biosens Bioelectron. 1997;12:205-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 161. | Trevin S, Bedioui F, Devynck J. Electrochemical and spectrophotometric study of the behavior of electropolymerized nickel porphyrin films in the determination of nitric oxide in solution. Talanta. 1996;43:303-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 162. | Ricciardolo FL, Vergnani L, Wiegand S, Ricci F, Manzoli N, Fischer A, Amadesi S, Fellin R, Geppetti P. Detection of nitric oxide release induced by bradykinin in guinea pig trachea and main bronchi using a porphyrinic microsensor. Am J Respir Cell Mol Biol. 2000;22:97-104. [PubMed] |
| 163. | Zhang X, Li H, Jin H, Ebin Z, Brodsky S, Goligorsky MS. Effects of homocysteine on endothelial nitric oxide production. Am J Physiol Renal Physiol. 2000;279:F671-F678. [PubMed] |
| 164. | Joshi MS, Lancaster JR, Liu X, Ferguson TB. In situ measurement of nitric oxide production in cardiac isografts and rejecting allografts by an electrochemical method. Nitric Oxide. 2001;5:561-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 165. | Griveau S, Dumézy C, Séguin J, Chabot GG, Scherman D, Bedioui F. In vivo electrochemical detection of nitric oxide in tumor-bearing mice. Anal Chem. 2007;79:1030-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 166. | Isik S, Castillo J, Blöchl A, Csöregi E, Schuhmann W. Simultaneous detection of L-glutamate and nitric oxide from adherently growing cells at known distance using disk shaped dual electrodes. Bioelectrochemistry. 2007;70:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 167. | Zhang X. Real time and in vivo monitoring of nitric oxide by electrochemical sensors--from dream to reality. Front Biosci. 2004;9:3434-3446. [PubMed] |
| 168. | Dalbasti T, Kilinc E. Microelectrode for in vivo real-time detection of NO. Methods Enzymol. 2005;396:584-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 169. | Friedemann MN, Robinson SW, Gerhardt GA. o-Phenylenediamine-modified carbon fiber electrodes for the detection of nitric oxide. Anal Chem. 1996;68:2621-2628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 200] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 170. | Wu WC, Wang Y, Su CK, Chai CY. The nNOS/cGMP signal transducing system is involved in the cardiovascular responses induced by activation of NMDA receptors in the rostral ventrolateral medulla of cats. Neurosci Lett. 2001;310:121-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 171. | Wu WC, Wang Y, Kao LS, Tang FI, Chai CY. Nitric oxide reduces blood pressure in the nucleus tractus solitarius: a real time electrochemical study. Brain Res Bull. 2002;57:171-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 172. | Zheng X, Ning G, Yang Y. Study on the technology of nitric oxide (NO) detection in vitro and in vivo. Clin Hemorheol Microcirc. 2006;34:347-352. [PubMed] |
| 173. | Alvin Koh WC, Rahman MA, Choe ES, Lee DK, Shim YB. A cytochrome c modified-conducting polymer microelectrode for monitoring in vivo changes in nitric oxide. Biosens Bioelectron. 2008;23:1374-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 174. | Brown FO, Finnerty NJ, Lowry JP. Nitric oxide monitoring in brain extracellular fluid: characterisation of Nafion-modified Pt electrodes in vitro and in vivo. Analyst. 2009;134:2012-2020. [PubMed] |
| 175. | Finnerty NJ, O’Riordan SL, Brown FO, Serra P, O’Neill RD, Lowry JP. In vivo characterisation of a Nafion®-modified Pt electrode for real-time nitric oxide monitoring in brain extracellular fluid. Analytical Methods. 2012;4:550-557. |
| 176. | Santos RM, Rodrigues MS, Laranjinha J, Barbosa RM. Biomimetic sensor based on hemin/carbon nanotubes/chitosan modified microelectrode for nitric oxide measurement in the brain. Biosens Bioelectron. 2013;44:152-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 177. | Rocchitta G, Serra PA. Direct monitoring of ethanol in the brain. OA Alcohol. 2013;1:15. |
| 178. | Hardman JG, Limbird LE. Goodman and Gilman’s the Pharmacological Basis of Therapeutics, 11th ed. New York: McGraw-Hill 2005; . |
| 179. | Brodie MS, Shefner SA, Dunwiddie TV. Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res. 1990;508:65-69. [PubMed] |
| 180. | Gessa GL, Muntoni F, Collu M, Vargiu L, Mereu G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res. 1985;348:201-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 521] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 181. | Theile JW, Morikawa H, Gonzales RA, Morrisett RA. GABAergic transmission modulates ethanol excitation of ventral tegmental area dopamine neurons. Neuroscience. 2011;172:94-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 182. | Karahanian E, Quintanilla ME, Tampier L, Rivera-Meza M, Bustamante D, Gonzalez-Lira V, Morales P, Herrera-Marschitz M, Israel Y. Ethanol as a prodrug: brain metabolism of ethanol mediates its reinforcing effects. Alcohol Clin Exp Res. 2011;35:606-612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 183. | Jamal M, Ameno K, Kumihashi M, Ameno S, Kubota T, Wang W, Ijiri I. Microdialysis for the determination of acetaldehyde and ethanol concentrations in the striatum of freely moving rats. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;798:155-158. [PubMed] |
| 184. | Munson PL, Mueller RA, Breese GR. Principles of Pharmacology: Basic Concepts and Clinical Application, 1st ed. New York: Chapman and Hall Inc 1995; . |
| 185. | Secchi O, Zinellu M, Spissu Y, Pirisinu M, Bazzu G, Migheli R, Desole MS, O’Neill RD, Serra PA, Rocchitta G. Further in-vitro characterization of an implantable biosensor for ethanol monitoring in the brain. Sensors (Basel). 2013;13:9522-9535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 186. | Rocchitta G, Secchi O, Alvau MD, Migheli R, Calia G, Bazzu G, Farina D, Desole MS, O’Neill RD, Serra PA. Development and characterization of an implantable biosensor for telemetric monitoring of ethanol in the brain of freely moving rats. Anal Chem. 2012;84:7072-7079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 187. | FCC (Federal Communication Commission) (U.S.A.). Amendment of Parts 2 and 95 of the Commission’s Rules to Create a Wireless Medical Telemetry Service. 2000;. |
| 188. | Crespi F, Dalessandro D, Annovazzi-Lodi V, Heidbreder C, Norgia M. In vivo voltammetry: from wire to wireless measurements. J Neurosci Methods. 2004;140:153-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 189. | Garris PA, Ensman R, Poehlman J, Alexander A, Langley PE, Sandberg SG, Greco PG, Wightman RM, Rebec GV. Wireless transmission of fast-scan cyclic voltammetry at a carbon-fiber microelectrode: proof of principle. J Neurosci Methods. 2004;140:103-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 190. | Garris PA, Greco PG, Sandberg SG, Howes G, Pongmaytegul S, Heidenreich BA, Casto JM, Ensman R, Poehlman J, Alexander A. In: Michael AC, Borland LM. Electrochemical Methods for Neuroscience- In vivo voltammetry with telemetry. Boca Raton (FL): CRC Press 2007; 233-260. |
| 191. | Johnson DA, Wilson GS. In: Michael A.C, Borland LM. Electrochemical Methods for Neuroscience- Telemetry for biosensor systems. Boca Raton (FL): CRC Press 2007; 451-464. |
| 192. | Kasasbeh A, Lee K, Bieber A, Bennet K, Chang SY. Wireless neurochemical monitoring in humans. Stereotact Funct Neurosurg. 2013;91:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 193. | Imeri L, De Simoni MG, Giglio R, Clavenna A, Mancia M. Changes in the serotonergic system during the sleep-wake cycle: simultaneous polygraphic and voltammetric recordings in hypothalamus using a telemetry system. Neuroscience. 1994;58:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 194. | Imeri L, Gemma C, De Simoni MG, Opp MR, Mancia M. Hypothalamic serotonergic activity correlates better with brain temperature than with sleep-wake cycle and muscle tone in rats. Neuroscience. 1999;89:1241-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 195. | De Simoni MG, De Luigi A, Imeri L, Algeri S. Miniaturized optoelectronic system for telemetry of in vivo voltammetric signals. J Neurosci Methods. 1990;33:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 196. | Annovazzi-Lodi V, Donati S. An optoelectronic interconnection for bidirectional transmission of biological signals. IEEE Trans Biomed Eng. 1988;35:595-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 197. | Rocchitta G, Migheli R, Dedola S, Calia G, Desole MS, Miele E, Lowry JP, O’Neill RD, Serra PA. Development of a distributed, fully automated, bidirectional telemetry system for amperometric microsensor and biosensor applications. Sensor Actuact B Chem. 2007;126:700-709. [DOI] [Full Text] |