Published online Apr 26, 2022. doi: 10.5495/wjcid.v12.i1.33
Peer-review started: July 2, 2021
First decision: October 18, 2021
Revised: November 13, 2021
Accepted: January 25, 2022
Article in press: January 25, 2022
Published online: April 26, 2022
Processing time: 297 Days and 7.4 Hours
Coronavirus disease 2019 (COVID-19) is a highly contagious viral illness which conventionally manifests with primarily respiratory symptoms and less commonly with cardiac involvement in various forms, such as pericarditis. Myocarditis and pericarditis have been reported in a variety of live and attenuated vaccines, such as smallpox and influenza. As of October 2021, no cases of pericarditis associated with COVID-19 vaccination have been published. We present two healthy male patients who present post COVID-19 vaccination with pericarditis diagnoses.
A 21-year-old male with no significant past medical history presented with myalgia, chills, mild headache, and chest pain for two days. Patient received the Moderna COVID-19 vaccine the day prior to symptom onset. On presentation, electrocardiogram (ECG) revealed sinus rhythm with ST elevation, and troponin was elevated. Emergent cardiac catheterization was not significant for abnormalities. The primary diagnosis was acute pericarditis, and the patient was discharged on colchicine and indomethacin. Additionally, a 35-year-old male with no pertinent past medical history presented with fever, chills, weakness, nausea, vomiting, diarrhea, and retrosternal chest pain for three days. He received the Moderna COVID-19 vaccine four days prior to symptom onset. On presentation, troponin was elevated, and ECG revealed mild ST elevation. Left ventricular dysfunction with ejection fraction of 41% was reported on transthoracic echocardiogram. Patient was started on ibuprofen and colchicine for diagnosis of myopericarditis.
These case reports highlight a potential unintended consequence, pericarditis, associated with COVID-19 vaccination that may not warrant invasive cardiac intervention.
Core Tip: Coronavirus disease 2019 (COVID-19) manifests with primarily respiratory symptoms and less commonly with cardiac involvement. Myocarditis and pericarditis have been reported in a variety of live and attenuated vaccines. However, to our knowledge, there are no published cases associated with COVID-19 vaccination as of October 2021. We present two cases of pericarditis following COVID-19 vaccination. Both patients were treated with colchicine and non-steroidal anti-inflammatory agents but with varying degrees of invasive work-up. The first patient had emergent cardiac catheterization, while the second patient underwent computed tomographic angiography of the coronary arteries. Neither patient required intervention, thus questioning the necessity of cardiac catheterization.
- Citation: Fydrych J, Hughes AP, Abuhasna S, Mekonen E. Pericarditis following COVID-19 vaccination: Two case reports. World J Clin Infect Dis 2022; 12(1): 33-40
- URL: https://www.wjgnet.com/2220-3176/full/v12/i1/33.htm
- DOI: https://dx.doi.org/10.5495/wjcid.v12.i1.33
On January 9, 2020, the World Health Organization announced a mysterious Coronavirus-related pneumonia in Wuhan, China[1]. Shortly after, cases were identified across the globe and quarantines were ordered to help prevent transmission. Despite early prevention efforts of social distancing and donning masks, coronavirus disease 2019 (COVID-19) was relentless[1]. As of November 2021, there are over 250 million confirmed cases and over five million deaths attributed to COVID-19[2]. The development of a vaccine was the light at the end of the tunnel but not without some associated risks.
Cardiac complications, such as myocarditis and pericarditis, after immunizations are extremely rare events in both younger and older patients but have been reported following smallpox, diphtheria, tetanus, polio, human papillomavirus (HPV), and influenza vaccines[3-6]. Through a PubMed search of pericarditis associated with the COVID-19 vaccine, no published cases have been identified. However, recently, the possible link between myocarditis and the COVID-19 vaccine has been under investigation by the Israel Health Ministry[7]. Sixty-two cases of myocarditis in young males were identified, but at this time, no conclusions have been made. The European Medicines Agency and United States Centers for Disease Control and Prevention (CDC) are also investigating the link between pericarditis and myocarditis with the COVID-19 vaccine, but no association has been found[8,9].
Here below, we present two cases of pericarditis in two young adult males following Moderna COVID-19 vaccination. A 21-year-old healthy male presenting three days post vaccination, and a 35-year-old male presenting seven days post vaccination. The purpose of this case report is to highlight an atypical response to the COVID-19 vaccine.
Case 1: In April 2021, a 21-year-old man was admitted to the hospital with myalgia, chills, mild headache, and chest pain for two days.
Case 2: In February 2021, a 35-year-old man presented to the emergency department with fever, chills, weakness, nausea, vomiting, diarrhea, and retrosternal chest pain for three days.
Case 1: Chest pain was rated three out of ten which worsened on inspiration and described as pleuritic in nature. The patient received the first dose of the Moderna COVID-19 vaccine series the day prior to symptom onset. No associated diaphoresis or arm pain. No tenderness on palpation. No history of known COVID-19 infection or current fevers, nausea, vomiting, diarrhea, abdominal pain, shortness of breath, cough, extremity swelling, travel, or sick contacts.
Case 2: He received his first Moderna COVID-19 vaccine four days prior to symptom onset. Patient denies recent travel, sick contacts, COVID-19 exposure, or symptoms associated with COVID-19.
Case 1: The patient has no pertinent past medical history or surgeries.
Case 2: Past medical history significant for obesity, chronic allergic rhinitis, and previous COVID-19 infection reported several months prior to vaccination.
No tobacco, illicit drugs, or alcohol use reported. No family history of heart disease for either of the two cases.
Case 1: In the emergency department (ED), on physical examination, no rub, normal rate, and regular rhythm noted.
Case 2: In the ED, on physical exam, the patient was noted to be tachycardic but otherwise had no abnormal physical exam findings, including pericardial rub.
Case 1: Troponin was elevated at 15.2 ng/mL, creatinine kinase elevated at 657 units/L, C-Reactive Protein (CRP) elevated at 6.3 mg/dL, and erythrocyte sedi
Case 2: Troponin was elevated at 7.58 ng/mL, CRP elevated at 26.8 mg/dL, and ESR elevated at 96 mm/hr. White blood cell (WBC) count was also elevated at 20.4 K/mcL. Bacterial and viral infectious etiologies were ruled out including COVID-19, RSV, influenza, bocavirus, adenovirus, parainfluenza, metapneumovirus, rhinovirus, enterovirus, Mycoplasma pneumoniae, and Chlamydophila pneumoniae using a sample from a nasopharyngeal swab tested on Cepheid Xpert Xpress SARS-CoV-2/Flu/RSV RT-PCR and Multiplex RT PCR amplification followed by Liquid Bead Array Hybridization. Hepatitis and human immunodeficiency viruses were also ruled out via antibody and antigen testing. Blood cultures had no growth.
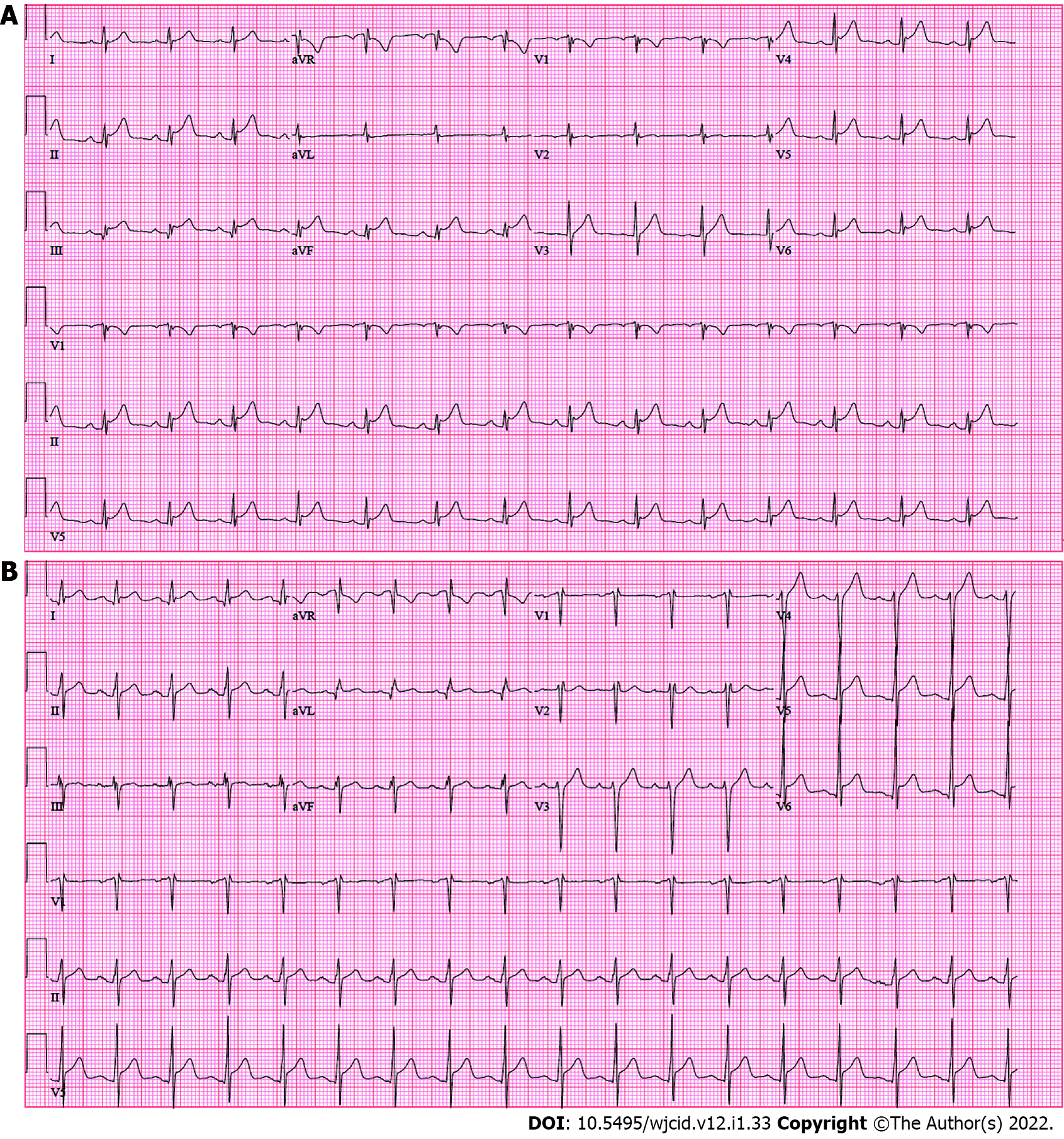
Case 1: Chest X-ray was unremarkable with clear lungs and normal heart size. Transthoracic echocardiogram (TTE) demonstrated an ejection fraction (EF) of 65% without wall motion abnormalities and no pericardial effusion. Original electrocardiogram (ECG) reported sinus rhythm with ST elevation (Figure 1A). On repeat ECG, there was disproportionate worsening of ST elevation in inferior leads with development of new ST depression in aVL lead, so the patient was worked up for acute inferior myocardial infarction to rule out obstructive etiology. Emergent cardiac catheterization demonstrated 40% stenosis of the midsegment of the left anterior descending artery (LAD), though the remaining coronary arteries appeared to be disease-free and no interventions warranted.
Case 2: Chest X-ray and computed tomographic angiography (CTA) of the chest with contrast were both unremarkable. ECG demonstrated mild diffuse ST/T elevation (Figure 1B). On TTE, left ventricular dysfunction with EF of 41% reported.
Case 1: The primary diagnosis was acute pericarditis.
Case 2: The final diagnosis was myopericarditis complicated by acute kidney injury (AKI) secondary to intravenous (IV) contrast.
Case 1: The patient was started on colchicine, indomethacin, and aspirin. He was discharged the following day on aspirin, colchicine for 14 d, and an indomethacin taper over 28 d.
Case 2: The patient was given acetaminophen and ketorolac in the ED. Cardiology was consulted and started the patient on ibuprofen and colchicine for myopericarditis. Ibuprofen was changed to prednisone due to contrast induced AKI. Colchicine and prednisone were continued for five additional days on discharge with instruction to follow-up with cardiology outpatient. Carvedilol and sacubitril/valsartan were initiated based on left ventricular dysfunction with plans to monitor and titrate outpatient. No antimicrobials were administered, and leukocytosis spontaneously resolved.
Case 1: Upon discharge, an appointment was scheduled with a new primary care provider with instruction to obtain a cardiologist referral. During follow-up with cardiology three months after discharge, repeat TTE was unremarkable with normal function and no regional wall motion abnormalities. Troponin, CRP, and ESR were normal as well. No further work-up or cardiology follow-up warranted. To our knowledge, there were no complications or readmissions associated with pericarditis following discharge.
Case 2: The patient’s course was complicated by AKI secondary to IV contrast which extended his hospitalization to four days. Coronary CTA was later completed outpatient with no notable findings. To our knowledge, there were no complications or readmissions associated with pericarditis following discharge.
As of November 2021, over three billion individuals are fully vaccinated for COVID-19[10]. Common side effects of the COVID-19 vaccine are pain, redness, and swelling of the injection site, fatigue, headache, muscle pain, chills, fever, and nausea. No case reports of pericarditis as a side effect of COVID-19 vaccines have been published as of November 2021. A hypothesized mechanism of action of vaccine induced myopericarditis is an autoimmune reaction that typically occurs after seven days. Our patients presented within seven days post vaccination; therefore, this mechanism is unlikely[11]. A possible mechanism for male predominance in myocarditis/pericarditis is presumably related to variations in sex hormones. Estrogen is thought to inhibit proinflammatory T cells, resulting in a decrease in immune related responses. Pericarditis in females may also be underdiagnosed, which could explain the male predominance[12].
On the contrary, active COVID-19 infection presenting as pericarditis and endocarditis have been reported[13,14]. A systematic review conducted in December 2020 identified 34 COVID-19 patients with pericarditis reported, and 62% were diagnosed with myopericarditis[15]. Pericarditis in COVID-19 patients is still poorly understood. A proposed explanation is the binding of the SARS-CoV-2 Spike protein to angiotensin-converting enzyme 2 which can be found on the heart. Thus, there is potential cause for myocarditis, pericardial effusion, and pericarditis[13,15].
While no case reports were published to our knowledge, as of May 2021, 133 cases of pericarditis and 119 cases of myocarditis had been reported to the CDC Vaccine Adverse Event Reporting System (VAERS)[16]. Cases of pericarditis were reported following each of the Food and Drug Administration approved COVID-19 vaccines Pfizer (67), Moderna (54), and Johnson and Johnson (12). Pfizer was the first vaccine to be approved as well as the most common manufacturer associated with reported pericarditis cases, followed by Moderna, then Johnson and Johnson. Of the 133 pericarditis cases, 77 reported cases are males, 53 are females, and 3 were not reported. The average age for males was 44.6 years old and ranged from 16 to 84 years old. The average age for females was 54.5 years old and ranged from 20 to 85 years old. The mean ages are likely falsely elevated based on vaccine rollout and earlier access to patients 65 years and older in the general public. The average reported time to symptom onset is 7.4 d and ranges from 0 to 63 d. Our patient cases consist of two males that are below the mean reported age and below the average time to symptom onset. As of November 2021, the number of adverse events with the word “pericarditis” associated with the COVID-19 vaccine has since substantially increased to 15895 reports[16].
Acute pericarditis is most commonly caused by viral infections and has been reported in patients with active COVID-19 infections[14,17-19]. Our case report is unique because we present two individuals diagnosed with pericarditis post COVID-19 vaccination who tested negative for active COVID-19 infection. Pericarditis is diagnosed when at least two of the four following criteria are present: Pericarditic chest pain, pericardial rubs, new widespread ST elevation or PR depression on ECG, or pericardial effusion[20]. Additional supporting findings include elevation of cardiac inflammatory markers and evidence of pericardial inflammation by imaging[20]. Both of our patients presented with ST elevation, pericarditic chest pain, along with several elevated cardiac inflammatory markers. In the second case, ECG does not show the typical patterns of acute pericarditis (i.e. diffuse ST elevations with PR depressions), which are often seen hours to days after symptom onset during the first stage. Considering the patient presented seven days post-vaccination and three days after symptom onset, it is plausible any atypical ECG patterns had since normalized. Neither patient had diagnosed cardiac history, infectious causes, noninfectious causes, or past medical history to predispose them to pericarditis; therefore, we believe these episodes of acute pericarditis are secondary to the COVID-19 vaccine.
Patients presenting with COVID-19 and elevated troponins often have worse outcomes[21]. However, if patients present following COVID-19 immunization with elevated cardiac inflammatory markers, ST elevation, but not diagnosed with COVID-19, should cardiac catheterization be standard of care? The 21-year-old patient was taken for emergent cardiac catheterization and was found to have 40% stenosis of the midsegment of the LAD, but remaining coronary arteries appeared to be disease-free. No lesions were identified, and no interventions warranted. While no complications occurred with this patient, possible complications of invasive cardiac catheterization include infection, vascular complications, bleeding, stroke, myocardial infarction, and death[22]. The 35-year-old patient did not undergo cardiac catheterization but did receive a CTA of the chest and coronary arteries instead, which was unremarkable. It is unknown if the pericarditis cases reported to CDC VAERS warranted catheterization or other invasive testing. But of the 133 individuals reported, 86 were hospitalized, 46 were not hospitalized, and one unknown, which leads us to believe many cases self-resolved without seeking out higher level of care.
Our patient cases do have inherent limitations. In the second case, since the patient reported a previous diagnosis of COVID-19, it is impossible to completely rule out myopericarditis as a complication from previous infection. The patient tested negative for active infection on admission, but past COVID-19 test results could not be found in the electronic medical record, so an exact date of infection is unknown. To our knowledge, neither patient has received the second immunization in the series; therefore, we are unable to assess outcomes when re-challenged with the second dose. Additionally, due to a lack of endomyocardial biopsy, a histopathological diagnosis for pericarditis or myocarditis cannot be confirmed. Both patients presented to a community hospital where there was not access to cardiac MRI, and based on rapid clinical improvement, myocardial biopsy was not warranted. Furthermore, other causes of pericarditis cannot be completely ruled out. The first patient tested negative for COVID-19, influenza, and RSV. Other infectious causes are less likely but were not tested for. COVID-19, RSV, influenza, bocavirus, adenovirus, parainfluenza, metapneumovirus, rhinovirus, enterovirus, Mycoplasma pneumoniae, and Chlamydophila pneumoniae were ruled out for the second patient, making viral and bacterial causes unlikely. Neither of these patients have a significant past medical history, including no likely medication causes, no trauma, nor autoimmune conditions. We also do not have long-term follow-up, thus no long-term outcomes for either patient. Despite these limitations, both patients scored a 5 on the Naranjo Algorithm, or Adverse Drug Reaction Probability Scale, resulting in a probable association. The reaction “followed a reasonable temporal sequence after a drug, followed a recognized response to the suspected drug, was confirmed by withdrawal but not by exposure to the drug, and could not be reasonably explained by the known characteristics of the patient’s clinical state.” [23].
During the time of these cases, the new mRNA COVID-19 vaccinations were under emergency use authorization. It is important to note, despite the skepticism and fear surrounding these novel vaccines, the benefits greatly outweigh the risk of rare side effects, including pericarditis and myocarditis. In fact, the CDC has reported the rare incidence of myocarditis/pericarditis as about 12.6 cases per million doses of second-dose mRNA vaccines in those age 12 to 39 years old (0.0000126%)[12]. However, a study investigating cardiovascular sequelae in COVID-19 infected patients revealed that 5.0% developed new-onset myocarditis and 1.5% developed pericarditis; therefore, there is much higher risk from active infection than vaccination[24]. The COVID-19 mRNA vaccines reduce the rate of severe infections, hospitalizations, and death from COVID-19. In a study conducted at five Veteran Affairs Medical Centers, mRNA vaccines were 86.8% effective at preventing COVID-19 associated hospitalizations in those who were over the age of 18 years old[25]. Most episodes of pericarditis are uncomplicated and can be managed in the outpatient setting[18]. Therefore, these cases should alleviate the fear of vaccine associated adverse effects and help guide the public on when to seek care.
Despite our findings and presumed correlation of COVID-19 vaccination and pericarditis, this should not deter individuals from being vaccinated, especially given the reported cardiac involvement from COVID-19 infections. Immunizations are essential for public health and achieving population immunity. Rather, these cases are intended to bring awareness to a potential etiology of pericarditis that should be considered in the differential that might not warrant invasive interventions with substantial risks. Further research and trials are needed to assess the linkage between COVID-19 vaccination and cardiac injury. Our cases highlight the importance of recognizing the possibility of COVID-19 vaccine side effects presenting as pericardial injury in young otherwise healthy individuals. The question remains, is cardiac catheterization necessary for every patient who presents with pericarditis secondary to COVID-19 vaccination?
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Infectious diseases
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ciccone MM, Spartalis M, Wong CP S-Editor: Li X L-Editor: A P-Editor: Li X
| 1. | A Timeline of COVID-19 Developments in 2020. Published online 2021. Available from: https://www.ajmc.com/view/a-timeline-of-covid19-developments-in-2020. |
| 2. | World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Published online 2020. Available from: https://covid19.who.int/. |
| 3. | Mei R, Raschi E, Forcesi E, Diemberger I, De Ponti F, Poluzzi E. Myocarditis and pericarditis after immunization: Gaining insights through the Vaccine Adverse Event Reporting System. Int J Cardiol. 2018;273:183-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 4. | Nalca A, Zumbrun EE. ACAM2000: the new smallpox vaccine for United States Strategic National Stockpile. Drug Des Devel Ther. 2010;4:71-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 148] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 5. | Hawkes D, Lea CE, Berryman MJ. Answering human papillomavirus vaccine concerns; a matter of science and time. Infect Agent Cancer. 2013;8:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Boccara F, Benhaiem-Sigaux N, Cohen A. Acute myopericarditis after diphtheria, tetanus, and polio vaccination. Chest. 2001;120:671-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Israel assesses myocarditis cases linked to Pfizer-BioNTech COVID-19 vaccine. Pharmacceutical Technology. Published 2021. Available from: https://www.pharmaceutical-technology.com/news/israel-myocarditis-pfizer-vaccine/. |
| 8. | European Medicines Agency. COVID-19 vaccine safety update. 28 January 2021. Available from: https://www.ema.europa.eu/en/documents/COVID-19-vaccine-safety-update/COVID-19-vaccine-safety-update-comirnaty-january-2021_en.pdf. |
| 9. | Achenbach J, Sun LH. CDC probes rare cases of heart inflammation in vaccinated teens, young adults. Published 2021. Available from: https://www.washingtonpost.com/health/2021/05/24/heart-inflammation-coronavirus-vaccines-young-adults/. |
| 10. | World Health Organization. Coronavirus disease (COVID-19): Vaccines. Published 2021. Available from: https://www.who.int/news-room/q-a-detail/coronavirus-disease-(COVID-19)-vaccines?adgroupsurvey=%7Badgroupsurvey%7D&gclid=Cj0KCQiA6Or_BRC_ARIsAPzuer84gbbRGBKcj2M76A-THyhJcXvZZ0wFZ80kdlnNVP1YeeK6J4T1C5UaAn1tEALw_wcB. |
| 11. | O'Leary ST, Maldonado YA. Myocarditis After SARS-CoV-2 Vaccination: True, True, and… Related? Pediatrics. 2021;148: e2021052644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Bozkurt B, Kamat I, Hotez PJ. Myocarditis With COVID-19 mRNA Vaccines. Circulation. 2021;144:471-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 556] [Cited by in RCA: 621] [Article Influence: 155.3] [Reference Citation Analysis (0)] |
| 13. | COVID-19 as a Possible Cause of Myocarditis and Pericarditis. Am Coll Cardiol. Published online 2021. Available from: https://www.acc.org/latest-in-cardiology/articles/2021/02/05/19/37/COVID-19-as-a-possible-cause-of-myocarditis-and-pericarditis. |
| 14. | Kumar R, Kumar J, Daly C, Edroos SA. Acute pericarditis as a primary presentation of COVID-19. BMJ Case Rep. 2020;13:e237617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 15. | Diaz-Arocutipa C, Saucedo-Chinchay J, Imazio M. Pericarditis in patients with COVID-19: a systematic review. J Cardiovasc Med (Hagerstown). 2021;22:693-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 16. | The Vaccine Adverse Event Reporting System (VAERS) Request. Available from: https://wonder.cdc.gov/vaers.html. |
| 17. | de Meester A, Luwaert R, Chaudron JM. Symptomatic pericarditis after influenza vaccination: report of two cases. Chest. 2000;117:1803-1805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Tingle LE, Molina D, Calvert CW. Acute pericarditis. Am Fam Physician. 2007;76:1509-1514. [PubMed] |
| 19. | Tung-Chen Y. Acute pericarditis due to COVID-19 infection: An underdiagnosed disease? Med Clin (Barc). 2020;155:44-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Adler Y, Charron P, Imazio M, Badano L, Barón-Esquivias G, Bogaert J, Brucato A, Gueret P, Klingel K, Lionis C, Maisch B, Mayosi B, Pavie A, Ristic AD, Sabaté Tenas M, Seferovic P, Swedberg K, Tomkowski W; ESC Scientific Document Group. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2015;36:2921-2964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1457] [Cited by in RCA: 1591] [Article Influence: 159.1] [Reference Citation Analysis (0)] |
| 21. | Sandoval Y, Januzzi JL Jr, Jaffe AS. Cardiac Troponin for Assessment of Myocardial Injury in COVID-19: JACC Review Topic of the Week. J Am Coll Cardiol. 2020;76:1244-1258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 301] [Article Influence: 60.2] [Reference Citation Analysis (0)] |
| 22. | Wanamaker BL, Seth MM, Sukul D, Dixon SR, Bhatt DL, Madder RD, Rumsfeld JS, Gurm HS. Relationship Between Troponin on Presentation and In-Hospital Mortality in Patients With ST-Segment-Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. J Am Heart Assoc. 2019;8:e013551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Adverse Drug Reaction Probability Scale (Naranjo) in Drug Induced Liver Injury. 2019 May 4. In: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012- . [PubMed] |
| 24. | Buckley BJR, Harrison SL, Fazio-Eynullayeva E, Underhill P, Lane DA, Lip GYH. Prevalence and clinical outcomes of myocarditis and pericarditis in 718,365 COVID-19 patients. Eur J Clin Invest. 2021;51:e13679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 25. | Bajema KL, Dahl RM, Prill MM, Meites E, Rodriguez-Barradas MC, Marconi VC, Beenhouwer DO, Brown ST, Holodniy M, Lucero-Obusan C, Rivera-Dominguez G, Morones RG, Whitmire A, Goldin EB, Evener SL, Tremarelli M, Tong S, Hall AJ, Schrag SJ, McMorrow M, Kobayashi M, Verani JR, Surie D; SUPERNOVA COVID-19; Surveillance Group; Surveillance Platform for Enteric and Respiratory Infectious Organisms at the VA (SUPERNOVA) COVID-19 Surveillance Group. Effectiveness of COVID-19 mRNA Vaccines Against COVID-19-Associated Hospitalization - Five Veterans Affairs Medical Centers, United States, February 1-August 6, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1294-1299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 87] [Article Influence: 21.8] [Reference Citation Analysis (0)] |