Published online Jun 18, 2020. doi: 10.5495/wjcid.v10.i2.24
Peer-review started: April 23, 2020
First decision: May 5, 2020
Revised: May 6, 2020
Accepted: May 21, 2020
Article in press: May 21, 2020
Published online: June 18, 2020
Processing time: 55 Days and 15.9 Hours
Coronavirus disease 2019 (COVID-19) has emerged as a public health crisis that was declared as a global pandemic by the World Health Organization. Although most cases have no or mild symptoms, around 10% of patients develop severe or critical illness that necessitates hospitalization and intensive care unit admission.
To assess the literature for the predictive factors that can identify patients having severe/critical COVID-19 disease.
A Preferred Reporting Items for Systematic Reviews and Meta-Analyses-compliant systematic search of the literature was conducted. Electronic databases including PubMed/MEDLINE, Scopus, and Cochrane Library were queried. The main outcome measures were the predictors of severe/critical COVID-19 and mortality.
Five studies including 583 patients of a median age of 50.5 years were reviewed. Patients were 346 (59.4%) male and 237 (40.6%) female. Of 583 hospitalized patients, 242 (41.5%) had critical illness. Acute respiratory distress disease occurred in 291 patients, accounting for 46.7% of total complications. One-hundred (17.1%) mortalities were recorded. The most commonly reported predictors of severe COVID-19 were older age, medical comorbidities, lymphopenia, elevated C-reactive protein, increased D-dimer, and increased neutrophil ratio. Findings on computed tomography (CT) scanning predictive of severe disease were bronchial wall thickening, CT score > 7, linear opacities, consolidation, right upper lobe affection, and crazy paving pattern.
Several demographic, clinical, laboratory, and radiologic factors can help predict severe and critical COVID-19 along with the potential need for mechanical ventilation. Factors that were more commonly reported were older age, medical comorbidities, lymphopenia, increased neutrophil ratio, elevated C-reactive protein, and increased D-dimer.
Core tip: After systematic literature search, several demographic, clinical, laboratory, and radiologic factors were found to be predictive of severe and critical coronavirus disease 2019 along with the potential need for mechanical ventilation. Factors that were more commonly reported were older age, medical comorbidities, lymphopenia, increased neutrophil ratio, elevated C-reactive protein, and increased D-dimer. Findings on computed tomography (CT) scanning predictive of severe disease were bronchial wall thickening, CT score > 7, linear opacities, consolidation, right upper lobe affection, and crazy paving pattern.
- Citation: Emile SH, Khan SM. Predictors of severe and critical COVID-19: A systematic review. World J Clin Infect Dis 2020; 10(2): 24-32
- URL: https://www.wjgnet.com/2220-3176/full/v10/i2/24.htm
- DOI: https://dx.doi.org/10.5495/wjcid.v10.i2.24
Coronaviruses are enveloped non-segmented positive-sense RNA viruses belonging to the family Coronaviridae[1]. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative organism of coronavirus disease 2019 (COVID-19) belongs to the family of beta coronavirus, akin to the Middle Eastern respiratory distress syndrome coronavirus and SARS-CoV[2].
By the end of 2019, several cases of pneumonia of unknown cause emerged in Wuhan, China[2]. The World Health Organization (WHO) announced that the official name of the disease caused by the virus as COVID-19[3]. As of March 1st 2020, according to WHO situation report worldwide there are 87137 confirmed cases of COVID-19[4].
COVID-19 has a wide spectrum of disease severity, ranging from mild disease to severe acute respiratory distress disease (ARDS). According to a study by Wang et al[5], 44.9% of patients with COVID-19 developed complications, with ARDS occurring in 61% of patients. According to a study by Lai et al[6] fever was the most common symptom, followed by a cough, dyspnea, myalgia, and headache.
Timely identification of patients who are having a severe disease can play a pivotal role in improving outcomes. Basic disease treatment, secondary infection prevention, and timely organ function support are needed for these patients. Therefore, it is crucial to evaluate the prognostic markers of COVID-19.
Several studies had pointed towards various risk factors of severe disease. Comorbidities such as diabetes mellitus, cardiovascular disease, chronic lung disease and advanced age have been linked with more severe disease[7]. Other studies have shown that laboratory parameters including d-dimer level and leucocyte count can have a prognostic significance. An interesting study by Huang et al[8] showed that COVID-19 patients can have exaggerated cytokine storm with effected patients having high amounts of IL1B, IFNγ, IP10, and MCP1, probably leading to activated T-helper-1 (Th1) cell responses. The authors also showed that this exaggerated immune response is linked to disease severity. During our literature search we did not find previous reviews that could uniformly address these risk factors, therefore we aimed to evaluate different risk factors that can identify patients having severe/critical COVID-19 disease.
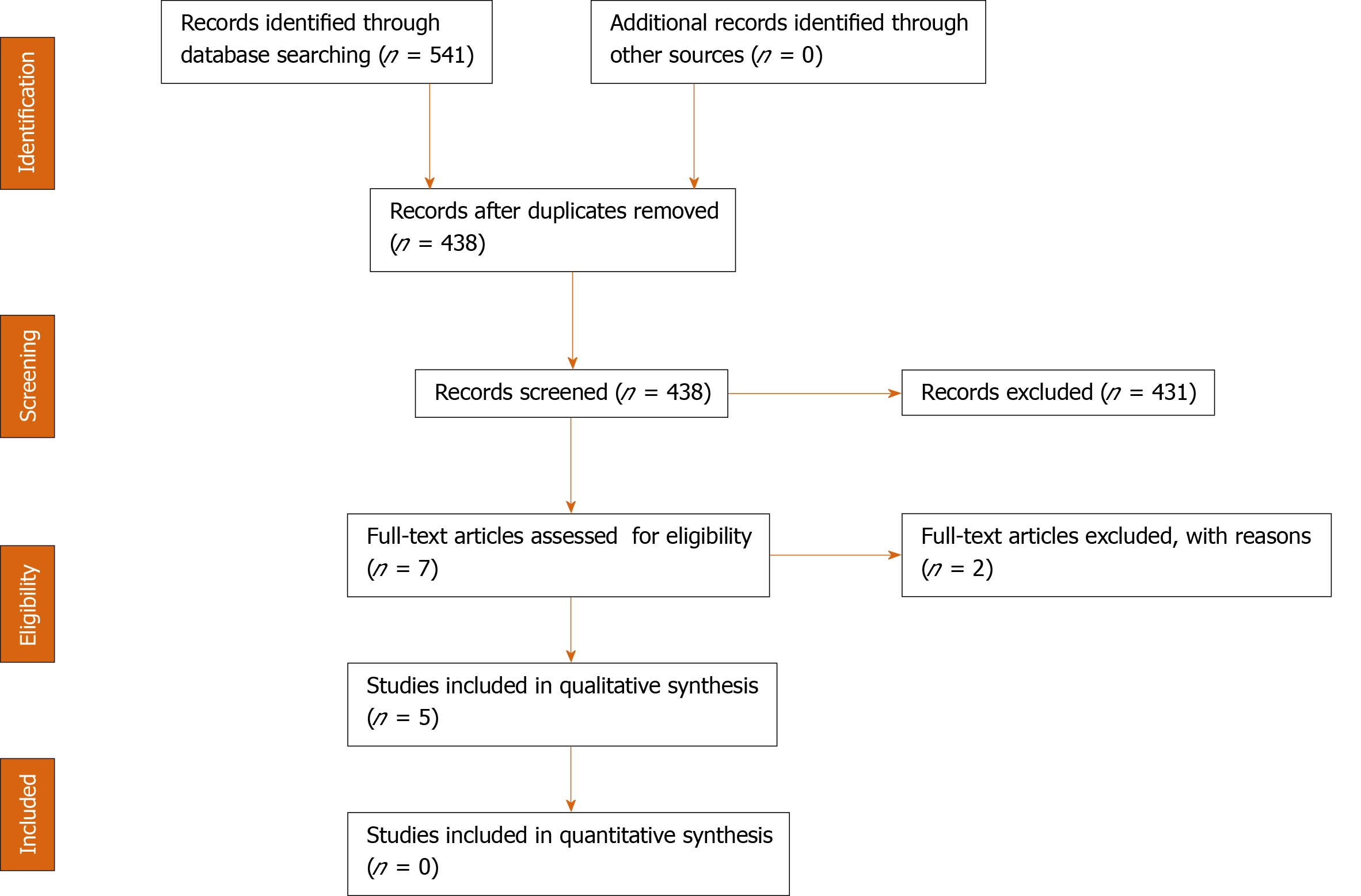
The guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRSIMA) have been followed when reporting this systematic review[9] (Figure 1). An organized, systematic literature search was conducted querying electronic databases. Two authors searched PubMed, Scopus and Cochrane Library for all relevant published and ahead-of-publication studies dating from December 2019 through March 2020.
There were no restrictions to study design and population and language. Using the “related articles” PubMed function further publications were retrieved and screened. The reference section of the retrieved studies was screened for other potentially eligible studies.
The keywords used for the literature search were: (“novel coronavirus” OR “severe acute respiratory syndrome-coronavirus 2” OR “SARS-CoV-2” OR “COVID-19” OR “coronavirus disease 2019”) AND (risk factors OR predictive factors OR predictors OR age OR comorbidities OR laboratory tests OR d-dimer OR WBC count) AND (outcomes OR mortality OR ARDS OR prognosis) were used in the search process. The medial subject headings terms “coronavirus”, “COVID-19” and “Outcome” were also used in the search process.
Two authors screened the articles first by the title and abstract and then full text screening was conducted. In the case of disagreement about the inclusion of an article, the final decision was made after mutual discussion and agreement.
After removing duplicates, the articles retrieved were screened on the basis of pre-defined inclusion and exclusion criteria. We included studies that reported predictive factors for severe/critical COVID-19 as odds ratio (OR) and 95% confidence interval (95%CI). Severe/critical COVID-19 was defined as COVID-19 that warranted mechanical ventilation, whether was associated with mortality or not.
We excluded case reports, editorial, letters to the editor, previous reviews and meta analyses, and articles that did not report the main parameters of the review clearly and completely.
Two authors independently appraised the selected articles for risk of bias (validity) and applicability. Judgments were discussed then a consensus was reached. The methodological index for non-randomized studies (MINORS) was used to assess the quality of the studies reviewed[10]. MINORS score consists of 12 items, the first eight being specifically for non-comparative studies and the last four items pertain to comparative studies. The maximum score for non-comparative studies is 16 and for comparative studies is 24. Non-comparative studies that score greater than 12 and comparative studies that score greater than 20 are considered of low risk of bias.
One author (Emile SH) extracted the following data: (1) Study design, duration, and country; (2) Total number of patients, male to female ratio, and age in years; (3) Clinical symptoms including fever, cough, dyspnea, myalgia and headache; (4) Number of patients in critical condition, number of complications of the disease, and mortality; and (5) The risk factors for severe COVID-19 expressed as odds OR and 95%CI.
The primary outcome of the review was the predictive factors of severe/critical COVID-19. Secondary outcomes comprised prevalence of clinical symptoms, number of complications and mortalities of COVID-19.
Data were analyzed using SPSS™ version 25 (IBM corp, Chicago, USA). Continuous variables were expressed as mean ± SD, or median and normal range. Categorical variables were expressed as numbers and percentages. P value less than 0.05 was considered significant.
Five studies[11-15] were included to this systematic review. All studies were retrospective series, were conducted in China, and were published in 2020. Although all studies have been conducted in China, no overlap of the study participants was noted. The studies included a total of 583 patients, who were 346 (59.4%) male and 237 (40.6%) female of a median age of 50.5 (range, 38-56) years (Table 1).
| Study | Duration | Design | Country | Number | Male | Age | MINORs score |
| Li et al[11], 2020 | Jan 2020- Feb 2020 | Retrospective | China | 83 | 44 | 45.5 | 14 (Low) |
| Zhou et al[12], 2020 | Dec 2019- Jan 2020 | Retrospective | China | 191 | 119 | 56 | 13 (High) |
| Liu et al[13], 2020 | Dec 2019- Jan 2020 | Retrospective | China | 78 | 39 | 38 | 12 (High) |
| Qu et al[14], 2020 | Dec 2019- Jan 2020 | Retrospective | China | 30 | 16 | 50.5 | 13 (High) |
| Wu et al[15], 2020 | Dec 2019- Jan 2020 | Retrospective | China | 201 | 128 | 51 | 12 (High) |
A total of 440 (75.5%) patients presented with fever, 413 (70.8%) with cough, 109 (18.7%) with myalgia, 89 (15.3%) with dyspnea, 16 (2.7%) with abdominal pain, and 9 (1.5%) with headache. Of 583 hospitalized patients, 242 (41.5%) had critical illness (Table 2).
| Study | Fever | Cough | Dyspnea | Abdominal | Myalgia | Headache | Critical cases |
| Li et al[11], 2020 | 72 (86.7) | 65 (78.3) | 9 (10.8) | 7 (8.4) | 15 (18.1) | 9 (10.8) | 25 (30.1) |
| Zhou et al[12], 2020 | 180 (94.2) | 151 (79) | NA | 9 (4.7%) | 29 (15.1) | NA | 119 (62.3) |
| Liu et al[13], 2020 | NA | 34 (34.6) | NA | NA | NA | NA | 11 (14.1) |
| Qu et al[14], 2020 | NA | NA | NA | NA | NA | NA | 3 (10) |
| Wu et al[15], 2020 | 188 (93.5) | 163 (81.1) | 80 (39.8) | NA | 65 (32.3) | NA | 84 (41.8) |
There were 622 recorded complications. ARDS occurred in 291 patients, accounting for 46.7% of total complications. Other complications of COVID-19 included: Heart failure (n = 44), septic shock (n = 38), coagulopathy (n = 37), acute cardiac injury (n = 33), acute kidney injury (n = 28), secondary infection (n = 28), hypoproteinemia (n = 22). One-hundred (17.1%) mortalities were recorded across the studies (Table 3).
Demographic and clinical predictors: (1) Age. Four studies[11-13,15] reported older age as a predictor for poor outcome. The odds ratios of having critical/severe COVID-19 with older age were as follows: (a) Age > 50: OR = 7.596 (2.664-21.659); (b) Age > 60: OR = 1.10 (1.03-1.17); (c) Age > 60: OR = 8.546 (1.628-44.864); and (d) Age > 65: OR = 3.26 (2.08-5.11); (2) Presence of comorbidities. Four studies[11-13,15] reported medical comorbidities as predictor for poor outcome. The odds ratios of having critical/severe COVID-19 with medical comorbidities were as follows: (a) Comorbidities of any type: OR = 10.607 (2.930-38.399); (b) Smoking: OR = 14.285 (1.577-25.000); (c) Coronary heart disease: OR = 2.14 (0.26-17.79); (d) Diabetes mellitus: OR = 2.34 (1.35-4.05); and (e) Hypertension: OR = 1.82 (1.13-2.95); and (3) Clinical symptoms. Three studies[11,13,15] reported clinical symptoms as predictor for poor outcome. The odds ratios of having poor outcome with clinical symptoms were as follows: (a) Dyspnea: OR = 10.899 (2.073-57.198); (b) Chest pain: OR = 10.85 (1.14-102.77); (c) Cough: OR = 9.95 (1.24-79.55); (d) Expectoration: OR = 4.87 (1.5-15.78); (e) Temp > 37.3: OR = 8.999 (1.036-78.147); (f) Temp > 39: OR = 1.77 (1.11-2.84); and (g) Respiratory failure: OR = 8.772 (1.942-40.000).
Laboratory parameters: (1) Reported by more than two studies. Lymphopenia was reported by three studies[11,12,15] as poor prognosticator of COVID-19 with the following odds ratio: OR = 12 (3.21-44.81), OR = 0.19 (0.02-1.62), and OR = 0.37 (0.21-0.63); (2) Reported by two studies: (a) Elevated D-dimer levels > 1: OR = 18.42 (2.64-128.55) and OR = 1.03 (1.01-1.04); (b) Neutrophilia: OR = 9.67 (3.27-28.57) and OR = 1.14 (1.09-1.19); and (c) Elevated C-reactive protein (CRP): OR = 13.2 (2.84-61.23) and OR = 4.81 (1.52-15.27); and (3) Reported by one study: (a) Decreased monocyte ratio: OR = 18 (2.03-159.1); (b) Decreased lymphocyte ratio: OR = 7.6 (2.48-23.28); (c) Increased procalcitonin: OR = 7.989 (2.426-26.305); (d) Decreased oxyhemoglobin saturation: OR = 8.329 (2.483-27.933); (e) Platelet lymphocyte ratio: OR = 0.993 (0.983-1.003); (f) Reduced CD3: OR = 0.83 (0.72-0.96); (g) Reduced CD4: OR = 0.74 (0.59-0.93); (h) Increased bilirubin: OR = 1.05 (1.02-1.08); (i) Elevated AST: OR = 1.02 (1.01-1.03); (j) Hypoalbuminemia: OR = 0.49 (0.37-0.66); (k) Hyperglobulinemia: OR = 2.32 (1.45-3.71); (l) Decreased prealbumin: OR = 0.99 (0.98-0.99); (m) Increased urea: OR = 1.13 (1.09-1.18); (n) Increased creatinine: OR = 1.05 (1.01-1.10); (o) Hypoglycemia: OR = 1.13 (1.08-1.19); (p) Increased cholinesterase: OR = 1.13 (1.08-1.19); (q) Increased cystatin: OR = 1.69 (1.31-2.19); (r) Increased LDH: OR = 1.61 (1.44-1.79); (s) Increased alpha HBDH: OR = 1.74 (1.52-1.99); (t) Increased LDL: OR = 0.63 (0.44-0.88); and (u) Increased serum ferritin: OR = 3.53 (1.52-8.16).
Radiologic parameters in CT scanning: (1) Bronchial wall thickening: OR = 32.593 (7.876-134.880); (2) CT score > 7: OR = 19.200 (5.820-63.336); (3) Linear opacities: OR = 10.016 (2.160-46.454); (4) Consolidation: OR = 6.387 (1.720-23.719); (5) Right upper lobe affection: OR = 5.603 (1.195-26.277); and (6) Crazy paving pattern: OR = 3.341 (1.257-8.878).
The odds ratios of all predictive factors of severe COVID-19 found after literature search were reviewed and the factors that had the highest odds (OR > 10) were selected to construct a prognostic scoring system. Each predictive factor was given points according to its relative weight and OR. The prognostic score ranged from 0 to 16. According to the probability to develop severe COVID-19, the outcome of the score was summarized as: Low probability (0-5 points), moderate probability (6-10 points), and high probability (11-16 points, Table 4).
| Predictive factor | Odds ratio | Score points |
| Bronchial wall thickening in CT scan | 32.593 | 3 |
| CT score > 7 | 19.200 | 2 |
| Elevated D-dimer | 18.42 | 2 |
| Decreased monocyte ratio | 18 | 2 |
| Smoking | 14.285 | 2 |
| Elevated C-reactive protein | 13.2 | 1 |
| Lymphopenia | 12 | 1 |
| Dyspnea | 10.899 | 1 |
| Chest pain | 10.85 | 1 |
| Medical comorbidities | 10.607 | 1 |
As the COVID-19 pandemic is one the rise, the numbers of people with critical illness increase and so does the pressure on hospitals and intensive care units[16]. Since the disease tends to progress rapidly once pulmonary affection has occurred, there is a pressing need to predict which patients are more vulnerable to succumb into critical illness and may require mechanical ventilation.
The present systematic review aimed to explore the available literature on COVID-19 on the risk factors for developing critical illness that may result in fatality. Several predictive factors were found and were classified into clinical, laboratory, and radiologic parameters.
Among the important demographic and clinical factors, older age was reported by almost all the studies reviewed. The cut-off point for age varied between 50, 60, and 65 years among the studies[11-13,15]. This observation can be attributed to the effect of aging on the respiratory system. This effect includes chest wall and thoracic spine deformities that tends to impair the total respiratory system compliance, loss of supporting structure of the lung causing dilation of air spaces, weakness of respiratory muscles that impairs effective cough and airway clearance, and diminished ventilatory response to hypoxia and hypercapnia, making elderly more prone to respiratory failure during high demand states[17]. In addition, impaired immune functions in individuals > 65 years, known as immunosenescence, is associated with increased susceptibility to diseases, infections and poor response to treatments[18].
Medical comorbidities are strongly linked to poor outcome with COVID-19 as reported by several investigators[11,12,15]. Patients with poor state of health may have weakened immunity against the SARS-COV2 as compared to other healthy individuals. A recent analysis demonstrated the impact of comorbidity on COVID-19 patients in China and reported that patients with comorbidities such as diabetes mellitus, hypertension, COPD, and malignancy had greater disease severity compared with those without comorbid conditions and the greater the number of comorbidities the greater the severity of COVID-19[19].
Smoking appeared to increase the odds of progression to critical illness 14 times as compared to non-smokers[13]. Smoking predisposes to COPD and small airway disease and has well-documented effects on the pulmonary functions that include decreased forced vital capacity (FVC), forced expiratory volume in one second (FEV1), FEV1/FVC, and forced expiratory flow[20]. Therefore, rapid progression of respiratory infection in active smokers into critical lung disease with impaired ventilation may be reasonable.
Some clinical symptoms including dyspnea, chest pain, and fever were also associated with more severe COVID-19. Chest pain is usually caused by inflammation of the pleural membrane. Dyspnea can be attributed to damage of the alveoli in severe illness and elevated temperature may indicate high activation of the immune system towards the intrusive pathogen[11].
Laboratory parameters can help inform the clinical about the progression of COVID-19 towards critical illness. Parameters reported in more than one study were lymphopenia, increased D-dimer and elevated CRP. Lymphopenia can be an important prognosticator of COVID-19 as it reflects poor immune response to the infection. The primary target cells of viral infection in general are lymphocytes and hence when viral infection starts to induce damage to the immune system it usually presents as a decrease in the absolute number of lymphocytes[21]. One study[11] concluded that COVID-19 patients with lymphopenia are 12 times more likely to develop critical illness as compared to people with normal lymphocyte count. Decreased lymphocyte count could be used as an important index of evaluation of severity of COVID-19[22].
Elevation of C-reactive protein along with increased neutrophil ratio and procalcitonin were associated with higher odds of developing more severe disease. These parameters may be related to the cytokine storm syndrome induced by viral infection[23]. Increased D-Dimer implies a coagulation dysfunction that is related to the development of ARDS and progression from ARDS to death. This may suggest that disseminated intravascular coagulation is a step on the pathway to death in some patients[15].
Findings in CT scanning of the lungs can greatly help clinicians understand the current disease state and possible outcome. Li et al[11] found that patients with severe disease have lung consolidation secondary to complete filling of the alveoli with inflammatory exudate. Extrapulmonary lesions including pleural and pericardial effusion and enlarged lymph nodes may indicate more severe inflammation. Moreover, the overall CT scores of patients with severe/critical illness were significantly higher than the patients with mild disease.
Limitation of the present review includes the small number and retrospective nature of the studies included. Owing to the statistical heterogeneity and lack of essential data, the conduction of formal meta-analysis was not possible. Given the limitations with regards to study execution and article selection, no solid conclusions can be reached.
In conclusion, several demographic, clinical, laboratory, and radiologic factors may help predict severe and critical COVID-19 along with the potential need for mechanical ventilation. Factors that were more commonly reported were older age, medical comorbidities, lymphopenia, increased neutrophil ratio, elevated C-reactive protein, and increased D-dimer. As CT scanning has paramount importance in the making the diagnosis and assessment of COVID-19, it may also have a role in predicting more severe course of COVID-19. Nonetheless, as more studies on the COVID-19 pandemic are being conducted, more data on the predictors assessed in this review in addition to other predictors may be obtained.
Coronavirus disease 2019 (COVID-19) has been declared by the World Health Organization as a global pandemic. Although the majority of patients have mild or no symptoms, about 10% of patients may present with severe or critical disease that necessitates mechanical ventilation and may progress to death.
Patients who develop severe/critical COVID-19 disease have higher morbidity and mortality rates. Predicting which patients who are more likely to develop severe COVID-19 is highly required in order to implement more aggressive treatment measures to prevent potential deterioration.
The main objectives of the study were the incidence of severe COVID-19, mortality rate, and predictive factors of severe/critical disease.
A Preferred Reporting Items for Systematic Reviews and Meta-Analyses-compliant systematic review of the existing literature was conducted. Three databases were searched and the articles reporting the predictors of severe/critical COVID-19 were retrieved. The quality of the articles was assessed with the methodological index for non-randomized studies index. Outcomes were summarized in a qualitative form.
Five studies including 583 patients of a median age of 50.5 years were included. 242 (41.5%) of 583 hospitalized patients had critical illness. Acute respiratory distress disease occurred in 291 patients, accounting for 46.7% of total complications. The most commonly reported predictors of severe COVID-19 were older age, medical comorbidities, lymphopenia, elevated C-reactive protein, increased D-dimer, and increased neutrophil ratio. Findings on computed tomography (CT) scanning predictive of severe disease were bronchial wall thickening, CT score > 7, linear opacities, consolidation, right upper lobe affection, and crazy paving pattern.
Several factors may help predict severe/critical COVID-19. Factors that were more commonly reported were older age, medical comorbidities, lymphopenia, increased neutrophil ratio, elevated C-reactive protein, and increased D-dimer. As CT scanning has paramount importance in the making the diagnosis and assessment of COVID-19, it may also have a role in predicting more severe course of COVID-19.
Manuscript source: Unsolicited manuscript
Specialty type: Infectious diseases
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wang YP S-Editor: Wang JL L-Editor: A E-Editor: Wu YXJ
| 1. | de Wilde AH, Snijder EJ, Kikkert M, van Hemert MJ. Host Factors in Coronavirus Replication. Curr Top Microbiol Immunol. 2018;419:1-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 246] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 2. | Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W; China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18987] [Cited by in RCA: 17648] [Article Influence: 3529.6] [Reference Citation Analysis (0)] |
| 3. | World Health Organization. WHO Director-General's remarks at the media briefing on 2019-nCoV on 11 February 2020. Available from: https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020. |
| 4. | World Health Organization. Coronavirus disease 2019 (COVID-19) - Situation Report - 41. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200301-sitrep-41-covid-19.pdf?sfvrsn=6768306d_2 CdC-SR. |
| 5. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14113] [Cited by in RCA: 14767] [Article Influence: 2953.4] [Reference Citation Analysis (0)] |
| 6. | Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Agents. 2020;55:105924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3736] [Cited by in RCA: 3197] [Article Influence: 639.4] [Reference Citation Analysis (0)] |
| 7. | Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11409] [Cited by in RCA: 11507] [Article Influence: 2301.4] [Reference Citation Analysis (0)] |
| 8. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30123] [Article Influence: 6024.6] [Reference Citation Analysis (3)] |
| 9. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 47198] [Article Influence: 2949.9] [Reference Citation Analysis (0)] |
| 10. | Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3743] [Cited by in RCA: 5641] [Article Influence: 256.4] [Reference Citation Analysis (0)] |
| 11. | Li K, Wu J, Wu F, Guo D, Chen L, Fang Z, Li C. The Clinical and Chest CT Features Associated With Severe and Critical COVID-19 Pneumonia. Invest Radiol. 2020;55:327-331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 882] [Cited by in RCA: 797] [Article Influence: 159.4] [Reference Citation Analysis (0)] |
| 12. | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17476] [Cited by in RCA: 18201] [Article Influence: 3640.2] [Reference Citation Analysis (0)] |
| 13. | Liu W, Tao ZW, Wang L, Yuan ML, Liu K, Zhou L, Wei S, Deng Y, Liu J, Liu HG, Yang M, Hu Y. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl). 2020;133:1032-1038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 562] [Cited by in RCA: 636] [Article Influence: 127.2] [Reference Citation Analysis (0)] |
| 14. | Qu R, Ling Y, Zhang YH, Wei LY, Chen X, Li XM, Liu XY, Liu HM, Guo Z, Ren H, Wang Q. Platelet-to-lymphocyte ratio is associated with prognosis in patients with coronavirus disease-19. J Med Virol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 272] [Cited by in RCA: 347] [Article Influence: 69.4] [Reference Citation Analysis (0)] |
| 15. | Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Zhou X, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;e200994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4960] [Cited by in RCA: 5518] [Article Influence: 1103.6] [Reference Citation Analysis (1)] |
| 16. | Lake MA. What we know so far: COVID-19 current clinical knowledge and research. Clin Med (Lond). 2020;20:124-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 442] [Article Influence: 88.4] [Reference Citation Analysis (0)] |
| 17. | Sharma G, Goodwin J. Effect of aging on respiratory system physiology and immunology. Clin Interv Aging. 2006;1:253-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 461] [Cited by in RCA: 586] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 18. | Pawelec G. Immunosenescence comes of age. Symposium on Aging Research in Immunology: The Impact of Genomics. EMBO Rep. 2007;8:220-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, Liu XQ, Chen RC, Tang CL, Wang T, Ou CQ, Li L, Chen PY, Sang L, Wang W, Li JF, Li CC, Ou LM, Cheng B, Xiong S, Ni ZY, Xiang J, Hu Y, Liu L, Shan H, Lei CL, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Cheng LL, Ye F, Li SY, Zheng JP, Zhang NF, Zhong NS, He JX, China Medical Treatment Expert Group for COVID-19. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55:2000547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1711] [Cited by in RCA: 2189] [Article Influence: 437.8] [Reference Citation Analysis (0)] |
| 20. | Tantisuwat A, Thaveeratitham P. Effects of smoking on chest expansion, lung function, and respiratory muscle strength of youths. J Phys Ther Sci. 2014;26:167-170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Wu CT, Hsia SH, Huang JL. Influenza B-associated rhabdomyolysis in Taiwanese children. Acta Paediatr. 2010;99:1701-1704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5228] [Cited by in RCA: 5786] [Article Influence: 1157.2] [Reference Citation Analysis (2)] |
| 23. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14869] [Cited by in RCA: 12977] [Article Influence: 2595.4] [Reference Citation Analysis (1)] |