Published online Feb 23, 2017. doi: 10.5494/wjh.v7.i1.10
Peer-review started: August 25, 2016
First decision: September 27, 2016
Revised: November 6, 2016
Accepted: December 27, 2016
Article in press: December 28, 2016
Published online: February 23, 2017
Processing time: 177 Days and 2 Hours
Hypertension represent one of the most important comorbid factors in chronic kidney disease (CKD) patients and its prevalence increases from 65% to 95% according to glomerular filtration rate decline. CKD patients need to maintain their blood pressure levels into 130/80 mmHg according to most recent guidelines. Despite of many therapeutic agents, achievement of ideal blood pressure levels remains so far from the ideal ones. Hypertensive disease represent most important risk factor to develop a type IV cardiorenal syndrome, while prevalence of end stage renal disease is still raising and it represents worldwide epidemiological challenge. Correct management of hypertensive disease can obtain better control on CKD progression.
Core tip: Treat hypertensive disease can delay chronic kidney disease progression and type IV cardiorenal syndrome onset.
- Citation: Di Lullo L, Bellasi A, De Pascalis A. Hypertension, type IV cardiorenal syndrome and chronic kidney disease: Pathophysiological and therapeutical approach. World J Hypertens 2017; 7(1): 10-18
- URL: https://www.wjgnet.com/2220-3168/full/v7/i1/10.htm
- DOI: https://dx.doi.org/10.5494/wjh.v7.i1.10
Hypertensive disease represents major risk factor in CKD-related cardiovascular disease. Table 1 shows risk factors involved in the pathogenesis of CKD-related hypertension. The prevalence of hypertension increases from 65% to 95% according to glomerular filtration rate (GFR) decline from 85 to 15 mL/min per 1.73 m²[1]. Hypertension itself is actually recognized as a risk factor for renal disease progression till to end-stage renal disease (ESRD)[2,3]. Hypertension can be also accounted for higher risk of all-cause and cardiovascular mortality in CKD patients, as referred in several randomized controlled clinical trials[4-9]. Current guidelines actually suggest to reach less than 130/80 mmHg BP values in CKD patients’ population. Despite of many therapeutic agents, achievement of ideal blood pressure levels remains so far from the ideal ones[10,11].
| Factor | Dominant mechanism |
| Impaired Na excretion | Expansion of ECF volume |
| Activation of RAS | Direct vasoconstriction |
| Sympathetic activation | |
| Sympathetic activation | Direct vasoconstriction |
| Stimulation of renin release | |
| Imbalance in PG or kinins | Vasoconstriction |
| Endothelin | Direct vasoconstriction |
| Renal injury | |
| Reduced nitric oxide | Loss of vasodilatator effect |
RAS is an important therapeutic target and drugs that block this system have been extensively developed, such as ACE inhibitors (ACE-I) and Angiotension II receptor blockers (ARB). This blocking has been postulated as the first choice for treatment of hypertension in CKD patients[12,13]. Several ARB inhibitor trials for CKD patients were conducted and showed a slower decline in renal function with the use of this class of antihypertensive medication related mainly to proteinuria reduction than to intensive blood pressure control[9,14-17]. Antiproteinuric effect was postulated as the corner stone of renoprotection and it is more effective if it’s associated to low sodium diet or to combination therapy with diuretics leading to extracellular volume (ECV) depletion. ECV depletion and RAS inhibition is particularly suitable in proteinuric CKD patients allowing to reach because the best renal outcomes[18].
RAS inhibitors are highly effective in diabetic patients with renal involvement, reducing protein excretion and preventing to shift from microalbuminuria to proteinuria and renal failure, as it occurs in proteinuric normotensive patients[19,20]. Renoprotective properties of ARBs has been pointed up in type 2 diabetic nephropathy, but combination therapy with ACEi is still a critical issue[21,22]. Additive antiproteinuric effect has been reported in proteinuric nondiabetic CKD patients affected by glomerular nephropathies (i.e., IgA nephropathy). At the same time an increased efficacy in terms of slowing CKD progression has been proven in the same patients’ population[13]. Combination therapy approach could be indicated in the in the majority of CKD patients because ACEi stand alone therapy doesn not allow to obtain less than 500 mg/d proteinuria[23]. Preliminary exclusion of patients suffering adverse effects of strong RAS inhibition (hyperkalemia, marked increase in serum creatinine concentration) has to be realized as far as extensive abuse of diuretics. Plasma creatinine and potassium concentrations should be measured in the first weeks of therapy.
Sodium and fluid retention paly fundament role in the pathogenesis of CKD-related hypertension, even if extracellular volume (ECV) expansion is not able to induce edema, as it occurs in heart failure patients. Urinary fractional excretion of sodium increases as GFR declines contributing to hypertension, especially in those patients undergoing on RAS inhibitors therapy[24]. CKD patients take benefits by small reduction of salt intake in respect of essential hypertensive patients undergoing major restriction of salt intake, probably due to basal ECV amount[25-27].
CKD patients compliance with the dietary prescription is generally poor in the setting of clinical practice. The determination of urinary excretion of sodium (target: ≤ 100 mEq/d, equal to ≤ 6 g NaCl/d), is very important to monitor the patient’s adherence to dietary prescriptions, specifically reducing added salt in the diet, cooking with spices rather than salt, choosing fresh food, eating low-salt bread.
Natriuretic agents become the cornerstone of treatment of CKD-related hypertension, especially in patients with poor compliance to salt restriction (urinary sodium excretion > 100 mEq/d)[28]. In patients with stage I to IIIa CKD, thiazide diuretics are indicated, since they can restore the antiproteinuric effect of ACE-I in patients not compliant to a low-salt diet. Thiazides could also prevent development of cardiovascular events in older people with isoled systolic hypertension and mild renal function impairment[29]. Loop diuretics are indicated when GFR falls under 40 mL/min and titrated until BP reaches guidelines recommended values (< 130/80 mmHg). Diuretics have to be carefully employed when so called patient’s “dry weight” is reached. Dry weight that is defined as the weight at which further fluid losses will lead to symptoms (orthostatic hypotension, cramps) or decreased tissue perfusion (an unexplained elevation of azotemia and plasma creatinine concentration can be observed). In Stage IIIa CKD patients torasemide (40 mg/d) or furosemide (80 mg/d) induce an antihypertensive effect closely linked to natriuretic response and ECV contraction[30]. Once sodium retention is corrected (induction phase), and the achievement of normal BP values is reached, down-titration of loop diuretic dosage can be started and maintained (maintenance phase). Maintenance dose of loop diuretic is lower than that of the induction one and it should be clear that therapeutic dose of furosemide is characterized by a large inter-individual variability due to different bioavailability. It’s good clinical practice to start with a low diuretic dose gradually increasing to achieve progressive body weight reduction. On the other side, maintenance phase is fundamental to downtitrate the dose and detect the lowest target dose.
In the real world nephrologists are not confident with loop diuretics in their hypertensive CKD patients, because of their side effects, that can be avoided if renal function and serum electrolyte levels are periodically checked in the first weeks of treatment.
Aldosterone antagonists can provide reduction in urine albumin levels excretion, especially in combination therapy for resistant hypertension in CKD patients. Aldosterone antagonists also provide clinical benefits in non-CKD patients with heart failure, including heart failure following myocardial infarction. Because of the risk of hyperkalemia and reduction in GFR, they should be used at lower doses (i.e., 25-50 mg/d) and with caution in CKD patients.
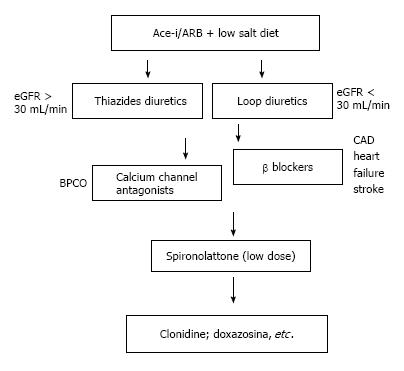
RAS inhibitors and diuretics are the cornerstones of therapy in hypertensive CKD patients, but they are not the only therapeutic strategies in CKD-related hypertensive disease. If specific cardiovascular disease and therapeutic targets are needed, additional agents shold be choosen in order to avoid side-effects and interactions, as it is showed in Figure 1[31].
Beta-blockers are especially indicated in patients with cardiac chronic ischemic disease, congestive heart failure (and consequent diastolic dysfunction), tachycardia, headaches, and glaucoma. These agents should in general avoided in patients with bradycardia, second- or third-degree heart block, asthma, chronic obstructive pulmonary disease, severe peripheral vascular disease, depression. In CKD patients beta-blockers can induce hyperkalemia due to impaired transcellular distribution of potassium, especially for whom concerning non-selective beta-blockers. All beta-blockers can induce hyperglycemia, due to insulin resistance, and dyslipidemia with a decrease in HDL cholesterol plasmatic levels.
Among calcium channel blockers (CCB), the nondihydropyridine ones show positive effects on CKD progression and cardiovascular outcomes. Reduction in proteinuria levels is observed in diabetic patients with renal disease treated with diltiazem and verapamil[31]. Nondihydropyridine calcium-channel blockers can provide poor cardiovascular outcomes due to negative effects on cardiac contractility and conduction. Therefore, they should not be used in patients with severe left ventricular dysfunction, sick sinus syndrome, or second- or third-degree heart block. Constipation represent very common side effects occurring in up to 25% of patients on verapamil treatment. Among long-acting dihydropyridine agents, some of them do not hold cardiac depressant activity, as amlodipine and lacidipine and they have to be preferred rather than short-acting CCB. Therefore dihydropyridines are associated with vasodilation-related side-effects as peripheral edema, dizziness, headache, and flushing[31].
Alpha-adrenergic agents should not be considered as first-line therapy in CKD patients because of higher side effects incidence, such as dry mouth, sedation, and sexual dysfunction[31]. Headache, weakness, dizziness, and syncope are frequent in patients on selective α-1 blockers. Dizziness and syncope can be minimized by starting with a low dose of a long-acting agent such as doxazosin and administering the initial dose at bedtime[31].
Direct powerful vasodilators, as minoxidil, are often administered together with beta-blocker and loop diuretic to minimize reflex tachycardia, hirsutism, pleural or pericardial effusion and lower extremity edema. It should be reserved for those patients on three drugs combination therapy who cannot achieve adequate BP levels according to international hypertension guidelines[31].
Type IV cardiorenal syndrome (CRS), also defined as chronic renocardiac, is characterized by cardiovascular involvement in patients affected by chronic kidney disease at any stage according to National Kidney Foundation (NKF) classification.
Hypertensive disease represent most important risk factor to develop a type IV CRS. Prevalence of end stage renal disease (ESRD) is still raising and it represents worldwide epidemiological challenge[32]. Last US data estimate up to 13% population present CKD at any stage of disease.
It’s well established how renal dysfunction is an independent risk factor for cardiovascular disease; CKD patients show higher mortality risk for myocardial infection and sudden death[32].
At present time pathophysiological mechanisms leading to increased cardiovascular risk in CKD patients are not completely known but we are confident in strict connections between heart and kidney.
Decline of glomerular filtration rate (GFR) leads to activation of RAAS and sympathetic nervous system and, on the other hand, it stimulates calcium-parathyroid axis; this can be due to primary diseases such as diabetes or hypertension, main causes of CKD development in western countries.
Loss of kidney function usually leads to accumulation of sodium and water with consequent stimulus to angiotensin II and aldosterone production and development of arterial hypertension. Hypertension, together with angiotensin and aldosterone, accelerates left ventricular hypertrophy and cardiac fibrosis.
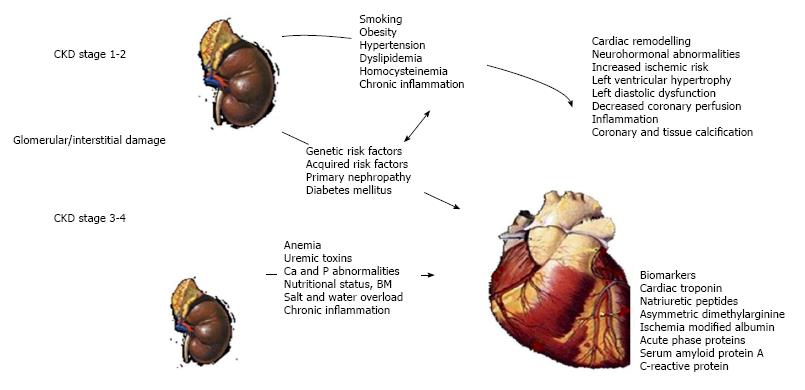
To better understand pathophysiological pathways underlying type-4 CRS (Figure 2), we have to consider various aspects of this cardio-renal syndrome from atherosclerotic damage to vascular calcifications development up to left ventricular hypertrophy development and cardiomyocites remodelling. Finally galectin-3 and FGF-23 roles will be cleared based on last experimental evidences.
Epidemiological and clinical evidences have been proved association between renal dysfunction and cardiovascular disease; it’s well established that late stages of CKD are closely associated to higher cardiovascular morbidity. On the other hand it’s still unclear increased incidence of cardiovascular disease at early stages of chronic kidney disease.
CKD patients present increased rates of atherosclerotic coronary disease, acute coronary syndrome, left ventricular hypertrophy and sudden death.
Cardiovascular risk for patients with eGFR less 30
mL/min per 1.73/m2 is ten fold higher in respect of patients with eGFRs above 60 mL/min per 1.73/m2.
These higher rates are in contrast with risk expected from typical risk factor present in CKD patients (hypertension, diabetes, dyslipidemia and so on); CKD is probably able to directly contribute to cardiovascular complications[33,34].
CKD patients present, at early and late stages of disease, higher prevalence of coronary artery disease at angiographic evaluation; these patients also show multivessel disease and ECG evidence of previous silent ischemia[35].
Recent data showed that dobutamine stress echocardiography presents best accuracy for non-invasive coronary artery disease (CAD) screening in renal transplant candidates[36].
To assess CAD prevalence in early stages of CKD, an accurate review has evaluated coronary catheterization in 261 patients with eGFR between 30 and 90 mL/min; despite preserved renal function, more than half patients with eGFR > 90 mL/min had a 70% stenosis in at least one coronary artery. On the other hand, more than 84% patients with late stages of CKD (eGFR < 30 mL/min) showed significant CAD with higher involvement of left coronary artery and multivessel disease[37].
Accelerated coronary atherosclerosis is not sufficient to completely explain higher rates of cardiovascular involvement in CKD patients.
We are now confident that osteoblastic transformation of smooth muscle cells is a key point in pathogenesis of vascular and valvular calcification during CKD.
Impaired vitamin D synthesis, secondary hyperparathyroidism and altered calcium-phosphate metabolism contribute to vascular calcification because of their direct effects on osteoblastic cells[38].
Coronary calcifications can predict major cardiac events contributing to reduced coronary reserve in CKD patients and higher risk of coronary acute syndromes[39,40] raising with progression of renal disease.
Clinical studies conducted with high resolution multislice computed tomography (CT) demonstrated early detection of coronary calcifications since CKD stage 3 according to NKF classification; patients data showed how 83% of them presented coronary calcification did not related to CKD stage[41]. Calcifications were also extended to low limbs arteries explaining high rates of lower extremity amputation among ESRD patients presenting also greater quantity and density of calcium deposits not limited to intima, but extended to vessels’ media[42]. In other studies immunostaining assay of calcified areas demonstrated presence of bone matrix proteins such as osteopontin, type I collagen and bone sialoprotein[43].
An autoptic evaluation showed how medial calcification was present in 16% of uremic patients but only in 3% of patients with normal renal function; medial calcification was also associated to presence of osteocalcin, inflammatory markers (TGF-α) and activated complement elements (C3 and C4)[44].
Increased calcium content can be accountable both reduced left ventricular compliance and prevalence of arrhythmias.
Aortic calcification is strongly associated to reduced aortic compliance and coronary artery perfusion leading to increased central pressure inducing sub-endocardial ischemia because of reduced diastolic filling[45].
Left ventricular hypertrophy (LVH) has been always recognized, together left ventricular systolic and diastolic dysfunction, main cardiovascular damage marker in CKD patients. LVH prevalence surely increases with declining renal function because of traditional risk factors as hypertension, diabetes and volume overload. More recent data have focused their attention of secondary hyperparathyroidism, malnutrition and even dialysis as further risk factors for development of LVH in CKD. LVH prevalence varies from 16%-31% in patients with GFR > 30 mL/min up to 60%-75% in ESRD and 90% prevalence in people starting renal replacement therapy[46].
Foley et al found that 74% of ESRD patients had echocardiographic evidence of LVH and 30% presented left ventricular failure[47].
In another survey including 596 incident hemodialysis patients with no history of cardiac disease, Foley demonstrated that, after 18 mo of dialysis, left ventricular mass index (LVMI) increased in 62% patients with left ventricular failure in 49% of them[48].
At present time mechanisms contributing to left ventricular dysfunction in CKD patients are unknown but many evidences suggest uremia products can directly affect cardiac structure; many of these toxins are highly protein bound and they present limited clearance by conventional dialyzers; these limitations could be accountable of dialysis effects on LVH and left heart failure[49].
Clinical conditions leading to LVH in CKD patients are similar to those observed in other clinical patterns including hypertension, atherosclerosis, pressure overload and RAAS activation. Atherosclerosis and hypertension directly promote myocites hypertrophy with consequent increased left ventricular mass, increased ventricular wall thickness, secondary myocardial fibrosis and compensatory hypertrophy[50].
In CKD patients aortic compliance is affected by accelerated atherosclerotic damage but other typical CKD variables, such as hyperphosphatemia, can affect aortic compliance[51].
In middle and end stage of CKD progression, progressive loss of nephron leads to salt and water accumulation with hypertension and volume/pressure overload; these changes up-regulate RAAS with release of pro-fibrotic factors such as galectin-3, TGF-β and endogenous cardiac steroids[52].
As a LVH consequence, myocytes enlarge capillaries density because of increased oxygen demand; myocite diameter and interstitial volume space are increased in CKD patients compared to other patients groups: Long lasting periods of hemodynamic load promote cardiac remodeling and increase cardiac expression of interstitial myofibroblasts not ever present in normal myocardium[53].
Reduction in myocardial capillary density may explain marked CKD patients susceptibility to myocardial ischemia, LVH and myocardial fibrosis[53].
Lot of evidence now suggest that CKD patients, especially late stages, develop particular pattern of cardiac fibrosis. CKD and ESRD patients present inter-myocardial fibrosis features quite different from those of hypertensive and chronic ischemic heart disease patients in which endocardial and epicardial fibrosis predominate[54].
Mechanisms leading to CKD cardiac fibrosis are still understood but recent evidences suggest that uremic toxins such as indoxyl sulfate and p-cresol can contribute to cardiac fibrosis in renal patients. In CKD patients indoxyl sulfate concentrations are 300 fold higher than control population and it directly contributes to cardiac fibrosis by synthesis of TGF-β, tissue inhibitor of metalloproteinase-1 (TIMP-1) and alpha-1 collagen[54].
Once verified close linkage between eGFR decline and cardiovascular structure changes, other further biomarkers have to be investigated.
One of them is represented by fibroblast growth factor-23 (FGF-23), member of fibroblast growth factor family (implicated in regulation, growth and differentiation of cardiac myocytes) holding paracrine functions in kidneys because of its phosphaturic properties; it blocks vitamin D3 synthesis and inhibits proximal nephron reabsorption[55].
During CKD progression, accumulation of phosphate leads to increase in FGF-23 incretion, which prolonged high levels can contribute to LVH and cardiac remodeling.
New data have shown that modest reduction in GFR can stimulate FGF-23 production; echocardiographic assays demonstrated a 5% LVMI rise for every log increase in plasma FGF-23 levels. Patients included in highest tertile of FGF-23 also have a 2.4 fold higher risk for coronary artery calcifications[56].
Type-4 CRS diagnosis is based on serological and instrumental diagnosis of both chronic heart and kidney disease.
On one hand, cardiac function is more widely assessed by NT-proBNP serum levels, while, on the other hand, eGFR represent most employed biochemical test to evaluate kidney function.
Based on recent evidence, evaluation of FGF-23 levels can be helpful in monitoring secondary hyperparathyroidism status but it is also involved in cardiac fibrotic remodeling. Ultrasound diagnosis of type-4 CRS is classically based upon kidney and heart evaluation. Kidneys ultrasound evaluation usually shows classic features of chronic nephropathy such as thin and hyperechogenic cortex with reduced cortico-medullary ratio. It’s quite frequent to observe small dilation of urinary tract and parapyelic cysts.
Echocardiographic assay allows to point out signs of volume overload, left ventricular dysfunction and right ventricular dysfunction especially in ESRD and hemodialysis patients.
At echocardiographic evaluation we can find increased atrial volumes or areas, pleural or pericardial effusion and lung comets (all signs of volume overload)[57].
Cardiac ultrasound also allow to discover presence of valvular calcifications (related to secondary hyperparathyroidism)[57] and possible right heart dysfunction features (high pulmonary artery pressure, low tricuspid annulus plane systolic excursion or right chamber dilation)[58].
Since type-4 CRS is characterized by chronic cardiovascular involvement in CKD patients, correction of traditional and non traditional cardiovascular risk factors is crucial.
Therapeutic interventions for traditional risk factors are less effective in patients with chronic kidney disease[59]. also for certain kind of “therapeutic nihilism” for which treatments with antiplatelets, statins, β-blockers and ACEi in CKD patients with coronary artery disease are often denied[59].
Strategies to reduce cardiovascular risk in CKD patients have to target both traditional (hypertension, dyslipidemia, diabetes, obesity) and non traditional (anemia, chronic inflammation, secondary hyperparthyroidism, LVH, oxydative stress, RAAS and SNS hyperactivity, renal replacement therapy complications).
Specific treatment targets are quite complicated especially in hemodialysis patients in which a lot of evidences support existence of a U-shaped curve associating mortality with blood pressure levels, BMI, dyslipidemia and hyperphosphatemia[60,61].
While it’s clearly established role of secondary anemia correction[62] controversies are aroused about other risk factors corrections such as secondary hyperparathyroidism, hypertension and dyslipidemia.
For whom to concern secondary hyperparathyroidism, EVOLVE study conduced in hemodialysis patients found that cinacalcet therapy did not significantly reduce the risk of death or major cardiovascular events in patients with moderate-to-severe secondary hyperparathyroidism who were undergoing dialysis[63].
SHARP study investigated dyslipidemia treatment in CKD patients and it has been able to demonstrate a significant reduction in cardiovascular events, such as myocardial infarction, stroke, or need for coronary artery revascularization, with the use of a combination of ezetimibe plus simvastatin[64].
Pre-dialysis patients are closely recommended to maintain blood pressure levels below 130/80 mmHg, HbA1c levels below 7%, hemoglobin levels between 11 and 12 g/dL, C-LDL below 90 mg/dL. Patients should avoid nephrotoxic drugs and follow low protein diet (0.6 g/kg per day)[10].
Patients on dialysis should keep their blood pressure below 140/90 before starting dialytic session and below 130/80 after dialysis session.
Special consideration have to be focused on mineral bone disorders preventing hyperphosphatemia and vascular calcifications, also in early stages of CKD[65].
Treatment of arrhythmias and sudden death is still a challenge for nephrologists and cardiologists; together with prior attention to electrolytes disorders prevention (low potassium dialysate), use of β-blockers appears beneficial. ACE inhibitors and ARBs efficacy have to be proven in more prospective trials[66].
Implantation of cardiac defibrillators in dialysis patients is associated with increased risk for bleeding and infection and does not significantly affect morbidity and mortality[66].
Hypertension management is crucial in CKD patients to achieve correct both renal and cardiovascular protection. Despite the availability of several drug classes, optimal BP control still remains an open question. Management of CKD-related hypertensive patients appears more complex when real world data of clinical practice are compared to those deriving from randomized controlled clinical trials. What clinicians should perform is to encourage the use of antihypertensive agents other than RAS inhibitors also acting on ECV expansion by salt restriction and appropriate diuretics prescription.
Manuscript source: Invited manuscript
Specialty type: Peripheral vascular disease
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Ferrer CFS, Salles G S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
| 1. | Buckalew VM, Berg RL, Wang SR, Porush JG, Rauch S, Schulman G. Prevalence of hypertension in 1,795 subjects with chronic renal disease: the modification of diet in renal disease study baseline cohort. Modification of Diet in Renal Disease Study Group. Am J Kidney Dis. 1996;28:811-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 141] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 2. | De Nicola L, Minutolo R, Chiodini P, Zoccali C, Castellino P, Donadio C, Strippoli M, Casino F, Giannattasio M, Petrarulo F. Global approach to cardiovascular risk in chronic kidney disease: reality and opportunities for intervention. Kidney Int. 2006;69:538-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 92] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Bakris GL, Weir MR, Shanifar S, Zhang Z, Douglas J, van Dijk DJ, Brenner BM. Effects of blood pressure level on progression of diabetic nephropathy: results from the RENAAL study. Arch Intern Med. 2003;163:1555-1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 311] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 4. | Jafar TH, Stark PC, Schmid CH, Landa M, Maschio G, de Jong PE, de Zeeuw D, Shahinfar S, Toto R, Levey AS. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med. 2003;139:244-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 790] [Cited by in RCA: 705] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 5. | Bakris GL, Williams M, Dworkin L, Elliott WJ, Epstein M, Toto R, Tuttle K, Douglas J, Hsueh W, Sowers J. Preserving renal function in adults with hypertension and diabetes: a consensus approach. National Kidney Foundation Hypertension and Diabetes Executive Committees Working Group. Am J Kidney Dis. 2000;36:646-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 970] [Cited by in RCA: 890] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 6. | Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154-2169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2429] [Cited by in RCA: 2577] [Article Influence: 117.1] [Reference Citation Analysis (0)] |
| 7. | Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med. 2004;164:659-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1123] [Cited by in RCA: 1155] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 8. | Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7995] [Cited by in RCA: 8508] [Article Influence: 405.1] [Reference Citation Analysis (0)] |
| 9. | Sarnak MJ, Greene T, Wang X, Beck G, Kusek JW, Collins AJ, Levey AS. The effect of a lower target blood pressure on the progression of kidney disease: long-term follow-up of the modification of diet in renal disease study. Ann Intern Med. 2005;142:342-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 330] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 10. | Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43:S1-290. [PubMed] |
| 11. | Marín R, Fernández-Vega F, Gorostidi M, Ruilope LM, Díez J, Praga M, Herrero P, Alcázar JM, Laviades C, Aranda P. Blood pressure control in patients with chronic renal insufficiency in Spain: a cross-sectional study. J Hypertens. 2006;24:395-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Zamboli P, De Nicola L, Minutolo R, Bertino V, Catapano F, Conte G. Management of hypertension in chronic kidney disease. Curr Hypertens Rep. 2006;8:497-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Cortinovis M, Ruggenenti P, Remuzzi G. Progression, Remission and Regression of Chronic Renal Diseases. Nephron. 2016;134:20-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW, Striker G. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med. 1994;330:877-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1620] [Cited by in RCA: 1542] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 15. | Ruggenenti P, Perna A, Loriga G, Ganeva M, Ene-Iordache B, Turturro M, Lesti M, Perticucci E, Chakarski IN, Leonardis D. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet. 2005;365:939-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 440] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 16. | Wright JT, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, Cheek D, Douglas-Baltimore JG, Gassman J, Glassock R. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421-2431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1439] [Cited by in RCA: 1438] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 17. | Appel LJ, Wright JT, Greene T, Agodoa LY, Astor BC, Bakris GL, Cleveland WH, Charleston J, Contreras G, Faulkner ML. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med. 2010;363:918-929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 578] [Cited by in RCA: 514] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 18. | Khosla N, Bakris G. Lessons learned from recent hypertension trials about kidney disease. Clin J Am Soc Nephrol. 2006;1:229-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329:1456-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3760] [Cited by in RCA: 3553] [Article Influence: 111.0] [Reference Citation Analysis (2)] |
| 20. | Barnett AH, Bain SC, Bouter P, Karlberg B, Madsbad S, Jervell J, Mustonen J. Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med. 2004;351:1952-1961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 625] [Cited by in RCA: 553] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 21. | Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5059] [Cited by in RCA: 5064] [Article Influence: 211.0] [Reference Citation Analysis (0)] |
| 22. | Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4068] [Cited by in RCA: 3986] [Article Influence: 166.1] [Reference Citation Analysis (1)] |
| 23. | Di Iorio BR, Minutolo R, De Nicola L, Bellizzi V, Catapano F, Iodice C, Rubino R, Conte G. Supplemented very low protein diet ameliorates responsiveness to erythropoietin in chronic renal failure. Kidney Int. 2003;64:1822-1828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Vasavada N, Agarwal R. Role of excess volume in the pathophysiology of hypertension in chronic kidney disease. Kidney Int. 2003;64:1772-1779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | He FJ, MacGregor GA. Effect of modest salt reduction on blood pressure: a meta-analysis of randomized trials. Implications for public health. J Hum Hypertens. 2002;16:761-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 556] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 26. | Cianciaruso B, Bellizzi V, Minutolo R, Colucci G, Bisesti V, Russo D, Conte G, De Nicola L. Renal adaptation to dietary sodium restriction in moderate renal failure resulting from chronic glomerular disease. J Am Soc Nephrol. 1996;7:306-313. [PubMed] |
| 27. | Koomans HA, Roos JC, Dorhout Mees EJ, Delawi IM. Sodium balance in renal failure. A comparison of patients with normal subjects under extremes of sodium intake. Hypertension. 1985;7:714-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Mees EJ. Volaemia and blood pressure in renal failure: have old truths been forgotten? Nephrol Dial Transplant. 1995;10:1297-1298. [PubMed] |
| 29. | Brater DC. Diuretic therapy. N Engl J Med. 1998;339:387-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 450] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 30. | Vasavada N, Saha C, Agarwal R. A double-blind randomized crossover trial of two loop diuretics in chronic kidney disease. Kidney Int. 2003;64:632-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1-266. [PubMed] |
| 32. | Redón J, Cea-Calvo L, Lozano JV, Fernández-Pérez C, Navarro J, Bonet A, González-Esteban J. Kidney function and cardiovascular disease in the hypertensive population: the ERIC-HTA study. J Hypertens. 2006;24:663-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Anavekar NS, McMurray JJ, Velazquez EJ, Solomon SD, Kober L, Rouleau JL, White HD, Nordlander R, Maggioni A, Dickstein K. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351:1285-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1380] [Cited by in RCA: 1406] [Article Influence: 67.0] [Reference Citation Analysis (1)] |
| 34. | Rostand SG, Kirk KA, Rutsky EA. Dialysis-associated ischemic heart disease: insights from coronary angiography. Kidney Int. 1984;25:653-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 159] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 35. | Joki N, Hase H, Nakamura R, Yamaguchi T. Onset of coronary artery disease prior to initiation of haemodialysis in patients with end-stage renal disease. Nephrol Dial Transplant. 1997;12:718-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 147] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 36. | Whalley GA, Marwick TH, Doughty RN, Cooper BA, Johnson DW, Pilmore A, Harris DC, Pollock CA, Collins JF. Effect of early initiation of dialysis on cardiac structure and function: results from the echo substudy of the IDEAL trial. Am J Kidney Dis. 2013;61:262-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 37. | Chonchol M, Whittle J, Desbien A, Orner MB, Petersen LA, Kressin NR. Chronic kidney disease is associated with angiographic coronary artery disease. Am J Nephrol. 2008;28:354-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 38. | McCullough PA, Agrawal V, Danielewicz E, Abela GS. Accelerated atherosclerotic calcification and Monckeberg’s sclerosis: a continuum of advanced vascular pathology in chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1585-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 39. | Gross ML, Meyer HP, Ziebart H, Rieger P, Wenzel U, Amann K, Berger I, Adamczak M, Schirmacher P, Ritz E. Calcification of coronary intima and media: immunohistochemistry, backscatter imaging, and x-ray analysis in renal and nonrenal patients. Clin J Am Soc Nephrol. 2007;2:121-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 109] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 40. | Ragosta M, Samady H, Isaacs RB, Gimple LW, Sarembock IJ, Powers ER. Coronary flow reserve abnormalities in patients with diabetes mellitus who have end-stage renal disease and normal epicardial coronary arteries. Am Heart J. 2004;147:1017-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 90] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 41. | Garland JS, Holden RM, Groome PA, Lam M, Nolan RL, Morton AR, Pickett W. Prevalence and associations of coronary artery calcification in patients with stages 3 to 5 CKD without cardiovascular disease. Am J Kidney Dis. 2008;52:849-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 42. | Adragao T, Pires A, Branco P, Castro R, Oliveira A, Nogueira C, Bordalo J, Curto JD, Prata MM. Ankle--brachial index, vascular calcifications and mortality in dialysis patients. Nephrol Dial Transplant. 2012;27:318-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 43. | Moe SM, O’Neill KD, Duan D, Ahmed S, Chen NX, Leapman SB, Fineberg N, Kopecky K. Medial artery calcification in ESRD patients is associated with deposition of bone matrix proteins. Kidney Int. 2002;61:638-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 310] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 44. | Campean V, Neureiter D, Nonnast-Daniel B, Garlichs C, Gross ML, Amann K. CD40-CD154 expression in calcified and non-calcified coronary lesions of patients with chronic renal failure. Atherosclerosis. 2007;190:156-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 45. | Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588-2605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4068] [Cited by in RCA: 4300] [Article Influence: 226.3] [Reference Citation Analysis (0)] |
| 46. | McGregor E, Jardine AG, Murray LS, Dargie HJ, Rodger RS, Junor BJ, McMillan MA, Briggs JD. Pre-operative echocardiographic abnormalities and adverse outcome following renal transplantation. Nephrol Dial Transplant. 1998;13:1499-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 47. | Foley RN, Parfrey PS, Harnett JD, Kent GM, Martin CJ, Murray DC, Barre PE. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int. 1995;47:186-192. [PubMed] |
| 48. | Foley RN, Curtis BM, Randell EW, Parfrey PS. Left ventricular hypertrophy in new hemodialysis patients without symptomatic cardiac disease. Clin J Am Soc Nephrol. 2010;5:805-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 49. | McIntyre CW, Burton JO, Selby NM, Leccisotti L, Korsheed S, Baker CS, Camici PG. Hemodialysis-induced cardiac dysfunction is associated with an acute reduction in global and segmental myocardial blood flow. Clin J Am Soc Nephrol. 2008;3:19-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 360] [Cited by in RCA: 341] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 50. | Parfrey PS, Harnett JD, Foley RN. Heart failure and ischemic heart disease in chronic uremia. Curr Opin Nephrol Hypertens. 1995;4:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 51. | Blacher J, Safar ME, Guerin AP, Pannier B, Marchais SJ, London GM. Aortic pulse wave velocity index and mortality in end-stage renal disease. Kidney Int. 2003;63:1852-1860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 380] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 52. | Bagrov AY, Shapiro JI. Endogenous digitalis: pathophysiologic roles and therapeutic applications. Nat Clin Pract Nephrol. 2008;4:378-392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 53. | Amann K, Breitbach M, Ritz E, Mall G. Myocyte/capillary mismatch in the heart of uremic patients. J Am Soc Nephrol. 1998;9:1018-1022. [PubMed] |
| 54. | Mall G, Huther W, Schneider J, Lundin P, Ritz E. Diffuse intermyocardiocytic fibrosis in uraemic patients. Nephrol Dial Transplant. 1990;5:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 183] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 55. | Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutiérrez OM, Aguillon-Prada R, Lincoln J, Hare JM. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393-4408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1413] [Cited by in RCA: 1529] [Article Influence: 109.2] [Reference Citation Analysis (0)] |
| 56. | Gutiérrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, Sarwar A, Hoffmann U, Coglianese E, Christenson R. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119:2545-2552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 696] [Cited by in RCA: 642] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 57. | Di Lullo L, Floccari F, Granata A, D’Amelio A, Rivera R, Fiorini F, Malaguti M, Timio M. Ultrasonography: Ariadne’s Thread in the Diagnosis of the Cardiorenal Syndrome. Cardiorenal Med. 2012;2:11-17. [PubMed] |
| 58. | Di Lullo L, Floccari F, Polito P. Right ventricular diastolic function in dialysis patients could be affected by vascular access. Nephron Clin Pract. 2011;118:c257-c261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 59. | McCullough PA. Why is chronic kidney disease the “spoiler” for cardiovascular outcomes? J Am Coll Cardiol. 2003;41:725-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 148] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 60. | Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int. 2003;63:793-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 838] [Cited by in RCA: 853] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 61. | Di Lullo L, Floccari F, Santoboni A, Barbera V, Rivera RF, Granata A, Morrone L, Russo D. Progression of cardiac valve calcification and decline of renal function in CKD patients. J Nephrol. 2013;26:739-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 62. | Dmitrieva O, de Lusignan S, Macdougall IC, Gallagher H, Tomson C, Harris K, Desombre T, Goldsmith D. Association of anaemia in primary care patients with chronic kidney disease: cross sectional study of quality improvement in chronic kidney disease (QICKD) trial data. BMC Nephrol. 2013;14:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 63. | Chertow GM, Block GA, Correa-Rotter R, Drüeke TB, Floege J, Goodman WG, Herzog CA, Kubo Y, London GM, Mahaffey KW. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367:2482-2494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 671] [Cited by in RCA: 633] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 64. | Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, Wanner C, Krane V, Cass A, Craig J. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181-2192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1946] [Cited by in RCA: 1754] [Article Influence: 125.3] [Reference Citation Analysis (0)] |
| 65. | Chapter 4. 1: Treatment of CKD-MBD targeted at lowering high serum phosphorus and maintaining serum calcium. Kidney Int. 2009;76113:S50-S99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 66. | Di Lullo L, Rivera R, Barbera V, Bellasi A, Cozzolino M, Russo D, De Pascalis A, Banerjee D, Floccari F, Ronco C. Sudden cardiac death and chronic kidney disease: From pathophysiology to treatment strategies. Int J Cardiol. 2016;217:16-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |