Published online May 23, 2016. doi: 10.5494/wjh.v6.i2.66
Peer-review started: March 4, 2016
First decision: April 15, 2016
Revised: May 12, 2016
Accepted: May 20, 2016
Article in press: May 21, 2016
Published online: May 23, 2016
Processing time: 101 Days and 11.8 Hours
Although it has known for some time that obesity is associated with salt sensitivity and hypertension, recent data suggests that the adipocyte may actually be the proximate cause of this physiological changes. In the following review, the data demonstrating this association as well as the potentially operative pathophysiological mechanisms are reviewed and discussed.
Core tip: Hypertension is a growing problem worldwide, and the problem is exacerbated by the growing obesity epidemic. This review looks into the complex relationship between these two diseases, outlining what current literature reports for treatment methods, hypotheses on cause, and potential cross talk between the two.
- Citation: Martin R, Shapiro JI. Role of adipocytes in hypertension. World J Hypertens 2016; 6(2): 66-75
- URL: https://www.wjgnet.com/2220-3168/full/v6/i2/66.htm
- DOI: https://dx.doi.org/10.5494/wjh.v6.i2.66
Hypertension is defined as elevated blood pressure-typically a systolic blood pressure of ≥ 140 mmHg or a diastolic pressure ≥ 90 mmHg (or both) in a relaxed state[1-3]. Currently, 29% (or 70 million) of Americans have been diagnosed with hypertension[4,5]. There are several stages of hypertension, as well as salt sensitive and salt resistant types of hypertension[6]. Hypertension may complicate and/or worsen other diseases such as diabetes, cardiovascular disease, chronic kidney disease, and obesity[2,7-9]. Obesity itself has reached pandemic proportions; according to the World Health Organization (WHO), over 500 million adults (10%-14% of world population) were obese in 2008, and this number keeps increasing[2,8,9]. As of 2014, this number has jumped to 600 million. There is an association between hypertension and obesity, but the mechanism(s) by which obesity predisposes to hypertension in humans has not be clearly established. In this review, we will explore some aspects of this important relationship.
Currently there are three known types of adipose tissue, each with it’s own specific characteristics: White, brown, and a mixture type, known as beige (or “brite”). The main purpose of adipose, regardless of the type, is to store excess energy that can be released as needed. The way the energy is stored varies between types. White adipose is the type one would think about when thinking about typical obesity. White adipocytes (WAT) are characterized by a spherical shape, a large lipid droplet that takes up 90% of the volume of the cell, very few mitochondria, and a flattened peripheral nucleus[10-15]. WAT can release triglycerides during a time of energy crisis in the body. WAT can be found virtually anywhere on the body, but are mainly located in subcutaneous abdomen, viscera, retroperitoneal, inguinal, and gonadal areas[10,16-19]. White adipocyte cells are known to secrete several kinds of proteins, such as inflammatory factors and the protein leptin[18-23].
Leptin is known as the satiety protein; when released, it inhibits feelings of hunger[13,14]. The antagonist of leptin is ghrelin; this is thought to be one of the hunger hormones[13,18,19,23]. It has clearly been shown by Sennello et al[24] and Friedman et al[25-27] that patients with an inability to produce leptin develop profound hyperphagia and obesity. However, in most obese subjects, leptin levels are high[25,26]. In these subjects, it is thought that as leptin levels are chronically elevated, responses to leptin are diminished[25]. Despite the high amount of energy already stored, the body ignores satiety signals and thinks it requires more energy to store; this higher level of leptin is consistent with a higher amount of adipocytes which are believed to be the primary source of leptin[25,26,28,29]. This hormone and protein releasing function therefore places white adipose tissue as an endocrine organ[14,30,31].
In addition to leptin, adipocytes also release other hormones and peptides, including tumor necrosis factor α (TNFα) and interleukin-6 (IL-6)[32-35]. These are inflammatory cytokines, and the increased levels are indicative of inflammation in the body[32-34]. Whether this inflammation leads to increased reactive oxygen species (ROS) production creating oxidative stress or oxidative stress from signaling leads to inflammation is currently unclear.
Brown adipocytes (BAT) are a bit different from WAT; they are polygonal in shape, contain fewer and smaller lipid molecules, have abundant mitochondria, and a central round nuclei[10,11,36]. BAT are mainly found in the subscapular region of rodents and human infants[10,37]. Whereas WAT use lipids to store energy, BAT store energy in the form of fat and break them down to produce heat in a process known as non-shivering thermogenesis[38-40]. Thermogenesis is the production of heat in an organism; non-shivering thermogenesis occurs in the brown adipose tissue because of the presence of thermogenin[41-44]. Thermogenin (also known as uncoupling protein 1) allows the uncoupling of protons moving down their gradient from adenosine triphosphate (ATP) synthesis; this energy is then dissipated as heat[43,44]. Free fatty acids from the brown adipose tissue remove any proteins that could inhibit thermogenin[41,42]. Thermogenin then causes an influx of H+ into the mitochondrial matrix, bypassing the ATP synthase normally used to make ATP[45-49]. This uncouples oxidative phosphorylation, and the energy normally used to convert adenosine diphosphate (ADP) to ATP is release as heat[46,50-52]. Interestingly, thermogenesis can also be produced ion pump leakage[50,53,54]. It is thought that a leaky ion pump in mitochondria releases H+ ions; the intensity of heat is proportional to the amount of H+ released during this process[41,43]. The ability of BAT to turn excess energy into heat is a property the WAT lack[4,55-57]. Circulating factors, such as irisin, FGF-21, and natriuretic peptides play a role in regulating BAT[4,55-57]. It is thought that these factors can encourage proliferation of BAT, and increase the amount of present beige adipocytes[55,56,58].
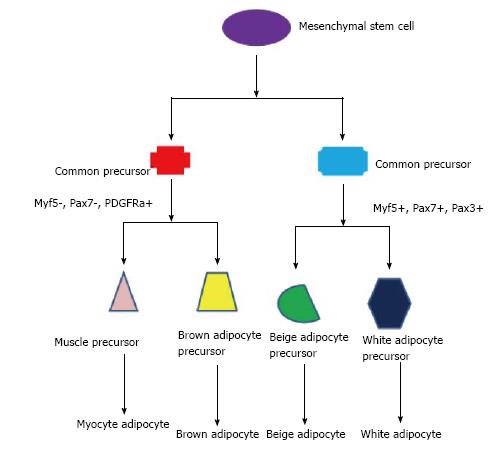
Beige adipocytes are a combination of brown and WAT[4]. Beige adipocytes are born through a browning process; WAT become more like BAT, the one large lipid droplet becomes many, and uncoupling protein 1 becomes expressed, and thermogenic activity increases[5,56,58]. All three types of adipocyte cells, along with muscle cells, come from the same precursor cell-a mesenchymal stem cell[4,5]. The expression of different genes at different points during the life cycle of these cells determines their fate[4,5], see Figure 1. The potential for obese adults to spontaneously form beige adipocytes from their WAT is unclear, but it brings to mind the possibility that such a phenomenon is possible.
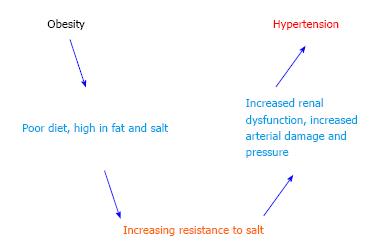
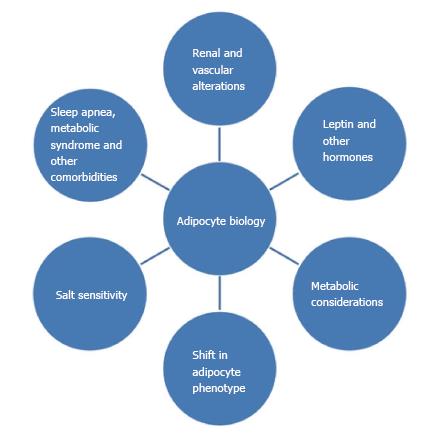
Obesity can increase the susceptibility to metabolic syndromes, cardiovascular diseases, type 2 diabetes, cancer, and hypertension[2,7-9]. Although some patients with hypertension are not obese, and vice versa, there is a strong correlation across populations[59]. The interactions between obesity, salt sensitivity and hypertension are shown schematically in Figure 2. When blood pressures reach the hypertensive range, there is almost always small vessel disease of the arterioles, or arteriolosclerosis, as well as kidney damage[60-62]. This strongly suggests that there are both vascular and renal components to the disease[63]. Hypertension has genetic and environmental factors in addition to those associated with obesity[4,29,64,65]. Salt sensitive hypertension refers to an increase in blood pressure related to an increase in salt (specifically sodium) intake[6,66,67]. Some workers in this field believe that all hypertension reflects either excessive sodium intake or some form of renal salt sensitivity, but this is admittedly still controversial[63,68-70].
Obesity appears to be associated with or complicated by “increased sympathetic nervous system (SNS) activity, activation of the renin-angiotensin aldosterone system (RAAS), and physical compression of the kidneys by extra-renal fat and by increased intrarenal extracellular matrix”[71-73]. This physical compression can directly activate the RAAS which, in turn, leads to increased SNS outflow as well as increased circulating concentrations of angiotensin II, a well-known vasoconstrictor and aldosterone, an anti-natriuretic hormone. The net affect is sodium retention and increased blood pressure[63,73-75]. Leptin levels, as discussed above, appear to be increased in obese patients. This hormone through several biochemical mechanisms, affects appetite as well as SNS outflow, and can cause increases in blood pressure[71,74]. The duration of obesity also plays a role; the longer one is obese, the more renal damage occurs which further impairs pressure natriuresis, exacerbating hypertension[63,71,72,76,77].
Another potentially contributing factor is obstructive sleep apnea (OSA), which is more than just another co-morbidity of obesity. OSA is much more common in people who are overweight or obese[1,2]. OSA occurs when the airway becomes blocked or constricted and can cause snoring, and lapses in breathing that are common to sleep apnea[2,78,79]. Untreated sleep apnea can lead to increases in blood pressure, obesity, heart attack risk, and diabetes, among other problems[2,7,75,80-82]. In addition to increasing the risk for hypertension, OSA can lead to other problems. Hypoxia, or lack of oxygen that occurs when breathing is stopped or obstructed, is also a risk factor for generating ROS and increasing oxidant stress and SNS activity[1,2,78,82].
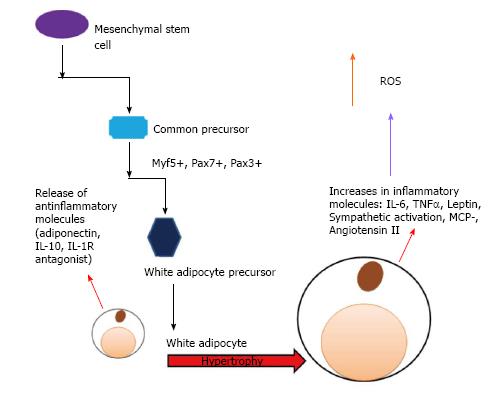
Recent work suggests that the adipocyte itself could play an important role in hypertension. Research has shown that high dietary sodium can increase the white adipocyte mass as well as leptin levels in rats[82], and blood pressure was significantly increased as well. The increase in adipocyte mass has a cascading effect; the mass increases, the release of additional adipokines occurs, and these lead to an increase in inflammation. This inflammation causes further exacerbation of disordered metabolism and insulin resistance[82-85]. These interactions are shown schematically in Figures 3 and 4.
Currently, there are several treatment methods to deal with obesity, and as it is so closely related with hypertension, treatments for the two can often overlap[86-88]. The treatments can be broken up into several categories; lifestyle changes (including nutritional changes and exercise addition), drug therapy, and surgical methods[89]. It has been shown that a reduction in a patient’s weight by 5%-10% is enough to reduce their risk of cardiovascular complications, including hypertension[89-91]. When looking at the drug treatment route, it is important to consider however that some drugs are not recommended for patients who have pre-existing conditions, such as hypertension or diabetes. Sibutramine, for example, has been associated with small increases in blood pressure and heart rate, and is not recommended for patients suffering from hypertension[89,92]. Some drugs that are used to treat hypertension can be used as a weight loss agent, such as the drug orlistat[89,93]. These drugs can work on multiple levels; some are known as feeder modulators, and change the way the patient receives signals that the body needs food[89,94]. Some effect the formation of agents such as angiotensin II and nitric oxide synthase (NOS)[89,95,96]. Still others work at the molecular level and effect the afferent signaling that can lead to obesity[89,95]. Serotonin drugs have been found to be an effective treatment of obesity, but the downside is they can cause an increased risk of primary hypertension because of their effects on vascular smooth muscle[97]. If we look at surgical approaches, the benefits of surgery on hypertension itself and the abnormal hormonal milieu appear to be huge, at least over the first year or so[98].
In addition to salt intake and obesity, nitrous oxide synthase and heme oxygenase (HO) both play a role in the cause and treatment of hypertension[99-101]. Obesity leads to an imbalance in the circulating level of nitic oxide (NO); this is due to increased oxidative stress and decreased NO production[100,102]. Decreasing the availability of the NO can predispose an individual to hypertension[99-101]. NO contributes to vasodilation, which is the relaxation of the vasculature[83,99-101]. If there is less NO present (because of a decrease in NO synthase), vasoconstriction can occur, which can exacerbate the damage of increased pressure from the other factors related to hypertension[99-101]. Human adipose tissue expresses angiotensinogen, angiotensin-converting enzyme (ACE) as well as angiotensin type 1 (AT1), and AT2 receptors[102-105]. The role of angiostatin is not well known, but it has some kind of redox purpose; it appears to involve inhibition of endothelial cell migration, proliferation and induction of apoptosis[65,99-101]. There is a link between NO synthase dysfunction and the ACE enzyme in the obese population[99-101]. Excessive NO formation by the inducible member of the NOS family (iNOS or Nos2) has been shown to cause nonspecific tissue damage; it is thought to be involved in the pathogenesis of inflammatory and autoimmune diseases[106-108]. By inhibiting this inducible factor, obesity still occurs but the pathologies associated are reduced[107]. A similar study showed that even though mice protected from pathologies associated with iNOS inhibition, they are still subjected to increased blood pressure and increased ROS[109,110]. iNOS is associated with increased inflammatory responses, which is related to the cascade of responses associated with obesity and hypertension[33,34,111,112]. It is known that increased NO can induce cellular stress, which can exacerbate the current problems present.
Similarly to NO synthase, HO has a role in amelioration of hypertension[1,100,104,107,113]. An increase in HO expression can cause reductions in ROS, or ROS. Increases in ROS, also called oxidant stress, are believed to be important in the progression of hypertension and associated cardiovascular diseases[65,81,87,93]. The isoform HO-1 is the inducible form of HO, and when induced it can cause a decrease in weight, and therefore a decrease in obesity[113-115]. HO does this by changing the phenotype of the adipocyte[113,116]. HO-1 can interact with NO in several ways, one of which is through AngII[99,117,118]. Increased AngII production causes an increase in ROS, which may inhibit the action of NO[100,119]. This can also increase salt reabsorption. When HO-1 expression is increased, the increases in AngII levels are attenuated; this decreases AngII’s downstream signaling effects[99,117,118]. Induction of HO-1 can also reduce the renal vasculature resistance that is increased with AngII level increases[99,117,118].
Several studies have shown that induction of HO-1 not only decreases weight and obesity, but it can also prevent the development of hypertension, even if its expression is limited to adipocytes[1,120]. It is not clear if blood pressure is lowered through the indirect effects on the vasculature, kidney, or through the release of other enzymes and factors. If induction of HO-1 is done at any step in the pathway described above, what are the specific effects? If induced during any stage of hypertension, will effects still be seen, or does it need to be induced early in obesity?
Abraham et al[1], look specifically at the role of HO-1 and the effects it can have on various aspects of obesity. One study specifically examines adipocyte dysfunction; induction of HO-1 can reverse adipocyte dysfunction and to an extent reverse effects of damage[121]. This lab has also shown significant findings of the role of HO-1 and the attenuating effects it can have with hypertension[1,122,123]. We have also looked from the other perspective, namely ROS generation. We have recently observed that attenuation of ROS generation with pNaKtide[124,125], a peptide designed to ameliorate the Na/K-ATPase mediated feed forward amplification of ROS[50], prevents phenotypical changes within adipocytes as well as ameliorates diet induced obesity in mice[35].
There is clearly a very strong relationship between obesity and hypertension. While a plethora of mechanisms potentially link obesity to hypertension, we are left with the provocative possibility that adipocyte biology may play an important role in blood pressure regulation, a topic which to date has not been systematically explored.
P- Reviewer: Bahlmann FH, Efstathiou SP, Mezalek ZT, Okumura K S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Abraham NG, Asija A, Drummond G, Peterson S. Heme oxygenase-1 gene therapy: recent advances and therapeutic applications. Curr Gene Ther. 2007;7:89-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | Adedayo AM, Olafiranye O, Smith D, Hill A, Zizi F, Brown C, Jean-Louis G. Obstructive sleep apnea and dyslipidemia: evidence and underlying mechanism. Sleep Breath. 2014;18:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 3. | Canning KL, Brown RE, Jamnik VK, Kuk JL. Relationship between obesity and obesity-related morbidities weakens with aging. J Gerontol A Biol Sci Med Sci. 2014;69:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Yang X, Bi P, Kuang S. Fighting obesity: When muscle meets fat. Adipocyte. 2014;3:280-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Cohen P, Spiegelman BM. Brown and Beige Fat: Molecular Parts of a Thermogenic Machine. Diabetes. 2015;64:2346-2351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 211] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 6. | Chen J, Gu D, Huang J, Rao DC, Jaquish CE, Hixson JE, Chen CS, Chen J, Lu F, Hu D. Metabolic syndrome and salt sensitivity of blood pressure in non-diabetic people in China: a dietary intervention study. Lancet. 2009;373:829-835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 193] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 7. | Albert U, Aguglia A, Chiarle A, Bogetto F, Maina G. Metabolic syndrome and obsessive-compulsive disorder: a naturalistic Italian study. Gen Hosp Psychiatry. 2013;35:154-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Brown TM, Vaidya D, Rogers WJ, Waters DD, Howard BV, Tardif JC, Bittner V. Does prevalence of the metabolic syndrome in women with coronary artery disease differ by the ATP III and IDF criteria? J Womens Health (Larchmt). 2008;17:841-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Herath Bandara SJ, Brown C. An analysis of adult obesity and hypertension in appalachia. Glob J Health Sci. 2013;5:127-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Alvarez R, de Andrés J, Yubero P, Viñas O, Mampel T, Iglesias R, Giralt M, Villarroya F. A novel regulatory pathway of brown fat thermogenesis. Retinoic acid is a transcriptional activator of the mitochondrial uncoupling protein gene. J Biol Chem. 1995;270:5666-5673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 146] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | Bonet ML, Serra F, Matamala JC, García-Palmer FJ, Palou A. Selective loss of the uncoupling protein from light versus heavy mitochondria of brown adipocytes after a decrease in noradrenergic stimulation in vivo and in vitro. Biochem J. 1995;311:327-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, Thome JJ, Farber DL, Lutfy K, Seale P. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2015;519:242-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 637] [Cited by in RCA: 771] [Article Influence: 70.1] [Reference Citation Analysis (0)] |
| 13. | Farooqi IS, O’Rahilly S. Leptin: a pivotal regulator of human energy homeostasis. Am J Clin Nutr. 2009;89:980S-984S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 213] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 14. | Fonseca-Alaniz MH, Takada J, Alonso-Vale MI, Lima FB. Adipose tissue as an endocrine organ: from theory to practice. J Pediatr (Rio J). 2007;83:S192-S203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Gil A, Olza J, Gil-Campos M, Gomez-Llorente C, Aguilera CM. Is adipose tissue metabolically different at different sites? Int J Pediatr Obes. 2011;6 Suppl 1:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 16. | Adams AE, Hanrahan O, Nolan DN, Voorheis HP, Fallon P, Porter RK. Images of mitochondrial UCP 1 in mouse thymocytes using confocal microscopy. Biochim Biophys Acta. 2008;1777:115-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Burgess A, Li M, Vanella L, Kim DH, Rezzani R, Rodella L, Sodhi K, Canestraro M, Martasek P, Peterson SJ. Adipocyte heme oxygenase-1 induction attenuates metabolic syndrome in both male and female obese mice. Hypertension. 2010;56:1124-1130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 18. | Cinti S, Frederich RC, Zingaretti MC, De Matteis R, Flier JS, Lowell BB. Immunohistochemical localization of leptin and uncoupling protein in white and brown adipose tissue. Endocrinology. 1997;138:797-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 120] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Cohen P, Ntambi JM, Friedman JM. Stearoyl-CoA desaturase-1 and the metabolic syndrome. Curr Drug Targets Immune Endocr Metabol Disord. 2003;3:271-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Abraham NG, Sodhi K, Silvis AM, Vanella L, Favero G, Rezzani R, Lee C, Zeldin DC, Schwartzman ML. CYP2J2 targeting to endothelial cells attenuates adiposity and vascular dysfunction in mice fed a high-fat diet by reprogramming adipocyte phenotype. Hypertension. 2014;64:1352-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 21. | Almabrouk TA, Ewart MA, Salt IP, Kennedy S. Perivascular fat, AMP-activated protein kinase and vascular diseases. Br J Pharmacol. 2014;171:595-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Bianco AC, Kieffer JD, Silva JE. Adenosine 3’,5’-monophosphate and thyroid hormone control of uncoupling protein messenger ribonucleic acid in freshly dispersed brown adipocytes. Endocrinology. 1992;130:2625-2633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Cohen P, Friedman JM. Leptin and the control of metabolism: role for stearoyl-CoA desaturase-1 (SCD-1). J Nutr. 2004;134:2455S-2463S. [PubMed] |
| 24. | Sennello JA, Fayad R, Morris AM, Eckel RH, Asilmaz E, Montez J, Friedman JM, Dinarello CA, Fantuzzi G. Regulation of T cell-mediated hepatic inflammation by adiponectin and leptin. Endocrinology. 2005;146:2157-2164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Friedman JM. Leptin and the regulation of body weigh. Keio J Med. 2011;60:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 26. | Friedman J. 20 years of leptin: leptin at 20: an overview. J Endocrinol. 2014;223:T1-T8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 174] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 27. | Friedman DJ, Talbert ME, Bowden DW, Freedman BI, Mukanya Y, Enjyoji K, Robson SC. Functional ENTPD1 polymorphisms in African Americans with diabetes and end-stage renal disease. Diabetes. 2009;58:999-1006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Zhou YT, Shimabukuro M, Koyama K, Lee Y, Wang MY, Trieu F, Newgard CB, Unger RH. Induction by leptin of uncoupling protein-2 and enzymes of fatty acid oxidation. Proc Natl Acad Sci USA. 1997;94:6386-6390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 259] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 29. | Simonds SE, Pryor JT, Ravussin E, Greenway FL, Dileone R, Allen AM, Bassi J, Elmquist JK, Keogh JM, Henning E. Leptin mediates the increase in blood pressure associated with obesity. Cell. 2014;159:1404-1416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 249] [Cited by in RCA: 275] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 30. | Wu MV, Bikopoulos G, Hung S, Ceddia RB. Thermogenic capacity is antagonistically regulated in classical brown and white subcutaneous fat depots by high fat diet and endurance training in rats: impact on whole-body energy expenditure. J Biol Chem. 2014;289:34129-34140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 149] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 31. | Villacorta L, Chang L. The role of perivascular adipose tissue in vasoconstriction, arterial stiffness, and aneurysm. Horm Mol Biol Clin Investig. 2015;21:137-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 32. | Yoda K, Sun X, Kawase M, Kubota A, Miyazawa K, Harata G, Hosoda M, Hiramatsu M, He F, Zemel MB. A combination of probiotics and whey proteins enhances anti-obesity effects of calcium and dairy products during nutritional energy restriction in aP2-agouti transgenic mice. Br J Nutr. 2015;113:1689-1696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 33. | Yoda K, Sun X, Kawase M, Kubota A, Miyazawa K, Harata G, Hosoda M, Hiramatsu M, He F, Zemel MB. Anti-inflammatory γ- and δ-tocotrienols improve cardiovascular, liver and metabolic function in diet-induced obese rats. Eur J Nutr. 2015;113:1689-1696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 34. | Sodhi K, Puri N, Kim DH, Hinds TD, Stechschulte LA, Favero G, Rodella L, Shapiro JI, Jude D, Abraham NG. PPARδ binding to heme oxygenase 1 promoter prevents angiotensin II-induced adipocyte dysfunction in Goldblatt hypertensive rats. Int J Obes (Lond). 2014;38:456-465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Sodhi K, Maxwell K, Yan Y, Liu J, Chaudhry MA, Getty M, Xie Z, Abraham NG, Shapiro JI. pNaKtide inhibits Na/K-ATPase reactive oxygen species amplification and attenuates adipogenesis. Sci Adv. 2015;1:e1500781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 36. | Alvarez R, Checa M, Brun S, Viñas O, Mampel T, Iglesias R, Giralt M, Villarroya F. Both retinoic-acid-receptor- and retinoid-X-receptor-dependent signalling pathways mediate the induction of the brown-adipose-tissue-uncoupling-protein-1 gene by retinoids. Biochem J. 2000;345 Pt 1:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 37. | Martin GS, Carstens GE, King MD, Eli AG, Mersmann HJ, Smith SB. Metabolism and morphology of brown adipose tissue from Brahman and Angus newborn calves. J Anim Sci. 1999;77:388-399. [PubMed] |
| 38. | Strack AM, Bradbury MJ, Dallman MF. Corticosterone decreases nonshivering thermogenesis and increases lipid storage in brown adipose tissue. Am J Physiol. 1995;268:R183-R191. [PubMed] |
| 39. | Klitsch T, Siemen D. Inner mitochondrial membrane anion channel is present in brown adipocytes but is not identical with the uncoupling protein. J Membr Biol. 1991;122:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 32] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 40. | Klingenspor M. Cold-induced recruitment of brown adipose tissue thermogenesis. Exp Physiol. 2003;88:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 194] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 41. | Rehnmark S, Néchad M, Herron D, Cannon B, Nedergaard J. Alpha- and beta-adrenergic induction of the expression of the uncoupling protein thermogenin in brown adipocytes differentiated in culture. J Biol Chem. 1990;265:16464-16471. [PubMed] |
| 42. | Puigserver P, Vázquez F, Bonet ML, Picó C, Palou A. In vitro and in vivo induction of brown adipocyte uncoupling protein (thermogenin) by retinoic acid. Biochem J. 1996;317:827-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 90] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 43. | Palou A, Picó C, Bonet ML, Oliver P. The uncoupling protein, thermogenin. Int J Biochem Cell Biol. 1998;30:7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 75] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 44. | Herron D, Rehnmark S, Néchad M, Loncar D, Cannon B, Nedergaard J. Norepinephrine-induced synthesis of the uncoupling protein thermogenin (UCP) and its mitochondrial targeting in brown adipocytes differentiated in culture. FEBS Lett. 1990;268:296-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 45. | Zietak M, Kozak LP. Bile acids induce uncoupling protein 1-dependent thermogenesis and stimulate energy expenditure at thermoneutrality in mice. Am J Physiol Endocrinol Metab. 2016;310:E346-E354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 46. | Zhou J, Cheng M, Boriboun C, Ardehali MM, Jiang C, Liu Q, Han S, Goukassian DA, Tang YL, Zhao TC. Inhibition of Sam68 triggers adipose tissue browning. J Endocrinol. 2015;225:181-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 47. | Xue R, Lynes MD, Dreyfuss JM, Shamsi F, Schulz TJ, Zhang H, Huang TL, Townsend KL, Li Y, Takahashi H. Clonal analyses and gene profiling identify genetic biomarkers of the thermogenic potential of human brown and white preadipocytes. Nat Med. 2015;21:760-768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 216] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 48. | Wang S, Wang X, Ye Z, Xu C, Zhang M, Ruan B, Wei M, Jiang Y, Zhang Y, Wang L. Curcumin promotes browning of white adipose tissue in a norepinephrine-dependent way. Biochem Biophys Res Commun. 2015;466:247-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 49. | Wang CZ, Wei D, Guan MP, Xue YM. Triiodothyronine regulates distribution of thyroid hormone receptors by activating AMP-activated protein kinase in 3T3-L1 adipocytes and induces uncoupling protein-1 expression. Mol Cell Biochem. 2014;393:247-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 50. | Yan Y, Shapiro AP, Haller S, Katragadda V, Liu L, Tian J, Basrur V, Malhotra D, Xie ZJ, Abraham NG. Involvement of reactive oxygen species in a feed-forward mechanism of Na/K-ATPase-mediated signaling transduction. J Biol Chem. 2013;288:34249-34258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 51. | Xing X, Yang M, Wang DH. The expression of leptin, hypothalamic neuropeptides and UCP1 before, during and after fattening in the Daurian ground squirrel (Spermophilus dauricus). Comp Biochem Physiol A Mol Integr Physiol. 2015;184:105-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 52. | Sacks HS, Fain JN, Holman B, Cheema P, Chary A, Parks F, Karas J, Optican R, Bahouth SW, Garrett E. Uncoupling protein-1 and related messenger ribonucleic acids in human epicardial and other adipose tissues: epicardial fat functioning as brown fat. J Clin Endocrinol Metab. 2009;94:3611-3615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 236] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 53. | Kopecký J, Baudysová M, Zanotti F, Janíková D, Pavelka S, Houstĕk J. Synthesis of mitochondrial uncoupling protein in brown adipocytes differentiated in cell culture. J Biol Chem. 1990;265:22204-22209. [PubMed] |
| 54. | Bednár J, Soukup T. Developmental changes in uncoupling protein 1 and F1-ATPase subunit levels in the golden hamster brown adipose tissue mitochondria as determined by electron microscopy in situ immunocytochemistry. Gen Physiol Biophys. 2003;22:477-486. [PubMed] |
| 55. | Vargas D, Shimokawa N, Kaneko R, Rosales W, Parra A, Castellanos Á, Koibuchi N, Lizcano F. Regulation of human subcutaneous adipocyte differentiation by EID1. J Mol Endocrinol. 2016;56:113-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 56. | Sharp LZ, Shinoda K, Ohno H, Scheel DW, Tomoda E, Ruiz L, Hu H, Wang L, Pavlova Z, Gilsanz V. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS One. 2012;7:e49452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 482] [Cited by in RCA: 511] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 57. | Sacks HS, Fain JN, Bahouth SW, Ojha S, Frontini A, Budge H, Cinti S, Symonds ME. Adult epicardial fat exhibits beige features. J Clin Endocrinol Metab. 2013;98:E1448-E1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 149] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 58. | Sharma A, Huard C, Vernochet C, Ziemek D, Knowlton KM, Tyminski E, Paradis T, Zhang Y, Jones JE, von Schack D. Brown fat determination and development from muscle precursor cells by novel action of bone morphogenetic protein 6. PLoS One. 2014;9:e92608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 59. | Shah PT, Shapiro AP, Khitan Z, Santhanam P, Shapiro JI. Why Does Obesity Lead to Hypertension? Further Lessons from the Intersalt Study. Marshall J Med. 2016;In Press. |
| 60. | Zhang Q, Davis KJ, Hoffmann D, Vaidya VS, Brown RP, Goering PL. Urinary biomarkers track the progression of nephropathy in hypertensive and obese rats. Biomark Med. 2014;8:85-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 61. | Watanabe H, Miyamoto Y, Honda D, Tanaka H, Wu Q, Endo M, Noguchi T, Kadowaki D, Ishima Y, Kotani S. p-Cresyl sulfate causes renal tubular cell damage by inducing oxidative stress by activation of NADPH oxidase. Kidney Int. 2013;83:582-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 287] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 62. | Sasaki N, Uchida E, Niiyama M, Yoshida T, Saito M. Anti-obesity effects of selective agonists to the beta 3-adrenergic receptor in dogs. II. Recruitment of thermogenic brown adipocytes and reduction of adiposity after chronic treatment with a beta 3-adrenergic agonist. J Vet Med Sci. 1998;60:465-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 63. | Hall JE, Granger JP, do Carmo JM, da Silva AA, Dubinion J, George E, Hamza S, Speed J, Hall ME. Hypertension: physiology and pathophysiology. Compr Physiol. 2012;2:2393-2442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 147] [Article Influence: 12.3] [Reference Citation Analysis (1)] |
| 64. | Zhang S, Yang L, Chen P, Jin H, Xie X, Yang M, Gao T, Hu C, Yu X. Circulating Adipocyte Fatty Acid Binding Protein (FABP4) Levels Are Associated with Irisin in the Middle-Aged General Chinese Population. PLoS One. 2016;11:e0146605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 65. | Shungin D, Winkler TW, Croteau-Chonka DC, Ferreira T, Locke AE, Mägi R, Strawbridge RJ, Pers TH, Fischer K, Justice AE. New genetic loci link adipose and insulin biology to body fat distribution. Nature. 2015;518:187-196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1401] [Cited by in RCA: 1172] [Article Influence: 117.2] [Reference Citation Analysis (0)] |
| 66. | Mori Y, Murakawa Y, Yokoyama J, Tajima N, Ikeda Y, Nobukata H, Ishikawa T, Shibutani Y. Effect of highly purified eicosapentaenoic acid ethyl ester on insulin resistance and hypertension in Dahl salt-sensitive rats. Metabolism. 1999;48:1089-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 67. | Gilibert S, Kwitek AE, Hubner N, Tschannen M, Jacob HJ, Sassard J, Bataillard A. Effects of chromosome 17 on features of the metabolic syndrome in the Lyon hypertensive rat. Physiol Genomics. 2008;33:212-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 68. | Fedorova OV, Shapiro JI, Bagrov AY. Endogenous cardiotonic steroids and salt-sensitive hypertension. Biochim Biophys Acta. 2010;1802:1230-1236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 69. | Hall JE, Guyton AC, Brands MW. Pressure-volume regulation in hypertension. Kidney Int Suppl. 1996;55:S35-S41. [PubMed] |
| 70. | Xie JX, Shapiro AP, Shapiro JI. The Trade-Off between Dietary Salt and Cardiovascular Disease; A Role for Na/K-ATPase Signaling? Front Endocrinol (Lausanne). 2014;5:97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 71. | Hall JE, da Silva AA, do Carmo JM, Dubinion J, Hamza S, Munusamy S, Smith G, Stec DE. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J Biol Chem. 2010;285:17271-17276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 350] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 72. | Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res. 2015;116:991-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 605] [Cited by in RCA: 804] [Article Influence: 80.4] [Reference Citation Analysis (0)] |
| 73. | Hall JE, Granger JP, Reckelhoff JF, Sandberg K. Hypertension and cardiovascular disease in women. Hypertension. 2008;51:951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 74. | Hall JE, Jones DW, Kuo JJ, da Silva A, Tallam LS, Liu J. Impact of the obesity epidemic on hypertension and renal disease. Curr Hypertens Rep. 2003;5:386-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 75. | Hall JE, Kuo JJ, da Silva AA, de Paula RB, Liu J, Tallam L. Obesity-associated hypertension and kidney disease. Curr Opin Nephrol Hypertens. 2003;12:195-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 76. | da Silva AA, do Carmo JM, Hall JE. Role of leptin and central nervous system melanocortins in obesity hypertension. Curr Opin Nephrol Hypertens. 2013;22:135-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 77. | Hall ME, do Carmo JM, da Silva AA, Juncos LA, Wang Z, Hall JE. Obesity, hypertension, and chronic kidney disease. Int J Nephrol Renovasc Dis. 2014;7:75-88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 322] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 78. | Al-Jehani HM, Hall JA, Maleki M. Decompressive laparotomy for treatment of refractory intracranial hypertension, thinking out of the box. Neurosciences (Riyadh). 2013;18:382-384. [PubMed] |
| 79. | Dharia SM, Unruh ML, Brown LK. Central Sleep Apnea in Kidney Disease. Semin Nephrol. 2015;35:335-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 80. | Allison DW, Gertsch JH, Mahan MA, Sheean GL, Brown JM. Anesthesia considerations for monitoring TCMEPs in adults diagnosed with poliomyelitis as children: a case report. Neurodiagn J. 2014;54:28-35. [PubMed] |
| 81. | Alonso-Galicia M, Brands MW, Zappe DH, Hall JE. Hypertension in obese Zucker rats. Role of angiotensin II and adrenergic activity. Hypertension. 1996;28:1047-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 144] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 82. | Fonseca-Alaniz MH, Brito LC, Borges-Silva CN, Takada J, Andreotti S, Lima FB. High dietary sodium intake increases white adipose tissue mass and plasma leptin in rats. Obesity (Silver Spring). 2007;15:2200-2208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 83. | Alon T, Friedman JM. Late-onset leanness in mice with targeted ablation of melanin concentrating hormone neurons. J Neurosci. 2006;26:389-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 84. | Enzi G, Busetto L, Sergi G, Coin A, Inelmen EM, Vindigni V, Bassetto F, Cinti S. Multiple symmetric lipomatosis: a rare disease and its possible links to brown adipose tissue. Nutr Metab Cardiovasc Dis. 2015;25:347-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 85. | Gutierrez DA, Puglisi MJ, Hasty AH. Impact of increased adipose tissue mass on inflammation, insulin resistance, and dyslipidemia. Curr Diab Rep. 2009;9:26-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 124] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 86. | Ying A, Arima H, Czernichow S, Woodward M, Huxley R, Turnbull F, Perkovic V, Neal B. Effects of blood pressure lowering on cardiovascular risk according to baseline body-mass index: a meta-analysis of randomised trials. Lancet. 2015;385:867-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 87. | Bray G. Drug treatment of obesity: don’t throw the baby out with the bath water. Am J Clin Nutr. 1998;67:1-2. [PubMed] |
| 88. | Brown AD, Barton DA, Lambert GW. Cardiovascular abnormalities in patients with major depressive disorder: autonomic mechanisms and implications for treatment. CNS Drugs. 2009;23:583-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 89. | Alemany M, Remesar X, Fernández-López JA. Drug strategies for the treatment of obesity. IDrugs. 2003;6:566-572. [PubMed] |
| 90. | Atkinson RL, Blank RC, Loper JF, Schumacher D, Lutes RA. Combined drug treatment of obesity. Obes Res. 1995;3 Suppl 4:497S-500S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 91. | Barja-Fernandez S, Leis R, Casanueva FF, Seoane LM. Drug development strategies for the treatment of obesity: how to ensure efficacy, safety, and sustainable weight loss. Drug Des Devel Ther. 2014;8:2391-2400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 92. | Ioannides-Demos LL, Proietto J, Tonkin AM, McNeil JJ. Safety of drug therapies used for weight loss and treatment of obesity. Drug Saf. 2006;29:277-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 93. | Charakida M, Tousoulis D, Finer N. Drug treatment of obesity in the cardiovascular patient. Curr Opin Cardiol. 2013;28:584-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 94. | Bray GA. Drug treatment of obesity. Baillieres Best Pract Res Clin Endocrinol Metab. 1999;13:131-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 95. | Charakida M, Finer N. Drug treatment of obesity in cardiovascular disease. Am J Cardiovasc Drugs. 2012;12:93-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 96. | Cheung BM. Drug treatment for obesity in the post-sibutramine era. Drug Saf. 2011;34:641-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 97. | Halford JC, Harrold JA, Lawton CL, Blundell JE. Serotonin (5-HT) drugs: effects on appetite expression and use for the treatment of obesity. Curr Drug Targets. 2005;6:201-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 153] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 98. | Ruano M, Silvestre V, Castro R, García-Lescún MC, Rodríguez A, Marco A, García-Blanch G. Morbid obesity, hypertensive disease and the renin-angiotensin-aldosterone axis. Obes Surg. 2005;15:670-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 99. | Zhou X, Ma L, Habibi J, Whaley-Connell A, Hayden MR, Tilmon RD, Brown AN, Kim JA, Demarco VG, Sowers JR. Nebivolol improves diastolic dysfunction and myocardial remodeling through reductions in oxidative stress in the Zucker obese rat. Hypertension. 2010;55:880-888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 100. | Ishima Y, Narisoko T, Kragh-Hansen U, Kotani S, Nakajima M, Otagiri M, Maruyama T. Nitration of indoxyl sulfate facilitates its cytotoxicity in human renal proximal tubular cells via expression of heme oxygenase-1. Biochem Biophys Res Commun. 2015;465:481-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 101. | De Simone R, Ajmone-Cat MA, Pandolfi M, Bernardo A, De Nuccio C, Minghetti L, Visentin S. The mitochondrial uncoupling protein-2 is a master regulator of both M1 and M2 microglial responses. J Neurochem. 2015;135:147-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 102. | Merial C, Bouloumie A, Trocheris V, Lafontan M, Galitzky J. Nitric oxide-dependent downregulation of adipocyte UCP-2 expression by tumor necrosis factor-alpha. Am J Physiol Cell Physiol. 2000;279:C1100-C1106. [PubMed] |
| 103. | Westphal S, Perwitz N, Iwen KA, Kraus D, Schick R, Fasshauer M, Klein J. Expression of ATRAP in adipocytes and negative regulation by beta-adrenergic stimulation of JAK/STAT. Horm Metab Res. 2008;40:165-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 104. | Peterson SJ, Frishman WH, Abraham NG. Targeting heme oxygenase: therapeutic implications for diseases of the cardiovascular system. Cardiol Rev. 2009;17:99-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 105. | Hilzendeger AM, Morgan DA, Brooks L, Dellsperger D, Liu X, Grobe JL, Rahmouni K, Sigmund CD, Mark AL. A brain leptin-renin angiotensin system interaction in the regulation of sympathetic nerve activity. Am J Physiol Heart Circ Physiol. 2012;303:H197-H206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 106. | Zaitone SA, Barakat BM, Bilasy SE, Fawzy MS, Abdelaziz EZ, Farag NE. Protective effect of boswellic acids versus pioglitazone in a rat model of diet-induced non-alcoholic fatty liver disease: influence on insulin resistance and energy expenditure. Naunyn Schmiedebergs Arch Pharmacol. 2015;388:587-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 107. | Rodella LF, Vanella L, Peterson SJ, Drummond G, Rezzani R, Falck JR, Abraham NG. Heme oxygenase-derived carbon monoxide restores vascular function in type 1 diabetes. Drug Metab Lett. 2008;2:290-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 108. | Marçano AC, Burke B, Gungadoo J, Wallace C, Kaisaki PJ, Woon PY, Farrall M, Clayton D, Brown M, Dominiczak A. Genetic association analysis of inositol polyphosphate phosphatase-like 1 (INPPL1, SHIP2) variants with essential hypertension. J Med Genet. 2007;44:603-605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 109. | Klein J, Fasshauer M, Benito M, Kahn CR. Insulin and the beta3-adrenoceptor differentially regulate uncoupling protein-1 expression. Mol Endocrinol. 2000;14:764-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 110. | Chen Y, Liu J, Zheng Y, Wang J, Wang Z, Gu S, Tan J, Jing Q, Yang H. Uncoupling protein 3 mediates H2O2 preconditioning-afforded cardioprotection through the inhibition of MPTP opening. Cardiovasc Res. 2015;105:192-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 111. | Cao J, Sodhi K, Puri N, Monu SR, Rezzani R, Abraham NG. High fat diet enhances cardiac abnormalities in SHR rats: Protective role of heme oxygenase-adiponectin axis. Diabetol Metab Syndr. 2011;3:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 112. | Sodhi K, Puri N, Inoue K, Falck JR, Schwartzman ML, Abraham NG. EET agonist prevents adiposity and vascular dysfunction in rats fed a high fat diet via a decrease in Bach 1 and an increase in HO-1 levels. Prostaglandins Other Lipid Mediat. 2012;98:133-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 113. | Vanella L, Sodhi K, Kim DH, Puri N, Maheshwari M, Hinds TD, Bellner L, Goldstein D, Peterson SJ, Shapiro JI. Increased heme-oxygenase 1 expression in mesenchymal stem cell-derived adipocytes decreases differentiation and lipid accumulation via upregulation of the canonical Wnt signaling cascade. Stem Cell Res Ther. 2013;4:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 114. | Kamble P, Litvinov D, Aluganti Narasimhulu C, Jiang X, Parthasarathy S. Aspirin may influence cellular energy status. Eur J Pharmacol. 2015;749:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 115. | Vanella L, Kim DH, Asprinio D, Peterson SJ, Barbagallo I, Vanella A, Goldstein D, Ikehara S, Kappas A, Abraham NG. HO-1 expression increases mesenchymal stem cell-derived osteoblasts but decreases adipocyte lineage. Bone. 2010;46:236-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 116. | Nicolai A, Li M, Kim DH, Peterson SJ, Vanella L, Positano V, Gastaldelli A, Rezzani R, Rodella LF, Drummond G. Heme oxygenase-1 induction remodels adipose tissue and improves insulin sensitivity in obesity-induced diabetic rats. Hypertension. 2009;53:508-515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 150] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 117. | Westenbrink BD, Ling H, Divakaruni AS, Gray CB, Zambon AC, Dalton ND, Peterson KL, Gu Y, Matkovich SJ, Murphy AN. Mitochondrial reprogramming induced by CaMKIIδ mediates hypertrophy decompensation. Circ Res. 2015;116:e28-e39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 118. | Valladares A, Roncero C, Benito M, Porras A. TNF-alpha inhibits UCP-1 expression in brown adipocytes via ERKs. Opposite effect of p38MAPK. FEBS Lett. 2001;493:6-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 119. | Wang P, Li B, Cai G, Huang M, Jiang L, Pu J, Li L, Wu Q, Zuo L, Wang Q. Activation of PPAR-γ by pioglitazone attenuates oxidative stress in aging rat cerebral arteries through upregulating UCP2. J Cardiovasc Pharmacol. 2014;64:497-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 120. | Cao J, Peterson SJ, Sodhi K, Vanella L, Barbagallo I, Rodella LF, Schwartzman ML, Abraham NG, Kappas A. Heme oxygenase gene targeting to adipocytes attenuates adiposity and vascular dysfunction in mice fed a high-fat diet. Hypertension. 2012;60:467-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 121. | Khitan Z, Harsh M, Sodhi K, Shapiro JI, Abraham NG. HO-1 Upregulation Attenuates Adipocyte Dysfunction, Obesity, and Isoprostane Levels in Mice Fed High Fructose Diets. J Nutr Metab. 2014;2014:980547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 122. | Abraham NG. Gene targeting and heme oxygenase-1 expression in prevention of hypertension induced by angiotensin II. Hypertension. 2008;52:618-620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 123. | Abraham NG, Kappas A. Mechanism of heme-heme oxygenase system impairment of endothelium contraction in the spontaneously hypertensive rat. Hypertension. 2011;58:772-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 124. | Li Z, Zhang Z, Xie JX, Li X, Tian J, Cai T, Cui H, Ding H, Shapiro JI, Xie Z. Na/K-ATPase mimetic pNaKtide peptide inhibits the growth of human cancer cells. J Biol Chem. 2011;286:32394-32403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 125. | Li Z, Cai T, Tian J, Xie JX, Zhao X, Liu L, Shapiro JI, Xie Z. NaKtide, a Na/K-ATPase-derived peptide Src inhibitor, antagonizes ouabain-activated signal transduction in cultured cells. J Biol Chem. 2009;284:21066-21076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 116] [Article Influence: 7.3] [Reference Citation Analysis (0)] |