Published online Nov 23, 2015. doi: 10.5494/wjh.v5.i4.129
Peer-review started: June 10, 2015
First decision: July 10, 2015
Revised: September 30, 2015
Accepted: October 23, 2015
Article in press: October 27, 2015
Published online: November 23, 2015
Processing time: 163 Days and 19.4 Hours
AIM: To investigate the associations of dietary acid-base load with prevalent and incident hypertension in community-living Chinese older adults in Hong Kong.
METHODS: Participants aged ≥ 65 years participating in a cohort study examining the risk factors for osteoporosis completed a validated food frequency questionnaire (FFQ) at baseline between 2001 and 2003. Estimated net endogenous acid production (NEAP) was calculated using Frassetto’s method based on the diet’s protein to potassium ratio derived from the FFQ. Prevalent and 4-year incident hypertension was defined as systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg and/or self-reported use of anti-hypertensive medications. Multivariable logistic regression was used for cross-sectional analysis (n = 3956) to assess the association between estimated NEAP and prevalent hypertension, and for longitudinal analysis (n = 795) on its association with 4-year incident hypertension, with adjustment for various potential socio-demographic and lifestyle factors.
RESULTS: Median estimated NEAP of the participants was 47.7 (interquartile range: 36.2, 60.9) g/mEq. Participants in the highest quartile of energy-adjusted estimated NEAP was associated with increased likelihood of prevalent hypertension than those in the lowest quartile of energy-adjusted estimated NEAP [multivariable OR = 1.66 (95%CI: 1.22 to 2.26, Ptrend = 0.002)]. No significant association was observed between energy-adjusted estimated NEAP and risk of incident hypertension.
CONCLUSION: A high dietary acid load was independently associated with an increased likelihood of prevalent hypertension in ambulant older Chinese people in Hong Kong. The longitudinal analyses failed to show any causal relationship between dietary acid load and hypertension in this population.
Core tip: This prospective study investigated the associations between baseline dietary acid-base load and prevalent and 4-year incident hypertension in community-dwelling Chinese older adults in Hong Kong. Baseline dietary data were collected using a validated food frequency questionnaire (FFQ). Estimated dietary net endogenous acid production (NEAP) was calculated based on the diet’s protein to potassium ratio from the FFQ. Higher quartile of energy-adjusted estimated NEAP was associated with increased likelihood of prevalent hypertension [multivariable OR = 1.66 (95%CI: 1.22 to 2.26, Ptrend = 0.002)]. No significant association was observed between energy-adjusted estimated NEAP and risk of incident hypertension.
- Citation: Chan R, Leung J, Woo J. Estimated net endogenous acid production and risk of prevalent and incident hypertension in community-dwelling older people. World J Hypertens 2015; 5(4): 129-136
- URL: https://www.wjgnet.com/2220-3168/full/v5/i4/129.htm
- DOI: https://dx.doi.org/10.5494/wjh.v5.i4.129
Hypertension is a global health challenge in view of its prevalence and burden on morbidity and mortality. Diet is one of the modifiable factors affecting blood pressure and hypertension[1]. A diet high in sodium content and low in potassium, calcium and magnesium intake is associated with an elevated blood pressure[1,2]. Other dietary approaches, like The Dietary Approaches to Stop Hypertension diet also play a prominent role in the etiology of hypertension[3].
A possible link between acid-base balance and cardiometabolic risk has been recently proposed[4]. Long-term excessive intake of acid-generating foods, like meat together with an inadequate consumption of the alkaline-producing foods, like fruits and vegetables may cause acidosis and have negative effects on blood pressure and hypertension[4]. However, there have been few studies investigating how dietary acidity was related to hypertension. Dietary acidity was positively linked with blood pressure in healthy young women[5], middle-aged women[6] as well as healthy children and adolescents[7,8]. In contrast, no association of baseline dietary acidity with incident hypertension was observed among Western older adults[9,10].
With ageing, the body’s ability to excrete acid drops to a great extent because of a decline in kidney function[11]. Therefore, consuming diets that induce minimal or no net acid load may be particularly vital when people are getting old. More importantly, the prevalence of hypertension rises with age, and recent data from China show a high prevalence of hypertension (58.2%) for the older adults as compared to the younger adults (17.5%)[12]. Therefore, identifying modifiable lifestyle factors that are associated with hypertension is important to determine the effective way for hypertension prevention and control. Considering the scanty evidences on this area and the differences in the dietary habits between Chinese and Caucasians, we explored how dietary acid-base load was linked with prevalent and incident hypertension in Chinese ambulatory older people. We expected that higher dietary acidity was linked with an elevated risk of hypertension.
The sample population was subjects from a longitudinal study investigating the risk factors for osteoporosis in Hong Kong and the study details have been reported elsewhere[13]. Briefly, 4000 Chinese (50% men) aged ≥ 65 years were recruited in a community health survey between 2001 and 2003. They attended the 4-year follow-up between 2005 and 2007. The 4-year follow-up was carried out through a mailed reminder and phone reminders for a follow-up health check appointment. The average follow-up year was 4 years. This study was carried out according to the Declaration of Helsinki. This study was granted an approval from the Clinical Research Ethics Committee of the Chinese University of Hong Kong. All subjects provided written informed consent.
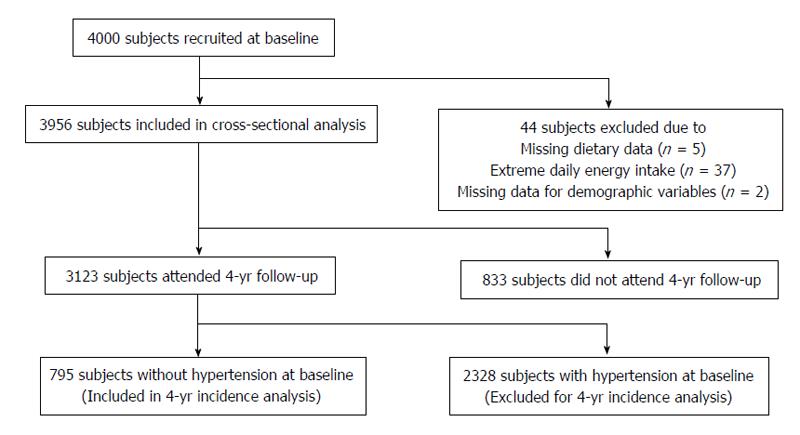
Forty-four participants were excluded because of baseline missing/invalid data on diet or demographics. A final sample of 3956 participants was included for the cross-sectional analysis. The 4-year longitudinal analysis further excluded participants with hypertension at baseline, thus 795 participants were finally included (Figure 1).
A structured interview was done to capture data on age, gender, education achievement, smoking habit, alcohol use and self-reported health conditions. Smoking status was categorized into three categories, namely former (100 or over cigarettes smoked in a lifetime), current or never. Alcohol use was categorized as never, past or current. Subjects self-reported their health conditions and the research staff validated the data by checking the relevant physician’s reports and the medications used.
The Physical Activity Scale of the Elderly (PASE) was used to assess the physical activity level[14]. The scale consists of twelve items and measures the average time (in hours) each day on leisure, household and occupational physical activities by participants in the past week. Higher summary scores indicates higher daily level of physical activity.
Baseline dietary intake was evaluated with a validated semi-quantitative food frequency questionnaire (FFQ)[15]. The FFQ consisted of 280 food items. Participants reported the frequency and the amount of consumption of each food over the previous year. Nine frequency categories were presented and ranged from never or seldom to more than once a day. A food photo album with pictures of standardized food portion size was presented to assist quantifying the amount of food consumption. Daily intake of various food groups covering cereals, egg and egg products, marine foods, fresh or dried fruits, legumes/nuts/seeds, meat and poultry, dairy and dairy products, and vegetables was derived. Average daily nutrient intake was generated with food composition tables of various sources[16,17]. Residual method was applied to generate energy-adjusted intakes[18].
Estimated net endogenous acid production (NEAP) of diet can be derived using different algorithms[19]. While Frassetto et al[20] derived the dietary estimated NEAP with reference to the dietary protein to potassium ratio, Remer et al[21] calculated the estimated NEAP based on the average intestinal absorption rates of the dietary intake of protein and other minerals and the anthropometry-based estimate for organic acid excretion. Each algorithm has its rationale and pitfalls[22]. Frassetto’s method was applied in the present study to make it consistent with our previous study[23]. The estimated NEAP by this method was expressed using g/mEq and could explain approximate 70% variation in renal net acid excretion[20]. Residual method was also applied to generate the energy-adjusted estimated NEAP[18].
Body weight and height were measured with the Physician Balance Beam Scale (Healthometer, Illinois, United States) and the Holtain Harpenden stadiometer (Holtain Ltd, Crosswell, United Kingdom) respectively. Body mass index (BMI) was calculated.
Trained staff measured participant’s blood pressure using a standard mercury sphygmomanometer (WA Baum Co. Inc., Copiague, NY, United States). The first and fifth Korotkoff phases were measured twice after 5 min rest in the sitting position and the average of the two readings was taken as systolic blood pressure (SBP) and diastolic blood pressure (DBP) respectively. Hypertension was defined as SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg and/or use of anti-hypertensive medication[24]. Participants were asked to bring and show all the drugs he/she was currently using and the interviewer recorded the names, types and doses accordingly.
SPSS version 21.0 (SPSS Inc., Illinois, United States) was used for the statistical analyses. Normality was checked using histograms and logarithmic transformation was performed where necessary. Independent t test and χ2 test were applied to check for differences in baseline characteristics between participants included and participants excluded for data analysis.
Since the distribution of the energy-adjusted estimated NEAP (continuous) was skewed, it was categorized using quartile values according to the distribution of the final sample. Differences across energy-adjusted estimated NEAP quartiles were checked using χ2 test for categorical variables and analysis of variance for continuous variables unless otherwise specified. Spearman’s correlation was applied for assessing the correlation of energy-adjusted nutrient intakes or food group intakes with the estimated NEAP.
Multivariable logistic regression was performed to calculate the odds ratio (OR) and 95%CIs for prevalent hypertension as well as 4-year incident hypertension according to the energy-adjusted estimated NEAP quartiles. The first model was controlled for age (years) and sex at baseline. The second model was further controlled for baseline BMI, PASE, education attainment, tobacco use, alcohol use, and baseline energy-adjusted intakes of fiber, sodium, magnesium, calcium and potassium. Ptrend was assessed by inputting quartiles of energy-adjusted estimated NEAP into all models. Since participants might have made dietary changes due to chronic diseases, several sensitivity analyses were further done by ruling out participants with a history of stroke, diabetes mellitus, or heart diseases, such as myocardial infarction. We also examined if the association between estimated NEAP and hypertension varied according to sex, age (≤ 69 years vs > 69 years), and BMI (underweight < 18.5 kg/m2vs normal 18.5 kg/m2 to < 23 kg/m2vs overweight/obese ≥ 23 kg/m2). Stratified multivariable analyses were also done and appropriate interaction terms were generated to test for the presence of signification interactions. An α level of 5%, 2-sided was considered as statistically significant.
There were no significant differences in the baseline characteristics between participants who were included and those who were excluded for baseline analysis. Those who did not attend 4-year follow-up were older, physically less active, had lower education level and lower BMI, and suffered from more chronic diseases (P < 0.05) than those who attended the follow-up (details not listed).
Mean (SD) baseline age of the studied sample (1979 men, 1977 women) was 72.5 (5.2) years. Mean (SD) baseline BMI was 23.7 (3.3) kg/m2. Mean (SD) baseline SBP and DBP was 142.6 (19.4) mmHg and 77.8 (9.2) mmHg respectively. Majority (75.2%) of the participants had hypertension at baseline. Among 795 participants included in the incidence analysis, 310 incident cases were identified and the cumulative incidence was 0.39. Median baseline estimated NEAP was 47.7 (interquartile range: 36.2, 60.9) g/mEq. Participants’ baseline characteristics according to the quartiles of energy-adjusted estimated NEAP are listed in Table 1. Those with higher energy-adjusted estimated NEAP were of lower BMI, physically more sedentary, higher education attainment, and were prone to be non-smokers, and had lower dietary intakes of fiber, magnesium, potassium and sodium.
| Variable | Quartile of energy adjusted estimated NEAP (g/mEq) | Ptrend1 | |||||||
| Q1 (n = 987) | Q2 (n = 991) | Q3 (n = 989) | Q4 (n = 989) | ||||||
| Mean | SD, % | Mean | SD, % | Mean | SD, % | Mean | SD, % | ||
| 2Estimated NEAP (g/mEq) | |||||||||
| Original | 26.9 | 21.3, 32.7 | 41.2 | 37.0, 45.7 | 53.4 | 48.7, 58.2 | 71.2 | 64.5, 79.7 | < 0.001 |
| Energy adjusted | 28.7 | 23.8, 32.7 | 41.8 | 39.0, 44.7 | 54.0 | 50.7, 57.3 | 71.2 | 65.7, 79.8 | < 0.001 |
| Age (yr) | 72.3 | 4.9 | 72.4 | 5.3 | 72.5 | 5.2 | 72.7 | 5.4 | 0.055 |
| BMI (kg/m2) | 23.8 | 3.3 | 23.7 | 3.3 | 23.6 | 3.3 | 23.5 | 3.3 | 0.027 |
| Male (%) | 50.1 | 49.9 | 50.1 | 50.1 | 0.989 | ||||
| Education level (%) | |||||||||
| Primary or below | 77.7 | 72.4 | 67.2 | 68.8 | < 0.001 | ||||
| Secondary/matriculation | 16.8 | 19.0 | 19.8 | 19.4 | |||||
| University or above | 5.5 | 8.7 | 12.9 | 11.8 | |||||
| Smoking habit (%) | |||||||||
| Never smoke | 58.5 | 62.6 | 65.4 | 66.5 | < 0.001 | ||||
| Former smoker | 33.0 | 31.1 | 29.3 | 26.1 | |||||
| Current smoker | 8.5 | 6.4 | 5.3 | 7.4 | |||||
| Alcohol use (%) | |||||||||
| Never | 84.1 | 84.8 | 84.6 | 86.6 | 0.155 | ||||
| Former drinker | 2.4 | 1.4 | 1.6 | 2.1 | |||||
| Current drinker | 13.5 | 13.8 | 13.8 | 11.3 | |||||
| 3Prevalent hypertension (%) | 72.8 | 74.9 | 75.2 | 77.8 | 0.014 | ||||
| Energy intake (kcal/d) | 1832.5 | 587.3 | 1847.5 | 555.1 | 1848.6 | 568.7 | 1821.6 | 561.2 | 0.696 |
| 2Energy adjusted fiber (g/d) | 9.1 | 7.0, 11.7 | 8.9 | 6.8, 11.2 | 8.5 | 6.6, 10.5 | 7.5 | 5.7, 8.9 | < 0.001 |
| 2Energy adjusted calcium (mg/d) | 535.6 | 418.9, 692.4 | 573.6 | 452.3, 725.8 | 590.7 | 460.1, 763.1 | 547.5 | 418.8, 726.2 | 0.098 |
| 2Energy adjusted magnesium (mg/d) | 358.5 | 297.9, 487.8 | 356.6 | 292.7, 471.5 | 344.4 | 291.2, 434.8 | 312.0 | 262.1, 373.2 | < 0.001 |
| 2Energy adjusted potassium (mg/d) | 3782.3 | 3180.4, 4507.6 | 2977.9 | 2536.0, 3437.3 | 2462.8 | 2092.3, 2935.4 | 2021.0 | 1677.8, 2440.5 | < 0.001 |
| 2Energy adjusted sodium (mg/d) | 1453.4 | 1036.9, 1963.4 | 1379.7 | 1040.7, 1886.0 | 1345.6 | 1030.3, 1793.3 | 1254.3 | 930.1, 1651.4 | < 0.001 |
| PASE score | 93.2 | 43.6 | 92.5 | 42.4 | 91.2 | 43.0 | 88.4 | 42.9 | 0.011 |
Estimated NEAP was positively correlated with total protein, calcium and phosphorus intake, and inversely linked with vitamin C, fiber, magnesium, vitamin K and potassium intake (P < 0.05, Table 2). Increasing estimated NEAP was linked with greater intake of protein rich animal foods, and lower fruits and vegetables consumption (P < 0.05, Table 2).
| Energy adjusted nutrients/ | Energy adjusted estimated NEAP (g/mEq) | |
| main food groups | rs | P |
| Total protein (g) | 0.27 | < 0.001 |
| Vitamin C (mg) | -0.27 | < 0.001 |
| Calcium (mg) | 0.04 | 0.015 |
| Phosphorus (mg) | 0.16 | < 0.001 |
| Fiber (g) | -0.23 | < 0.001 |
| Magnesium (mg) | -0.20 | < 0.001 |
| Vitamin K (mcg) | -0.17 | < 0.001 |
| Potassium (mg) | -0.68 | < 0.001 |
| Sodium (mg) | -0.10 | < 0.001 |
| Cereals (g) | -0.03 | 0.063 |
| Egg and egg products (g) | 0.03 | 0.034 |
| Fish and shellfish (g) | 0.07 | < 0.001 |
| Fruits and dried fruits (g) | -0.31 | < 0.001 |
| Legumes, seeds and nuts (g) | 0.01 | 0.678 |
| Meat and poultry (g) | 0.13 | < 0.001 |
| Milk and milk products (g) | 0.03 | 0.041 |
| Vegetables (g) | -0.18 | < 0.001 |
Participants in the highest quartile of energy-adjusted estimated NEAP had significantly increased likelihood of having prevalent hypertension than those in the lowest quartile in unadjusted and adjusted models (Table 3). The multivariable OR comparing those in the highest quartile with those in the lowest quartile was 1.66 (95%CI: 1.22 to 2.26, Ptrend = 0.002). Although increasing trend was detected between energy-adjusted estimated NEAP and risk of incident hypertension, the trend did not reach statistical significance (Table 4).
| Quartiles of energy adjusted estimated NEAP (g/mEq) | Ptrend1 | ||||
| Q1 (n = 987) | Q2 (n = 991) | Q3 (n = 989) | Q4 (n = 989) | ||
| No. of case and control | 719/268 | 742/249 | 744/245 | 769/220 | |
| Unadjusted OR (95%CI) | 1 (reference) | 1.11 (0.91-1.36) | 1.13 (0.93-1.38) | 1.30 (1.06-1.60) | 0.014 |
| Age and sex adjusted OR (95%CI) | 1 (reference) | 1.11 (0.91-1.36) | 1.13 (0.92-1.38) | 1.29 (1.05-1.58) | 0.020 |
| 2Multivariable adjusted OR (95%CI) | 1 (reference) | 1.22 (0.97-1.52) | 1.35 (1.03-1.76) | 1.66 (1.22-2.26) | 0.002 |
| Quartiles of energy adjusted estimated NEAP (g/mEq) | Ptrend1 | ||||
| Q1 (n = 198) | Q2 (n = 201) | Q3 (n = 198) | Q4 (n = 198) | ||
| No. of case and control | 78/120 | 80/121 | 75/123 | 77/121 | |
| Unadjusted OR (95%CI) | 1 (reference) | 1.02 (0.68-1.52) | 0.94 (0.63-1.41) | 0.98 (0.65-1.47) | 0.842 |
| Age and sex adjusted OR (95%CI) | 1 (reference) | 1.03 (0.69-1.54) | 0.95 (0.63-1.43) | 0.99 (0.66-1.49) | 0.873 |
| 2Multivariable adjusted OR (95%CI) | 1 (reference) | 1.15 (0.74-1.80) | 1.11 (0.66-1.86) | 1.32 (0.72-2.43) | 0.436 |
Sensitivity analyses ruling out participants with some major chronic diseases showed similar results (details not listed). Risk estimates for the relationship of estimated NEAP with prevalent hypertension tended to be higher in male, in those aged ≥ 69 years, and in those with BMI below 18.5 kg/m2, but the differences did not reach statistical significance (all with P-interaction > 0.05) (details not listed).
Our study indicated that higher estimated NEAP was associated with greater likelihood of prevalent hypertension but was not with the risk of incident hypertension in older Chinese adults. To our knowledge, such association in Chinese population has not been previously reported.
Few studies examined the link between dietary acid-base load and hypertension risk in older adults. Our cross-sectional findings were consistent with those reported in healthy children and adolescents[7] and young women[5], but different from those reported among community-based older Swedish men[10]. A cross-sectional analysis in 267 healthy children and adolescents showed that various markers of a higher dietary acidity were associated with higher blood pressure independent of BMI and other potential factors[7]. Similar findings were reported from a cross-sectional study investigating the relationship of dietary acid-base load with cardiometabolic risk factors in apparently healthy young female adults[5]. Several possible mechanisms by which acid-base balance affects blood pressure have been suggested. Diet-induced mild metabolic acidosis may influence blood pressure possibly through increased cortisol production[25], increased calcium excretion[26,27] or reduced citrate excretion[28,29].
The absence of association in our prospective analysis was in line with the results by Engberink et al[9] and Luis et al[10] but was different from the findings by Zhang et al[6]. Several reasons may explain these inconclusive findings. Different study design and participants’ characteristics may lead to these mixed findings. First, both Engberink’s and Luis as well as our studies included older men and women whereas Zhang et al[6] recruited middle-aged women in their study. However, this age difference seems to be unlikely to explain the null findings as older people are expected to be more vulnerable to dietary acid base load in view of their declining renal function. In contrast, we are uncertain whether there is age-dependent difference regarding the influence of dietary acidity on hypertension through other mechanisms. Second, multiple measures of dietary intakes were available in Zhang’s study whereas dietary data were only collected at a single time at baseline in Engberink’s study, Luis’s study and our study. Although sensitivity analyses not including participants with chronic diseases that might lead to dietary alterations did not change the null findings between estimated NEAP and incident hypertension in Engberink’s and our studies, we cannot rule out the possibility that some participants might have changed their diets for other reasons during the follow-up. Third, different dietary habits of the study participants of various studies might explain these different findings. The median estimated NEAP in our study (approximately 47 mEq/d), Engberink’s study (39 mEq/d) and Luis’s study (40.7 mEq/d) might be too low to have an effect on blood pressure. Zhang et al[6] showed an elevated risk of incident hypertension starting at an estimated NEAP of 44 mEq/d or above.
Several methods have been derived to estimate dietary NEAP, thus we compared the Frassetto’s model with the Remer’s model. Estimated NEAP derived from both methods were strongly correlated (rs = 0.95, P < 0.001). By including both protein and potassium as independent variables in a multivariate regression model, estimated NEAP derived using the latter varied proportionally with the protein intake (P < 0.001) and inversely with the potassium intake (P < 0.001). The multiple correlation coefficient was 0.95. Therefore, the Remer’s model’s ability in predicting estimated NEAP was mainly from the dietary protein and potassium contents. Furthermore, the results were consistent when all analyses were repeated using estimated NEAP by the Remer’s model.
Our study had some limitations. Unlike previous similar studies among older adults, our study did not have data on kidney function but kidney function was unlikely to modify the associations between dietary acid load and blood pressure[10]. Moreover, recall bias may arise from dietary data captured using FFQ. The assessment of salt intake using FFQ but not using 24-h urine method was a limitation. Dietary assessment of the salt intake, in particular the discretionary salt intake often results in an underestimation and this may partially account for the unexpected inverse association between estimated NEAP and sodium intake in our study. Another possibility may be due to the differences in the way of serving vegetables in Chinese diets as compared to Western diets. While vegetables are commonly served raw or boiled in Western diets, stir fried vegetables with salt added during cooking is a common way of serving vegetables in Chinese diets[30]. Furthermore, we did not have dietary data at 4 years whereas participants might have altered their diet over the 4 years period. We performed sensitivity analyses ruling out participants with major chronic diseases and the results were similar. Moreover, we did not include markers, like serum anion gap or bicarbonate in the study, which are more reflective of acid base load. Although we controlled for different factors in the analysis, residual potential confounding from other factors related to hypertension, like family history and sleep patterns might still be present. The differences in demographic and lifestyle characteristics between those included and those excluded for the analysis, and between those who attended and those who did not attend the follow-up may also limit the study generalizability. Finally, our study may be underpowered in view of the small sample size for the prospective analysis.
In summary, our results suggest an increased likelihood of prevalent hypertension in older adults with an elevated dietary acid load. However, as limited by the small number of the participants and the study methodology, our prospective analyses were unable to demonstrate a causal relationship between dietary acid load and hypertension in this population. Further longitudinal studies in populations of different dietary habits are required to confirm the influence of dietary acid load on hypertension. Moreover, the underlying mechanisms linking dietary acid-base load and blood pressure require further investigations.
We wish to thank all subjects for their participation and Dr. Edith Lau for her contribution in setting up the cohort.
Long-term excessive intake of acid-generating foods in combination with a low intake of the alkalizing fruits and vegetables may lead to acidosis and have negative effects on blood pressure and hypertension. The authors studied the association of dietary acid-base load with risk of prevalent and incident hypertension in Chinese community-dwelling older adults in Hong Kong. The authors speculated that higher dietary acid-base load was associated with an elevated risk of hypertension.
Diet is one of the modifiable factors for blood pressure and hypertension. The ability to excrete acid drops significantly with age because of a decline in kidney function. Therefore, consuming diets that induce minimal or no net acid load may be vital at the older age, and identifying modifiable lifestyle factors that are associated with hypertension is important to determine the effective way for hypertension prevention and control.
There have been few studies investigating the association between dietary acid-base load and hypertension. Positive associations have been reported in healthy young women, middle-aged women as well as healthy children and adolescents. Negative findings were observed among Western older adults. However, no such study has been conducted in Chinese population. In this study, data from participants aged 65 years or above participating in a cohort study examining the risk factors for osteoporosis at baseline and 4-year follow-up were examined. Baseline estimated net endogenous acid production (NEAP) was calculated based on the diet’s protein to potassium ratio derived from the Food Frequency Questionnaire and was related to the hypertension status at baseline (n = 3956) and 4-year follow-up (n = 795). The authors’ findings show that participants in the highest quartile of energy-adjusted estimated NEAP was associated with increased likelihood of prevalent hypertension than those in the lowest quartile of energy-adjusted estimated NEAP [multivariable OR = 1.66 (95%CI: 1.22 to 2.26, Ptrend = 0.002)]. No significant association was observed between energy-adjusted estimated NEAP and risk of incident hypertension.
This study serves as an additional evidence supporting the potential link between dietary acid-base load and hypertension. The authors’ findings show that a diet lower in dietary acid-base load might be beneficial for lowering risk of hypertension. However, further prospective studies in populations with different dietary habits are warranted to confirm the role of dietary acid-base balance in hypertension as well as the underlying mechanisms linking dietary acid-base load to blood pressure.
Estimated NEAP: A diet’s net acid load that is estimated from the composition of the diet based on collected dietary data; Hypertension: Abnormally high blood pressure.
The authors had declared some of the limitation in which may affects the generalizability of this study, such as the differences in demographic and lifestyle characteristics between those included and those exclude for the analysis; and vegetables intake of those participants in their study groups. It is also an interesting prospective cohort study which may accept for publication.
P- Reviewer: Cosmi E, Wong KL S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Woo J. Relationships among diet, physical activity and other lifestyle factors and debilitating diseases in the elderly. Eur J Clin Nutr. 2000;54 Suppl 3:S143-S147. [PubMed] |
| 2. | Miura K, Okuda N, Turin TC, Takashima N, Nakagawa H, Nakamura K, Yoshita K, Okayama A, Ueshima H. Dietary salt intake and blood pressure in a representative Japanese population: baseline analyses of NIPPON DATA80. J Epidemiol. 2010;20 Suppl 3:S524-S530. [PubMed] |
| 3. | Bazzano LA, Green T, Harrison TN, Reynolds K. Dietary approaches to prevent hypertension. Curr Hypertens Rep. 2013;15:694-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 4. | Adeva MM, Souto G. Diet-induced metabolic acidosis. Clin Nutr. 2011;30:416-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 207] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 5. | Murakami K, Sasaki S, Takahashi Y, Uenishi K. Association between dietary acid-base load and cardiometabolic risk factors in young Japanese women. Br J Nutr. 2008;100:642-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 115] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 6. | Zhang L, Curhan GC, Forman JP. Diet-dependent net acid load and risk of incident hypertension in United States women. Hypertension. 2009;54:751-755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Krupp D, Shi L, Maser-Gluth C, Pietzarka M, Remer T. 11β Hydroxysteroid dehydrogenase type 2 and dietary acid load are independently associated with blood pressure in healthy children and adolescents. Am J Clin Nutr. 2013;97:612-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Krupp D, Shi L, Remer T. Longitudinal relationships between diet-dependent renal acid load and blood pressure development in healthy children. Kidney Int. 2014;85:204-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Engberink MF, Bakker SJ, Brink EJ, van Baak MA, van Rooij FJ, Hofman A, Witteman JC, Geleijnse JM. Dietary acid load and risk of hypertension: the Rotterdam Study. Am J Clin Nutr. 2012;95:1438-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 10. | Luis D, Huang X, Riserus U, Sjögren P, Lindholm B, Arnlöv J, Cederholm T, Carrero JJ. Estimated dietary acid load is not associated with blood pressure or hypertension incidence in men who are approximately 70 years old. J Nutr. 2015;145:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Frassetto LA, Morris RC, Sebastian A. Effect of age on blood acid-base composition in adult humans: role of age-related renal functional decline. Am J Physiol. 1996;271:F1114-F1122. [PubMed] |
| 12. | Wang J, Zhang L, Wang F, Liu L, Wang H. Prevalence, awareness, treatment, and control of hypertension in China: results from a national survey. Am J Hypertens. 2014;27:1355-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 304] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 13. | Wong SY, Kwok T, Woo J, Lynn H, Griffith JF, Leung J, Tang YY, Leung PC. Bone mineral density and the risk of peripheral arterial disease in men and women: results from Mr. and Ms Os, Hong Kong. Osteoporos Int. 2005;16:1933-1938. [PubMed] |
| 14. | Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153-162. [PubMed] |
| 15. | Woo J, Leung SSF, Ho SC, Lam TH, Janus ED. A food frequency questionnaire for use in the Chinese population in Hong Kong: Description and examination of validity. Nutr Res. 1997;17:1633-1641. [RCA] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 131] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Paul AA, Southgate DAT. McCance & Widdowson’s: The Composition of Foods. 4th ed. London: HMSO 1978; . |
| 17. | Yang Y, Wang G, Pan X. China Food Composition 2002. 2002 ed. Peking: University Medical Press 2002; . |
| 18. | Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:1220S-1228S; discussion 1229S-1231S. [PubMed] |
| 19. | Frassetto LA, Lanham-New SA, Macdonald HM, Remer T, Sebastian A, Tucker KL, Tylavsky FA. Standardizing terminology for estimating the diet-dependent net acid load to the metabolic system. J Nutr. 2007;137:1491-1492. [PubMed] |
| 20. | Frassetto LA, Todd KM, Morris RC, Sebastian A. Estimation of net endogenous noncarbonic acid production in humans from diet potassium and protein contents. Am J Clin Nutr. 1998;68:576-583. [PubMed] |
| 21. | Remer T, Dimitriou T, Manz F. Dietary potential renal acid load and renal net acid excretion in healthy, free-living children and adolescents. Am J Clin Nutr. 2003;77:1255-1260. [PubMed] |
| 22. | Frassetto LA, Morris RC, Sebastian A. A practical approach to the balance between acid production and renal acid excretion in humans. J Nephrol. 2006;19 Suppl 9:S33-S40. [PubMed] |
| 23. | Chan RS, Woo J, Chan DC, Cheung CS, Lo DH. Estimated net endogenous acid production and intake of bone health-related nutrients in Hong Kong Chinese adolescents. Eur J Clin Nutr. 2009;63:505-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520. [PubMed] |
| 25. | Maurer M, Riesen W, Muser J, Hulter HN, Krapf R. Neutralization of Western diet inhibits bone resorption independently of K intake and reduces cortisol secretion in humans. Am J Physiol Renal Physiol. 2003;284:F32-F40. [PubMed] |
| 26. | Cappuccio FP, Kalaitzidis R, Duneclift S, Eastwood JB. Unravelling the links between calcium excretion, salt intake, hypertension, kidney stones and bone metabolism. J Nephrol. 2000;13:169-177. [PubMed] |
| 27. | Oshima T, Young EW. Systemic and cellular calcium metabolism and hypertension. Semin Nephrol. 1995;15:496-503. [PubMed] |
| 28. | Taylor EN, Mount DB, Forman JP, Curhan GC. Association of prevalent hypertension with 24-hour urinary excretion of calcium, citrate, and other factors. Am J Kidney Dis. 2006;47:780-789. [PubMed] |
| 29. | Mandel EI, Taylor EN, Curhan GC. Dietary and lifestyle factors and medical conditions associated with urinary citrate excretion. Clin J Am Soc Nephrol. 2013;8:901-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Albala K. Food cultures of the world encyclopedia. Santa Barbara, California: Greenwood 2011; . |