Published online Nov 20, 2013. doi: 10.5493/wjem.v3.i4.100
Revised: July 12, 2013
Accepted: September 18, 2013
Published online: November 20, 2013
Processing time: 190 Days and 7.2 Hours
AIM: To investigate the (-)-epigallocatechin-3-gallate (EGCG) binding to transforming growth factor-β (TGF-β) type II receptor (TGFRII).
METHODS: The expression of α-smooth muscle actin (α-SMA) was used as a marker for fibrotic change in human lung fibroblast MRC-5 cells. The α-SMA expression level was determined by western blotting and immunohistological analysis. We examined whether the anti-fibrotic effects of EGCG on MRC-5 cells was dependent on antioxidant mechanism by using edaravone and N-acetylcysteine (NAC). The suppression effects of EGCG on Smad2/3 activation were studied by confocal fluorescence microscopy. The binding of EGCG to recombinant TGFRII protein was analyzed by immunoprecipitation and affinity chromatography.
RESULTS: When MRC-5 cells were treated with TGF-β, EGCG decreased the expression of α-SMA in a dose dependent manner, whereas catechin did not influence the α-SMA expression in the cells. Except for EGCG, antioxidant compounds (e.g., edaravone and NAC) had no effects on the TGF-β-induced α-SMA expression. Nuclear localization of phosphorylated Smad2/3 was observed after TGF-β treatment; however, EGCG treatment attenuated the nuclear transportation of Smad2/3 in the presence or absence of TGF-β. After a TGFRII expression vector was introduced into COS-7 cells, cell lysates were untreated or treated with EGCG or catechin. The immunoprecipitation experiments using the lysates showed that EGCG dose-dependently bound to TGFRIIand that catechin did not at all. Affinity chromatography study indicated that EGCG would bind to TGFRII.
CONCLUSION: Our results demonstrate that EGCG interacts with TGFRII and inhibits the expression of α-SMA via the TGF-β-Smad2/3 pathway in human lung fibroblast MRC-5 cells.
Core tip: (-)-Epigallocatechin-3-gallate (EGCG) binds to transforming growth factor-β (TGF-β) type II receptor (TGFRII) and inhibits TGF-β action by interfering with the interaction between TGF-β and TGFRII. Because TGF-β is considered to be the strongest inducer of tissue fibrosis, the obtained data from this investigation suggest that EGCG may be a new therapeutic agent for organ fibrosis.
- Citation: Tabuchi M, Hayakawa S, Honda E, Ooshima K, Itoh T, Yoshida K, Park AM, Higashino H, Isemura M, Munakata H. Epigallocatechin-3-gallate suppresses transforming growth factor-beta signaling by interacting with the transforming growth factor-beta type II receptor. World J Exp Med 2013; 3(4): 100-107
- URL: https://www.wjgnet.com/2220-315X/full/v3/i4/100.htm
- DOI: https://dx.doi.org/10.5493/wjem.v3.i4.100
(-)-epigallocatechin-3-gallate (EGCG), the most biologically active constituent in green tea, has been recognized as a component that provides the beverage with potential benefits for human health[1]. The reported health-promoting properties of green tea include anti-cancer[1-3], anti-obesity[4], anti-diabetic[5,6], anti-atherosclerotic[7], anti-viral[8-10], anti-bacterial[11-13] and neuroprotective[14-16] effects. The anti-fibrotic effects of green tea and its constituents, especially EGCG, on liver fibrosis[17-19], pancreatic fibrosis[20] and pulmonary fibrosis[21] have been also reported.
Activation of myofibroblasts is the one of the critical events during fibrosis development. Transforming growth factor-beta (TGF-β) is a multifunctional cytokine that is pivotal in the regulation of myofibroblast activation, differentiation, migration, and extracellular matrix production; it also plays an important role in the initiation and progression of fibrosis[22]. However, the mechanisms by which EGCG influences TGF-β action on myofibroblast activation remain incompletely defined.
Tachibana et al[23] identified a catechin receptor for EGCG, and showed that this receptor partially mediates the function of EGCG. It is also known that EGCG shows its biological action by interacting with receptors other than the catechin receptor[24,25]. In the present study, we investigated the possibility that EGCG might bind to the TGF-β type II receptor (TGFRII).
The MRC-5 and COS-7 cell lines were obtained from the Riken Cell Bank (Tsukuba, Japan), and were maintained in Dullbecco’s modified Eagle’s medium (DMEM) (Sigma, St. Louis, MO, United States) supplemented with 10% fetal bovine serum (FBS) (JRH Biosciences, Lenexa, KS, United States) at 37 °C under 5% carbon dioxide and 95% air.
Catechin and EGCG were obtained from Funakoshi Co. (Tokyo, Japan) and dissolved in PBS. N-acetylcysteine (NAC) was purchased from Wako Pure Chemical Industries Ltd. (Osaka, Japan) and dissolved in dimethyl sulfoxide. Edaravone was the product of Mitsubishi Tanabe Pharma (Osaka, Japan). TGF-β was obtained from R&D Systems (Minneapolis, MN, United States).
The following antibodies were used in this study: monoclonal anti-FLAG antibody produced in mouse (anti-Flag) (Sigma); monoclonal anti-α-smooth muscle actin antibody (anti-α-SMA) produced in mouse (Sigma); monoclonal anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody (anti-GAPDH) produced in mouse (Sigma); rabbit anti-Smad2/3 antibody (anti-Smad2/3) (Cell Signaling Technology, Danvers, MA, United States); and goat anti-human TGFRII antibody (anti-TGFRII), which recognizes extracellular domain of the receptor (R and D).
After washing with ice-cold phosphate buffer saline (PBS), cells were treated with 0.25% trypsin-EDTA solution (Invitrogen, Carlsbad, CA, United States), suspended in growth medium and collected by centrifugation at 700 g for 5 min. The pellets were washed with PBS, resuspended in lysis buffer (20 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 0.1% SDS, 1% Triton X-100, 0.5% sodium deoxycholate), which contained a cocktail of protease inhibitors (Roche Molecular Biochemicals, Mannheim, Germany) on ice, and centrifuged at 18000 g at 4 °C for 10 min.
Protein concentration was determined by a BCA protein assay kit (Pierce, Rockford, IL, United States). Cell lysates were suspended in SDS electrophoresis sample buffer and boiled for 5 min. Samples (2.5 μg of protein per lane) were separated on 10% polyacrylamide gels and then transferred to an Immobilon P membrane (Millipore, Billerica, MA, United States). Antibody binding was detected by ECL Plus (GE Healthcare, Buckinghamshire, United Kingdom).
Cells were seeded on BD Falcon 8-well CultureSlide. Cells were cultured under indicated conditions. Medium was removed, and cells were washed with PBS, fixed by 3% paraformaldehyde in PBS for 10 min. After washing with PBS, cells were permeabilized by 0.1% Triton X-100 in PBS. Fixed cells were sequentially treated with anti-α-smooth muscle actin (SMA) antibody (1/100, 37 °C, 1 h), and fluorescein isothiocyanate conjugated goat anti-mouse immunoglobulin G (37 °C, 30 min). Actin stress fibers were visualized by rhodamine-labeled phalloidin (1/50, 37 °C, 10 min). For staining the nuclei, cells were treated with 4',6-diamidino-2-phenylindole (DAPI) (1 μg/mL) for
20 min. Cells were examined with a fluorescence microscope (Nikon ECLIPSE E-800, Nikon Corporation, Tokyo, Japan) equipped with a fluoresecence digital microscope camera controller (VB-7000; Keyence Co., Osaka, Japan).
Plasmid was constructed according to standard recombinant DNA techniques. The fragment encoding the human TGFRII cDNA (Met1-Lys567, GenBank accession no. M85079) was amplified from a human fetal liver cDNA library (OriGene Technologies, Rockville, MD, United States) by polymerase chain reaction (PCR) with KOD Plus DNA polymerase (Toyobo Co., Ltd., Osaka, Japan) using the primers 5’-TTTGAATTCGCCATGGGTCGGGGGCTGCTC-3’ (forward) and 5’-TTTGGATCCTTTGGTAGTGTTTAGGGAGCC-3’ (reverse). The forward and reverse primers were designed to introduce an EcoRI and a BamHI restriction site (underlined), respectively, for subcloning purposes. The PCR product was cloned into the pFlag-CMV-5a vector (Sigma). The construct was verified by DNA sequencing.
COS-7 cells, grown to 50%-70% confluence, were transfected using Lipofectamine plus (Invitrogen) according to the manufacturer’s instructions. The transfectants were grown in DMEM containing 10% FBS. After 3 d, the medium was removed and expression of TGFRII in the cells was examined by western blotting.
Cell lysates were treated with Protein G Sepharose (GE Healthcare) for 30 min at 4 °C to remove proteins nonspecifically bound to Protein G Sepharose. Anti- TGFRII antibody was then added to the above lysate, and incubated for 2 h at 4 °C. Next, Protein G Sepharose was added and incubated for 1 h at 4 °C. Protein G Sepharose was recovered by centrifugation and washed three times with PBS. The immunoprecipitated proteins were removed from the Protein G Sepharose by boiling in sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer for 5 min and then separated by electrophoresis.
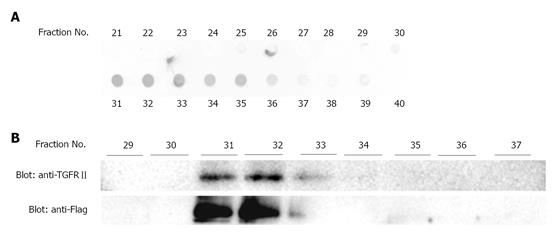
EGCG was coupled to CNBr-activated Sepharose 4B (GE Healthcare) at a concentration of 5 mg/mL of wet gel. Cell lysate was applied to a column of EGCG-Sepharose 4B and washed with PBS. Bound proteins were eluted with 4 mol/L urea, 1 mol/L NaCl in PBS, and fractions of 0.25 mL were collected. An aliquot of each fraction was spotted onto polyvinylidene difluoride (PVDF) membrane and stained with Coomassie Brilliant Blue. A portion of each fraction was also examined by western blot analysis after SDS-PAGE using anti-TGFRII antibody.
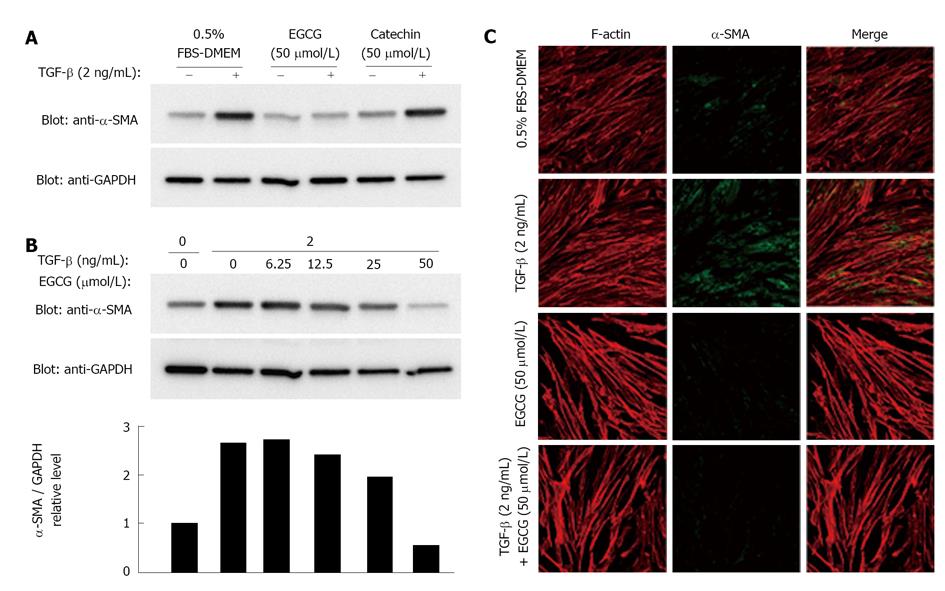
Effects of EGCG on the expression ofα-smooth muscle actin
The MRC-5 cell line, which is derived from human fetal lung fibroblasts, expresses α-SMA and is considered to be a myofibroblast cell line[26,27]. Therefore, this cell line was used in this study.
MRC-5 cells were grown to 85% confluence, and then serum-starved (0.5% FBS) for 48 h. After serum starvation, cells were treated with TGF-β. We and others usually use 1-2 ng/mL of TGF-β in culture media[27-31]. A representative and frequently used marker of myofibroblast activation is α-SMA[32,33]. Western blot analysis and immunohistological examination showed that expression of α-SMA was increased by TGF-β (Figure 1). Whereas a catechin control showed no effects on α-SMA expression, EGCG dose-dependently abolished the increase in expression of α-SMA induced by TGF-β (Figure 1B). The EGCG concentration used in this study was reasonable[34]. The expression of GAPDH also seemed to be decreased by a high dose of EGCG. The band densities of α-SMA and GAPDH were compared (Figure 1B), and the result clearly showed the effects of EGCG on α-SMA.
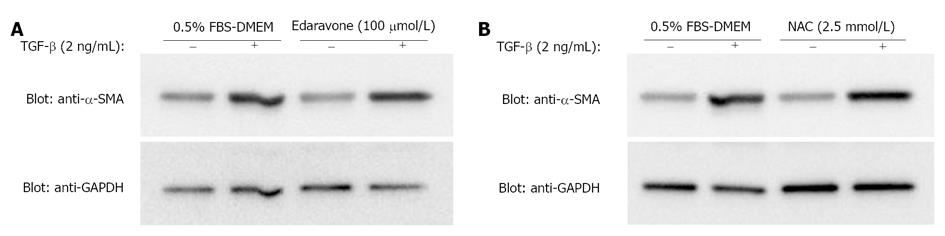
Because EGCG is an antioxidant compound, we examined whether edaravone and NAC, two well-known antioxidant compounds, have similar effects. Neither treatment with edaravone (Figure 2A) nor treatment with NAC (Figure 2B) affected the increase in expression of α-SMA induced by TGF-β.
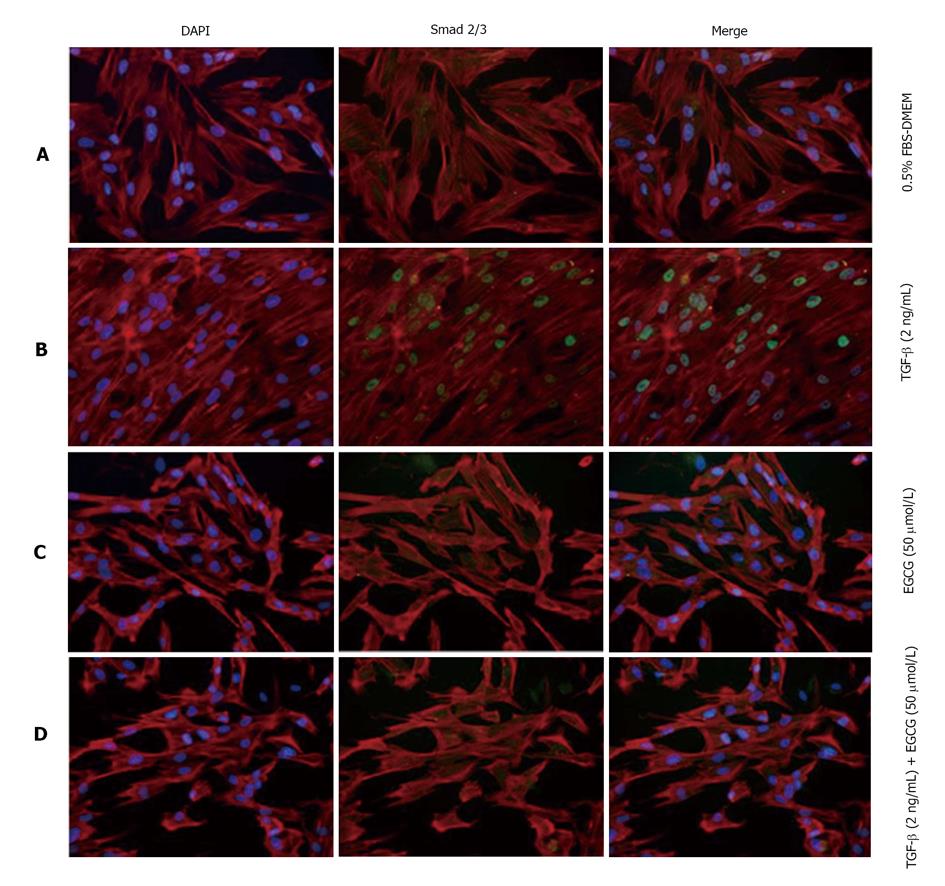
The effects of TGF-β are largely mediated by Smad proteins. TGF-β causes phosphorylation of Smad2/3, and then phosphorylated Smads enter into the nucleus. After treatment with TGF-β, MRC-5 cells were examined by confocal fluorescence microscopy. Nuclear localization of phosphorylated Smad2/3 was observed after TGF-β treatment, whereas EGCG treatment clearly decreased the nuclear transportation of Smad2/3 (Figure 3).
EGCG binds to TGFRII
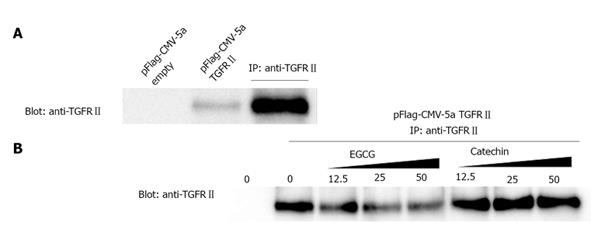
Next, we examined the possibility that EGCG interferes with binding of TGF-β to the TGFRII. To this end, cells expressing large amounts of the receptor are preferable. Because COS-7 cells showed high transformation efficiency and marked expression of exogenous cDNA, these cells were used for transformation experiments. A TGFRII expression vector was introduced into COS-7 cells. Cell lysates were untreated or treated with EGCG or catechin, and then subjected to immunoprecipitation with anti-TGFRII antibody. In untreated lysate and lysate treated with catechin, TGFRII was precipitated by the antibody. When lysate was treated with EGCG, however, anti-TGFR did not precipitate TGFRII (Figure 4).
To confirm the binding of EGCG to TGFRII, we next performed affinity chromatography. Namely, cell lysates were applied to an EGCG-conjugated agarose column and proteins bound to the column were examined by western blotting. Figure 5 shows that TGFRII bound to the column, indicating that EGCG binds to TGFRII.
In this study, we have demonstrated that EGCG both inhibits the signal transduction of TGF-β by binding to TGFRII and attenuates the expression of α-SMA in MRC-5 cells, which is a myofibroblast cell line, when it is stimulated by TGF-β. Myofibroblasts play crucial roles in the pathogenesis of tissue fibrosis[35]. Stimulation by TGF-β and other cytokines leads myofibroblasts to an activated state[36]. Activated myofibroblasts then secrete collagen and other components of the extracellular matrix, which can result in fibrosis[37].
TGF-β is the most potent cytokine causing fibrosis. Both Smad-dependent and Smad-independent TGF-β signaling pathways are known. Initiation of both pathways takes place via binding of TGF-β to its receptor. TGF-β binds to a type II receptor, which then phosphorylates a TGF-β type I receptor. Subsequently, the type I receptor phosphorylates R-Smads (receptor-regulated Smads), and phosphorylated R-Smads bind to Co-Smad (common-mediator Smad). R-Smad/Co-Smad complexes translocate into the nucleus, where they act as transcription factors[38]. In this manner, regulation of TGF-β target gene expression is carried out. Expression of many proteins in MRC-5 cells changes after stimulation by TGF-β[39]. A frequently used marker of the activation of myofibroblast is α-SMA; therefore, this protein was also used as a marker in this study. Expression changes after TGF-β stimulation in cells other than MRC-5 has been observed, for example, in IMR-90 human lung fibroblasts[33] and WI38-VA13 cells[40]. Besides α-SMA, upregulation of collagen I[41], fibronectin[27]and CTGF[42] has been reported when human lung fibroblasts are treated with TGF-β.
The expression of α-SMA is regulated by Smad[43]. TGF-β increases the nuclear translocation of Smad and expression of α-SMA. We examined the influence of EGCG treatment on Smad2/3 appearance in MRC-5 cells. Immunohistological experiments indicated that EGCG inhibits the nuclear transportation of Smad2/3.
Moreover, we found that EGCG had suppressive effects on the expression of α-SMA in MRC-5 cells, whereas catechin did not. These data suggest that the effects are dependent on the gallate or pyrogallol moiety of EGCG.
Next, we investigated the mechanism of the inhibitory effect on the Smad2/3 pathway. EGCG is a potent antioxidant and a lot of its health benefit effects are thought to be due to its antioxidative action[44-46]. EGCG attenuated the increase in α-SMA expression brought about by TGF-β, whereas edaravone and NAC did not. These results indicate that the inhibitory effect of EGCG on α-SMA expression is independent of its antioxidative action.
We thought that part of the EGCG’s effects on α-SMA expression might arise through interference with receptor-ligand binding. Indeed, EGCG treated cell lysate containing TGFRII showed no immunoprecipitation with anti-TGFRII antibody. The interaction between EGCG and TGFRII was also confirmed by the affinity chromatography experiment. A likely explanation for this observation is that EGCG binds to TGFRII, thereby blocking the antibody from binding to TGFRII. Similarly, if EGCG binds to the TGF-β receptor, TGF-β would not be able to bind to its receptor and downstream signaling pathways would be ineffective.
In conclusion, we have shown that EGCG interacts with TGFRII and inhibits the expression of α-SMA via the TGF-β-Smad2/3 pathway in MRC-5 cells, which are human lung fibroblasts. These results suggest that EGCG has anti-fibrotic effects that are crucial for the control of myofibroblast differentiation and extracellular matrix deposition, which are involved in fibrosis. The evidence that EGCG is effective in the suppression of fibrosis may lead not only to better understanding of the biological roles of EGCG but also to clinical applications of this flavonoid.
Fibrosis is an intractable disease. Effective treatments for it have not been developed yet. Catechin is a substance with a variety of physiological effects. However, the investigation on the antifibrotic effect of catechin has not been fully performed.
Various physiological effects of catechin have been intensely studied. It has been reported that catechin has a variety of physiological activity (e.g., regulation of blood pressure, blood cholesterol, blood sugar; antioxidant, anti-aging, anti-cancer effects).
Many studies have been performed about (-)-epigallocatechin-3-gallate (EGCG) relationship with transforming growth factor-β (TGF-β) and its antifibrotic properties. We demonstrated that EGCG inhibits the TGF-β activity through its binding to TGF-β type II receptor (TGFRII).
TGF-β is believed to be the strongest inducer of tissue fibrosis. EGCG inhibits TGF-β activity by interacting with TGFRII. Therefore, EGCG may become an antifibrotic agent.
Green tea contains four main catechin substances: Epicatechin, epigallocatechin, epicatechin gallate, and epigallocatechin gallate, all of which are inclusively called catechin. Organ fibrosis is a clinical condition caused by an excessive deposition of extracellular matrix. The progression of fibrosis resulted in a loss of normal function.
This paper reports a novel, interesting and important study. This is a basic work which shows that EGCG could bind to the TGFRII abolishing myofibroblast activation. The original point in this work is the analysis that is done on the cytokine receptor. The authors soundly demonstrated the binding EGCG to TGFRIIby immunoprecipitation and affinity chromatography experiments.
P- Reviewers: Carrillo MC, Nakajima N, Liu ZH, Slomiany BL, Swierczynski J S- Editor: Gou SX L- Editor: A E- Editor: Wu HL
| 1. | Suzuki Y, Miyoshi N, Isemura M. Health-promoting effects of green tea. Proc Jpn Acad Ser B Phys Biol Sci. 2012;88:88-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 2. | Fujiki H, Suganuma M, Imai K, Nakachi K. Green tea: cancer preventive beverage and/or drug. Cancer Lett. 2002;188:9-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 130] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 3. | Fujiki H. Green tea: Health benefits as cancer preventive for humans. Chem Rec. 2005;5:119-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Brown AL, Lane J, Holyoak C, Nicol B, Mayes AE, Dadd T. Health effects of green tea catechins in overweight and obese men: a randomised controlled cross-over trial. Br J Nutr. 2011;106:1880-1889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | Fukino Y, Ikeda A, Maruyama K, Aoki N, Okubo T, Iso H. Randomized controlled trial for an effect of green tea-extract powder supplementation on glucose abnormalities. Eur J Clin Nutr. 2008;62:953-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 107] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 6. | Fu Z, Zhen W, Yuskavage J, Liu D. Epigallocatechin gallate delays the onset of type 1 diabetes in spontaneous non-obese diabetic mice. Br J Nutr. 2011;105:1218-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Miura Y, Chiba T, Tomita I, Koizumi H, Miura S, Umegaki K, Hara Y, Ikeda M, Tomita T. Tea catechins prevent the development of atherosclerosis in apoprotein E-deficient mice. J Nutr. 2001;131:27-32. [PubMed] |
| 8. | Mukoyama A, Ushijima H, Nishimura S, Koike H, Toda M, Hara Y, Shimamura T. Inhibition of rotavirus and enterovirus infections by tea extracts. Jpn J Med Sci Biol. 1991;44:181-186. [PubMed] |
| 9. | Weber JM, Ruzindana-Umunyana A, Imbeault L, Sircar S. Inhibition of adenovirus infection and adenain by green tea catechins. Antiviral Res. 2003;58:167-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 152] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 10. | Park M, Yamada H, Matsushita K, Kaji S, Goto T, Okada Y, Kosuge K, Kitagawa T. Green tea consumption is inversely associated with the incidence of influenza infection among schoolchildren in a tea plantation area of Japan. J Nutr. 2011;141:1862-1870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Zhao WH, Hu ZQ, Okubo S, Hara Y, Shimamura T. Mechanism of synergy between epigallocatechin gallate and beta-lactams against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2001;45:1737-1742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 260] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 12. | Kim JS, Kim Y. The inhibitory effect of natural bioactives on the growth of pathogenic bacteria. Nutr Res Pract. 2007;1:273-278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Hatano T, Tsugawa M, Kusuda M, Taniguchi S, Yoshida T, Shiota S, Tsuchiya T. Enhancement of antibacterial effects of epigallocatechin gallate, using ascorbic acid. Phytochemistry. 2008;69:3111-3116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Jang S, Jeong HS, Park JS, Kim YS, Jin CY, Seol MB, Kim BC, Lee MC. Neuroprotective effects of (-)-epigallocatechin-3-gallate against quinolinic acid-induced excitotoxicity via PI3K pathway and NO inhibition. Brain Res. 2010;1313:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Itoh T, Imano M, Nishida S, Tsubaki M, Hashimoto S, Ito A, Satou T. (-)-Epigallocatechin-3-gallate protects against neuronal cell death and improves cerebral function after traumatic brain injury in rats. Neuromolecular Med. 2011;13:300-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Itoh T, Tabuchi M, Mizuguchi N, Imano M, Tsubaki M, Nishida S, Hashimoto S, Matsuo K, Nakayama T, Ito A. Neuroprotective effect of (-)-epigallocatechin-3-gallate in rats when administered pre- or post-traumatic brain injury. J Neural Transm. 2013;120:767-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Kim HK, Yang TH, Cho HY. Antifibrotic effects of green tea on in vitro and in vivo models of liver fibrosis. World J Gastroenterol. 2009;15:5200-5205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Hung GD, Li PC, Lee HS, Chang HM, Chien CT, Lee KL. Green tea extract supplementation ameliorates CCl4-induced hepatic oxidative stress, fibrosis, and acute-phase protein expression in rat. J Formos Med Assoc. 2012;111:550-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Chen A, Zhang L. The antioxidant (-)-epigallocatechin-3-gallate inhibits rat hepatic stellate cell proliferation in vitro by blocking the tyrosine phosphorylation and reducing the gene expression of platelet-derived growth factor-beta receptor. J Biol Chem. 2003;278:23381-23389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Meng M, Li YQ, Yan MX, Kou Y, Ren HB. Effects of epigallocatechin gallate on diethyldithiocarbamate-induced pancreatic fibrosis in rats. Biol Pharm Bull. 2007;30:1091-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Sriram N, Kalayarasan S, Sudhandiran G. Epigallocatechin-3-gallate exhibits anti-fibrotic effect by attenuating bleomycin-induced glycoconjugates, lysosomal hydrolases and ultrastructural changes in rat model pulmonary fibrosis. Chem Biol Interact. 2009;180:271-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2357] [Cited by in RCA: 2378] [Article Influence: 76.7] [Reference Citation Analysis (0)] |
| 23. | Tachibana H, Koga K, Fujimura Y, Yamada K. A receptor for green tea polyphenol EGCG. Nat Struct Mol Biol. 2004;11:380-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 518] [Cited by in RCA: 523] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 24. | Masuda M, Suzui M, Lim JT, Deguchi A, Soh JW, Weinstein IB. Epigallocatechin-3-gallate decreases VEGF production in head and neck and breast carcinoma cells by inhibiting EGFR-related pathways of signal transduction. J Exp Ther Oncol. 2002;2:350-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 191] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 25. | Andriamanalijaona R, Kypriotou M, Baugé C, Renard E, Legendre F, Raoudi M, Boumediene K, Gatto H, Monginoux P, Pujol JP. Comparative effects of 2 antioxidants, selenomethionine and epigallocatechin-gallate, on catabolic and anabolic gene expression of articular chondrocytes. J Rheumatol. 2005;32:1958-1967. [PubMed] |
| 26. | Ohkawa T, Ueki N, Taguchi T, Shindo Y, Adachi M, Amuro Y, Hada T, Higashino K. Stimulation of hyaluronan synthesis by tumor necrosis factor-alpha is mediated by the p50/p65 NF-kappa B complex in MRC-5 myofibroblasts. Biochim Biophys Acta. 1999;1448:416-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Honda E, Yoshida K, Munakata H. Transforming growth factor-beta upregulates the expression of integrin and related proteins in MRC-5 human myofibroblasts. Tohoku J Exp Med. 2010;220:319-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Yoshida K, Munakata H. Connective tissue growth factor binds to fibronectin through the type I repeat modules and enhances the affinity of fibronectin to fibrin. Biochim Biophys Acta. 2007;1770:672-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Frankel SK, Cosgrove GP, Cha SI, Cool CD, Wynes MW, Edelman BL, Brown KK, Riches DW. TNF-alpha sensitizes normal and fibrotic human lung fibroblasts to Fas-induced apoptosis. Am J Respir Cell Mol Biol. 2006;34:293-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Stockert J, Adhikary T, Kaddatz K, Finkernagel F, Meissner W, Müller-Brüsselbach S, Müller R. Reverse crosstalk of TGFβ and PPARβ/δ signaling identified by transcriptional profiling. Nucleic Acids Res. 2011;39:119-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Araya J, Kojima J, Takasaka N, Ito S, Fujii S, Hara H, Yanagisawa H, Kobayashi K, Tsurushige C, Kawaishi M. Insufficient autophagy in idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2013;304:L56-L69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 241] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 32. | Uhal BD, Kim JK, Li X, Molina-Molina M. Angiotensin-TGF-beta 1 crosstalk in human idiopathic pulmonary fibrosis: autocrine mechanisms in myofibroblasts and macrophages. Curr Pharm Des. 2007;13:1247-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 117] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 33. | Arribillaga L, Dotor J, Basagoiti M, Riezu-Boj JI, Borrás-Cuesta F, Lasarte JJ, Sarobe P, Cornet ME, Feijoó E. Therapeutic effect of a peptide inhibitor of TGF-β on pulmonary fibrosis. Cytokine. 2011;53:327-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | Yasui K, Tanabe H, Miyoshi N, Suzuki T, Goto S, Taguchi K, Ishigami Y, Paeng N, Fukutomi R, Imai S. Effects of (-)-epigallocatechin-3-O-gallate on expression of gluconeogenesis-related genes in the mouse duodenum. Biomed Res. 2011;32:313-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Desmoulière A. Factors influencing myofibroblast differentiation during wound healing and fibrosis. Cell Biol Int. 1995;19:471-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 201] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 36. | Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol. 1999;277:C1-C9. [PubMed] |
| 37. | Evans RA, Tian YC, Steadman R, Phillips AO. TGF-beta1-mediated fibroblast-myofibroblast terminal differentiation-the role of Smad proteins. Exp Cell Res. 2003;282:90-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 301] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 38. | Moustakas A, Souchelnytskyi S, Heldin CH. Smad regulation in TGF-beta signal transduction. J Cell Sci. 2001;114:4359-4369. [PubMed] |
| 39. | Honda E, Park AM, Yoshida K, Tabuchi M, Munakata H. Myofibroblasts: Biochemical and proteomic approaches to fibrosis. Tohoku J Exp Med. 2013;230:67-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | He X, Wang L, Szklarz G, Bi Y, Ma Q. Resveratrol inhibits paraquat-induced oxidative stress and fibrogenic response by activating the nuclear factor erythroid 2-related factor 2 pathway. J Pharmacol Exp Ther. 2012;342:81-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 41. | Jones B, Bucks C, Wilkinson P, Pratta M, Farrell F, Sivakumar P. Development of cell-based immunoassays to measure type I collagen in cultured fibroblasts. Int J Biochem Cell Biol. 2010;42:1808-1815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 42. | Kucich U, Rosenbloom JC, Herrick DJ, Abrams WR, Hamilton AD, Sebti SM, Rosenbloom J. Signaling events required for transforming growth factor-beta stimulation of connective tissue growth factor expression by cultured human lung fibroblasts. Arch Biochem Biophys. 2001;395:103-112. [PubMed] |
| 43. | Li Z, Xie WB, Escano CS, Asico LD, Xie Q, Jose PA, Chen SY. Response gene to complement 32 is essential for fibroblast activation in renal fibrosis. J Biol Chem. 2011;286:41323-41330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 44. | Nanjo F, Goto K, Seto R, Suzuki M, Sakai M, Hara Y. Scavenging effects of tea catechins and their derivatives on 1,1-diphenyl-2-picrylhydrazyl radical. Free Radic Biol Med. 1996;21:895-902. [PubMed] |
| 45. | Nanjo F, Mori M, Goto K, Hara Y. Radical scavenging activity of tea catechins and their related compounds. Biosci Biotechnol Biochem. 1999;63:1621-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 163] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 46. | Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003;43:89-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1258] [Cited by in RCA: 1181] [Article Influence: 53.7] [Reference Citation Analysis (1)] |