INTRODUCTION
Aging is the time-related deterioration of essential physiological functions for survival and fertility, occurring universally in various organisms and leading to natural death. This process involves gradual dysfunctions in all organs, affecting unicellular organisms, plants, animals, and humans alike. It is an inevitable stage of development and life. Zhang et al[1] define aging as the reduction of the body's capacity, both physically and psychologically, to adapt to the environment, and the gradual tendency towards death.
Recent gerontological research has delved into the complex biochemical mechanisms associated with the gradual decline in bodily functions. It emphasizes the involvement of multiple processes at the cellular, molecular, and systemic level, requiring comprehensive approaches for understanding aging. Despite being a physiological and universal process, aging occurs at an individual rate in each person[2,3].
Differentiating between chronological and biological age is important due to the varying rates of aging. Chronological age merely indicates the time passed since birth, while biological age encompasses a broad spectrum of physical, physiological, and cognitive functions, influenced by molecular and cellular processes[4]. Despite ongoing discussions, the biology of aging remains controversial, posing challenges in establishing a universally accepted definition of normal aging[5]. However, biological aging relies on various theories, including those related to the effects of free radicals, telomere shortening, and the mitochondrial theory.
In the broader context of anti-aging research, healthy aging is characterized by the attainment of an extended period of robust physiological and mental well-being. This entails an ongoing effort to seize opportunities for enhancing physical and mental health, preserving independence, and elevating overall quality of life across the lifespan. Seals et al[6] proposed the concept of optimal longevity, incorporating a compressed disease period at life's end. This innovative perspective has led to the emergence of geroscience, a new field in aging research. Geroscience is dedicated to identifying and intervening in biological mechanisms to enhance health span and actively promote healthy aging in individuals.
Aging itself is initiated by a combination of genetic and environmental factors that can influence organisms from birth. The signs of aging encompass various aspects, including impaired vision, hearing loss, muscle strength decline, reduced bone density, weakened immune system, cognitive decline, less efficient metabolism, reduced energy, hair loss, diminished balance, and overall decreased mobility[7].
Understanding these aging indicators is crucial within the setting of geroscience in order to promote extended health span and well-being throughout the aging process. In this review, we analyze the multifaceted roles of stem cells in tissue maintenance, disease pathogenesis, and the regulation of aging by comprehensively examining their properties, functions, and regulatory mechanisms in vivo.
AGING AND IMPAIRED VISION
Older adults commonly face three visual problems: Impaired spatial contrast sensitivity, scotopic sensitivity loss, and delayed rod-mediated dark adaptation, along with reduced visual processing speed. While the extent of deficits varies among individuals, older adults are likely to experience one or more of these disturbances. These visual challenges impact daily tasks. In severe cases, these aging-related visual issues may indicate the emergence of visual pathway conditions and diseases common in the elderly[8].
AGING AND IMPAIRED HEARING
Age-related hearing loss (ARHL), or presbycusis, is a common occurrence in mammals, including humans, with varying onset times and degrees of loss. It manifests through reduced sensitivity to sound, especially high pitches, and a diminished ability to understand speech in background noise[9].
ARHL involves both peripheral structures of the inner ear and central acoustic pathways, with oxidative stress identified as a key pro-aging mechanism in the human cochlea[10].
LOSS OF BONE DENSITY AND MUSCLE STRENGTH
Muscle and bone aging contribute significantly to morbidity and mortality in older populations, affecting the overall quality of life. Skeletal aging begins after reaching peak bone mass, with varying onset ages and rates between sexes. Two primary factors contribute to the decline in muscle mass and function with age: Muscle fiber atrophy and muscle fiber loss. It is evident that both these components exert influence on the regulation of muscle atrophy and dysfunction, impacting either individual muscles or groups of muscles[11].
Bone loss is accelerated during the perimenopausal period in women and gradually progresses in men of advanced age. Changes in both bone quantity and quality occur throughout growth and aging, impacting microarchitecture, size, and geometry. Genetic and epigenetic factors may predispose individuals to osteoporosis, characterized by weakened bones and an increased risk of fractures[12].
WEAKENED IMMUNE SYSTEM
The concept of immune senescence reflects age-related changes in immune responses, both cellular and serological, affecting the process of generating specific responses to foreign and self-antigens. The decline of the immune system with age is reflected in the increased susceptibility to infectious diseases, weaker responses to vaccination, increased prevalence of cancer, autoimmune and other chronic diseases[13].
AGING AT THE CELLULAR LEVEL
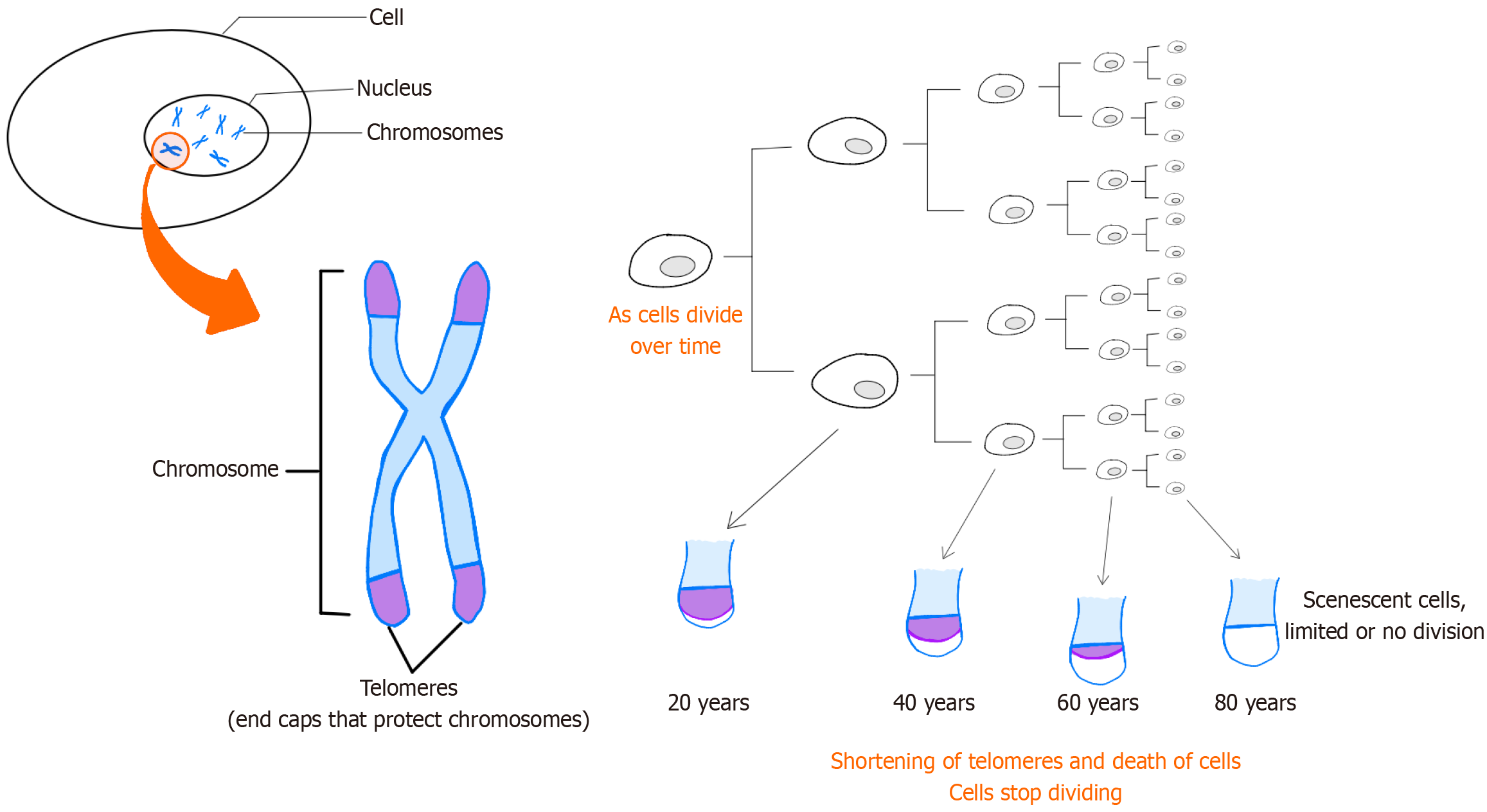
In 2013, López-Otín et al[14] defined nine cellular and molecular hallmarks of aging, laying a crucial foundation for future research in the field. These hallmarks encompass genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient-sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication (Figure 1). Expanding on this framework, a research symposium entitled "New Hallmarks of Aging" convened in Copenhagen on March 22, 2022. This symposium concentrated on presenting innovative findings and recontextualizing the original nine hallmarks, introducing potential new hallmarks such as compromised autophagy, dysregulation of RNA processing, microbiome disturbances, altered mechanical properties, and inflammation[14,15].
Figure 1 Telomeres on the end of chromosomes are believed to protect the DNA strands and prevent them from fusing with other strands.
Telomeres lose a little of their length during each cell division. Since replicative DNA polymerases are not able to replicate telomeres, and telomerase is not expressed in normal mammalian somatic cells, telomeres become too short to replicate after a fixed number of cell divisions. Eventually, the cell will stop growing and enter cellular senescence.
STEM CELLS AND MESENCHYMAL STEM CELLS IN AGING
Stem cells, characterized by their immature nature, possess the remarkable ability to undergo infinite self-renewal and differentiate into various cell lineages. Their unique capacity to function as a reservoir for the production, maintenance, repair, and regeneration of diverse tissues distinguishes them from other cell types[16].
There are two main subtypes of stem cells: Embryonic stem cells, derived from early-stage embryos, and mesenchymal stem cells (MSCs), found in adults and isolated from various tissues such as bone marrow and adipose tissue.
As a subtype of adult stem cells, MSCs are multipotent stem cells with remarkable regenerative potential found in various tissues throughout the body. They play a pivotal role in tissue regeneration and can differentiate into multiple cell lineages. Amongst the myriad roles played by MSCs, one of the most captivating and promising aspects lies in their remarkable ability to counteract the effects of aging. MSCs can go beyond their traditional roles in tissue repair, immune modulation, and paracrine signaling to intricately engage in fighting the multifaceted processes associated with aging, making them particularly promising for future clinical trials, especially in the fields of regenerative medicine and anti-aging treatments[17-19].
In fact, MSCs are known for their important proliferation, especially if the donor is young since proliferation capacity declines with age. Also, they can be differentiated into osteoblasts, chondrocytes, myoblasts, adipocytes, and fibroblasts. Additionally, MSCs exhibited anti-aging benefits through the secretion of cytokines and growth factors that act as promoters of angiogenesis, anti-inflammatory agents, and inhibitors of apoptosis[20,21].
In a recent study, Wang et al[22] highlighted the anti-aging and anti-obesity effects of MSCs from healthy mice when injected into older mice, providing new insights into potential anti-aging treatments. Moreover, MSCs, especially adipose stem cells, have been reported to improve aged skin by increasing angiogenesis growth factors[23].
MSCs reportedly release a wide array of bioactive molecules that collectively form the secretome. Within this complex mixture, an important subset is the exosomes which are tiny vesicles enriched with molecular cargo. These extracellular components, derived from the endosomal pathway within MSCs, constitute a vital facet of the broader secretome. The secretome, encompassing soluble factors such as growth factors and cytokines alongside exosomes, acts as a comprehensive communication system. Through paracrine signaling, MSCs influence neighboring and distant cells, fostering an environment conducive to tissue repair, immunomodulation, and anti-inflammatory responses. The interplay between MSCs, secretome, and exosomes highlights the interconnected web of regenerative mechanisms, underscoring their potential in therapeutic applications, from promoting wound healing to addressing aging-related degeneration[24-28].
These anti-aging effects encompass a spectrum of activities, from mitigating chronic inflammation to enhancing tissue regeneration and modulating cellular senescence[29]. The unique capacity of MSCs to address the underlying mechanisms of aging positions them at the forefront of regenerative medicine, holding immense potential for interventions, aiming to promote not just longevity, but a healthier and more vibrant aging process[30,31].
TISSUE REGENERATION AND STEM CELL DIFFERENTIATION
MSCs are a subset of non-hematopoietic adult stem cells capable of differentiating into various cell types, including osteoblasts, chondrocytes, and adipocytes. This differentiation capacity and immunomodulatory properties contribute to tissue regeneration by replacing damaged or aging cells with new, functional cells. The differentiation potential of MSCs makes them promising candidates for regenerative medicine applications in repairing fragile tissues associated with the musculoskeletal system, nervous system, myocardium, liver, cornea, trachea, and skin[32-34].
SECRETOME PRODUCTION AND IMMUNOMODULATION
As chronic inflammation is a hallmark of aging, MSCs exhibit immunomodulatory properties, suppressing excessive inflammatory responses by release of the secretome which contains a variety of anti-inflammatory cytokines and growth factors. These factors stimulate cell proliferation, enhance tissue repair, and contribute to overall health by modulating the immune system and creating an anti-inflammatory environment[35-37].
Intercellular communication
Exosomes, derived from MSCs, contain bioactive molecules, including proteins, lipids, and nucleic acids. They act as messengers, transferring information between cells which can influence neighboring cells, promoting tissue repair and rejuvenation[38,39].
Anti-fibrotic effects
MSCs and their secretome may have anti-fibrotic effects, reducing the accumulation of fibrotic tissue in organs. Fibrosis is associated with aging leading to impaired organ function. Hence, the therapeutic potential of MSCs resides in their capability to target multiple fibrogenesis parameters. This includes their capacity for immunosuppression, inhibition of the TGF-β1 pathway and mitigation of hypoxia and oxidative stress[40].
Cellular protection
MSC-derived exosomes may carry antioxidant enzymes and other protective factors. This cargo can help protect cells from oxidative stress, which is associated with cellular damage and an accelerated aging process[41].
Mitochondrial function
MSCs and exosomes may influence mitochondrial function, by enhancing energy production within cells. Indeed, improved mitochondrial function is associated with increased cellular resilience and longevity[42,43].
Senescence modulation
MSCs and exosomes may modulate cellular senescence, the process by which cells lose their ability to divide and function properly. By influencing senescence, MSCs contribute to maintaining youthful cellular functions[36,44].
Extracellular matrix remodeling
MSCs and secretome contribute to remodeling of the extracellular matrix, ensuring the maintenance of tissue structure, elasticity, and functionality[45].
Stem cell niche maintenance
MSCs and secretome play a role in maintaining stem cell niches in tissues. This ensures a continuous pool of functional cells for tissue repair and regeneration[46].
Enhanced wound healing
MSCs and exosomes contribute to enhanced wound healing, which is essential for preventing age-related complications and maintaining the integrity of the skin and other tissues in the elderly[38,46].
Angiogenesis promotion
Growth factors found in the secretome can stimulate angiogenesis and the formation of new blood vessels. Improved blood flow is crucial for supplying nutrients to tissues and therefore supporting overall healthy tissues[47,48].
Promotion of autophagy
Exosomes may stimulate autophagy, a cellular process that removes damaged components. Enhanced autophagy can contribute to cellular rejuvenation, thereby underlining their therapeutic efficacy. Consequently, strategies aimed at modulating autophagy through MSC-based therapies hold significant promise for enhancing therapeutic outcomes in various human diseases, including cancer, autoimmune disorders, and neurodegenerative conditions[49,50].
CONCLUSION
As we delve into the intricacies of MSCs and their multifaceted roles, it becomes evident that these cells hold immense promise in revolutionizing the landscape of regenerative medicine especially in the elderly. MSCs have important characteristics that make them ideal candidates for use in regenerative medicine, such as immunomodulatory capability valuable for improving immune system abnormalities, paracrine or autocrine roles that produce growth factors, and the vital potential to differentiate into various cells. Also, there is a lack of anti-aging treatment derived from the mechanism of aging. This review showed that MSCs have a significant effect on delaying aging. This exploration of their roles provides a foundation for understanding their potential applications in addressing a wide array of health-related diseases and challenges. It is essential to note that research in this field is ongoing, and clinical applications are still being explored. Further studies are needed to understand the optimal conditions for MSC-based therapies and their long-term effects on aging-related conditions.
ACKNOWLEDGEMENTS
We thank all who contributed to this work from all institutions.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country of origin: Lebanon
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade B
Creativity or Innovation: Grade C
Scientific Significance: Grade C
P-Reviewer: Bonartsev A S-Editor: Lin C L-Editor: Webster JR P-Editor: Wang WB