Published online Sep 20, 2024. doi: 10.5493/wjem.v14.i3.95540
Revised: May 22, 2024
Accepted: June 12, 2024
Published online: September 20, 2024
Processing time: 139 Days and 0.7 Hours
Acute ischemic stroke (AIS) retains a notable stance in global disease burden, with thrombolysis via recombinant tissue plasminogen activator (rtPA) serving as a viable management approach, albeit with variable outcomes and the potential for complications like hemorrhagic transformation (HT). The platelet-to-neutrophil ratio (P/NR) has been considered for its potential prognostic value in AIS, yet its capacity to predict outcomes following rtPA administration demands further exploration.
To elucidate the prognostic utility of P/NR in predicting HT and clinical out
Data from 418 AIS patients treated with intravenous rtPA at Thammasat Uni
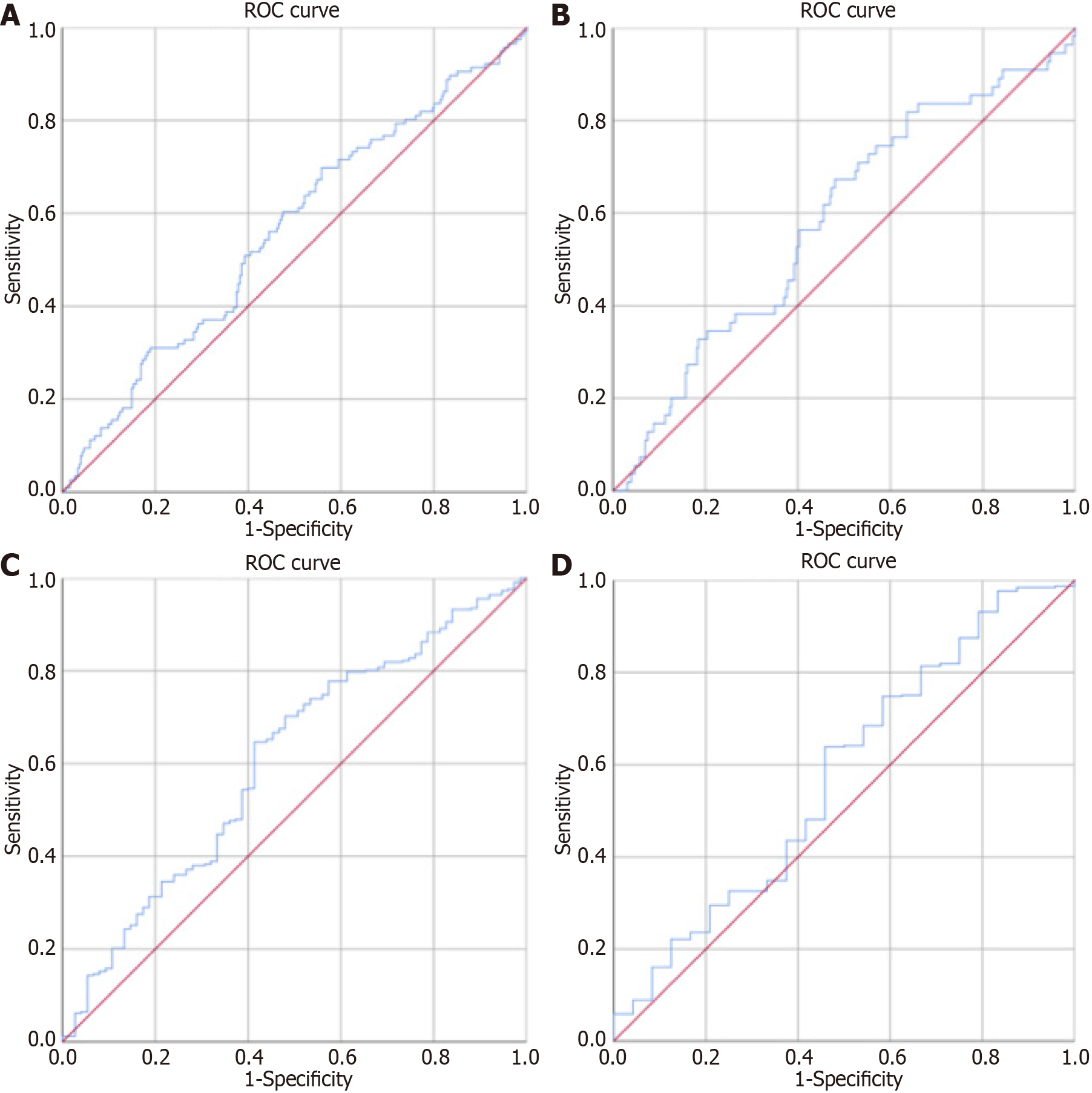
Notable variables, such as age, diabetes, and stroke history, exhibited statistical disparities when comparing patients with and without E-ND, HT, D-ND, and 3-mo outcomes. P/NR prognostication revealed an optimal cutoff of 43.4 with a 60.3% sensitivity and a 52.5% specificity for 90-d outcomes. P/NR prognostic accuracy was statistically significant for 90-d outcomes [area under the curve (AUC) = 0.562], D-ND (AUC = 0.584), and HT (AUC = 0.607).
P/NR demonstrated an association with adverse 3-mo clinical outcomes, HT, and D-ND in AIS patients post-rtPA administration, indicating its potential as a predictive tool for complications and prognoses. This infers that a diminished P/NR may serve as a novel prognostic indicator, assisting clinicians in identifying AIS patients at elevated risk for unfavorable outcomes following rtPA therapy.
Core Tip: The study explored the prognostic value of the platelet-to-neutrophil ratio (P/NR) in patients with acute ischemic stroke (AIS) who underwent thrombolysis with recombinant tissue plasminogen activator (rtPA). It aimed to determine if P/NR could predict hemorrhagic transformation and clinical outcomes following rtPA treatment. An optimal P/NR cutoff value was identified for predicting 90-d outcomes with moderate sensitivity and specificity. The study concluded that P/NR is associated with negative 3-mo outcomes, suggesting it could be a useful indicator for predicting risks post-rtPA treatment in AIS patients.
- Citation: Chaiwisitkun A, Muengtaweepongsa S. Platelet-to-neutrophil ratio predicts hemorrhagic transformation and unfavorable outcomes in acute ischemic stroke with intravenous thrombolysis. World J Exp Med 2024; 14(3): 95540
- URL: https://www.wjgnet.com/2220-315x/full/v14/i3/95540.htm
- DOI: https://dx.doi.org/10.5493/wjem.v14.i3.95540
Stroke has perpetually maintained a predominant position, ranking within the top three, regarding disease burden over the past two decades, as assessed through disability-adjusted life-years (DALYs)[1,2]. The gravity of cerebral infarction, witnessed during acute ischemic stroke (AIS), bears a correlation with the disease's burden[3]. Ensuring successful AIS management proves pivotal in mitigating its associated burdens[4]. Cardioembolic and atherothrombotic strokes are the subtypes of ischemic infarct associated with the highest in-hospital mortality. The short-term prognosis for patients with these types of strokes is poorer compared to other ischemic stroke subtypes[5]. Despite the absence of a mortality re
In the AIS pathogenesis, platelet activation and aggregation emerge as critical elements. Amidst pathological circumstances, an excessive activation and aggregation of platelets can precipitate thrombosis and vascular occlusion, thereby instigating ischemic stroke or heart disease[12]. A plethora of studies have authenticated a decrement in platelet count within the circulatory system of AIS patients, whereas the platelet distribution width and mean platelet volume experience an elevation[13]. The immune response is recognized as imperative in the pathological alterations observed in AIS. Ischemic and anoxic brain tissue instigates the infiltration of peripheral blood leukocytes into the afflicted area. Neutrophils, the initial cells to be recruited into the brain following a stroke, discharge inflammatory mediators within the ischemic brain area, exacerbate brain damage[14] and foster the incidence of ischemia by inducing thrombosis via various mechanisms, such as interfacing with platelets, coagulation factors, and discharging proteases[15]. The platelet-to-neutrophil ratio (P/NR) emerges as a novel biomarker that amalgamates platelets and neutrophil counts. Contrasting with singular platelet and neutrophil counts, P/NR mirrors the severity of both thrombosis and inflammation, elucidating the liaison between the two processes. Within the realm of stroke, a recent study posited that the level of P/NR upon admission is associated with the prognosis of AIS patients[16]. Furthermore, another study advocated that P/NR surpasses other complete blood count ratios in prognosticating an adverse outcome in AIS patients[17]. Within this retrospective study, our objective was to illustrate the clinical value of P/NR in prognosticating the outcome in AIS patients who have been treated with intravenous rtPA.
This retrospective study was conducted utilizing data procured from Thammasat University Hospital (TUH). The patient cohort comprised individuals diagnosed with AIS who underwent intravenous thrombolysis treatment, specifically utilizing intravenous rtPA, in adherence to the TUH protocol between January 2018 and June 2021. A complete blood count (CBC) was mandated before confirming the decision to administer intravenous rtPA[18].
Inclusion criteria: Patients formally diagnosed with AIS who met the criteria for and consequently received intravenous rtPA treatment within 4.5 h of stroke onset, in line with the stroke fast-track criteria established by TUH. Age range between 18 years and 85 years.
Exclusion criteria: Patients with a history of infection or surgery within the preceding 2 wk. An underlying disease condition such as malignancy, rheumatoid arthritis, or connective tissue disease. Chronic liver disease (Child-Pugh Score > B). Chronic kidney disease (serum creatinine > 2.0 mg/dL). Prior abnormalities in platelet and white blood cell counts. Ultimately, 418 patients were incorporated into the study, forming the basis for subsequent analysis and findings.
The Human Research Ethics Committee of Thammasat University (Medicine) bestowed approval for this study and granted a waiver for the requirement of informed consent, under approval number: 284/2564. All methodologies employed throughout the study adhered scrupulously to pertinent guidelines and regulations.
Data procurement entailed an assessment by proficient clinicians, predicated on clinical manifestations, to verify alignment with the diagnostic criteria for acute stroke. Stroke severity upon admission was gauged using the National Institute of Health Stroke Scale (NIHSS). To exclude hemorrhagic stroke, all patients underwent emergent imaging via computerized tomography (CT) scan or magnetic resonance imaging (MRI) prior to intravenous rtPA administration. Pre-rtPA intravenous CBC was obtained. Additionally, baseline clinical attributes, inclusive of alternative laboratory examinations within 24 h of admission [e.g., fasting blood glucose and low-density lipoprotein (LDL)] and demographic data, were amassed for all patients[19].
Criteria were as follows: Hypertension was characterized by recurrent systolic blood pressure readings ≥ 140 mmHg upon admission or antecedent hypertension history. Diabetes encapsulated either a prior diagnosis or admission with diabetes mellitus, and either fasting plasma glucose ≥ 126 mg/dL or HbA1C ≥ 6.5%. Atrial fibrillation (AF) required precedent AF episodes or admission-time AF electrocardiogram recordings. Hyperlipidemia entailed hyperlipidemia history or admission with dyslipidemia, and either LDL ≥ 100 mg/dL or triglyceride ≥ 150 mg/dL[20].
Infarct volume was calculated employing 418 cases examined via CT scans or MRI, utilizing the formula 0.5 × a × b × c (where a is the maximum longitudinal diameter, b is the maximum transverse diameter perpendicular to a, and c denotes 10 mm slices with infarction), with volumes < 5 cm³ and ≥ 5 cm³ defining small and large infarct volumes, respectively[21].
Clinical outcomes encompassed early neurological deterioration (E-ND), HT, delayed ND (D-ND), and 3-mo poor outcomes. HT was delineated as any perceptible hemorrhage discerned on brain CT or MRI within 24 h post-throm
Analytical procedures were executed utilizing the Statistical Program for Social Sciences (SPSS), version 22.0 (IBM, West Grove, PA, United States). The Mann-Whitney U-test facilitated the evaluation of disparities between two groups for variables demonstrating a nonparametric distribution, while the Chi-square test was employed to discern variations between categorical variables. Continuous and categorical variables were depicted utilizing medians with interquartile ranges (IQR) and percentages, respectively. The prognostic impact of P/NR was appraised by employing the receiver operating characteristic (ROC) curve, with a P-value < 0.05 establishing statistical significance in all comparative group analyses.
This investigation encompassed 418 patients, comprising 169 females (40.4%) and 249 males (59.6%), with a mean age of 64.5 years (range: 53-72 years) and a mean NIHSS score upon admission of 10 (IQR: 6-16). The predominant risk factors identified were hypertension (71.5%), hyperlipidemia (66%), and diabetes (33.7%). The mean time from stroke onset to intravenous rtPA administration was 170 min (IQR: 124-218 minutes). Laboratory findings included hemoglobin at 13.3 g/dL (IQR: 12.2-14.4) and white blood cell count at 8.4 × 109/L (IQR: 6.81-10.51 × 109/L), among other parameters. Antihypertensive therapy was the most prevalent current medication at 45.6%. Patient outcomes following intravenous rtPA at various time points (24 h, 24 h to 7 d, and 3 mo post-thrombolysis) were also analyzed. Of note, 24 (5.7%) exhibited E-ND, while 75 (18%) manifested HT within the initial 24 h following intravenous rtPA. Twelve patients (2.87%) died in the hospital. Of these, eight patients' deaths were due to neurologic complications.
Eligible patients were stratified into groups according to the presence or absence of distinct clinical outcomes (E-ND, HT, D-ND, and 3-mo outcomes). In the E-ND assessment, statistically significant disparities were observed between groups with and without E-ND in terms of age, diabetes prevalence, current alcohol consumption, baseline blood glucose, and infarct volume, as further detailed. For instance, a statistically higher age was observed in the E-ND group (70 vs 64; P = 0.045).
Differences were also evident when comparing patients with and without HT. Variables that demonstrated statistical variance encompassed previous stroke, stroke etiology, NIHSS upon admission, and LDL, among others. For instance, individuals without a prior stroke manifested a higher proportion of HT than those with a previous stroke (13% vs 4%; P = 0.027).
Differences in clinical characteristics between the presence and absence of D-ND highlighted variables such as age, sex, and hypertension as statistically significant. For example, the D-ND group exhibited a higher mean age than the non-D-ND group (67 vs 60 years; P < 0.001).
In distinguishing between favorable and unfavorable 3-mo clinical outcomes, statistically significant variations were found in variables such as age, sex, and hypertension. Notably, patients with poor 3-mo outcomes had a mean age of 69 years, contrasted with 60 years for those with favorable outcomes (P < 0.001).
The ROC and area under the curve (AUC) analyses for P/NR in prognosticating 90-d outcomes post-ischemic stroke following intravenous thrombolysis revealed an optimal P/NR cutoff value of 43.4, with a 60.3% sensitivity and a 52.5% specificity, 32.86% (95%CI: 26.56-39.17) positive predictive value and a 77.45% (95%CI: 71.72-83.19) negative predictive value. P/NR demonstrated a statistically significant prognostic accuracy of 56.2% for 90-d outcomes (AUC = 0.562, 95%CI: 0.501-0.624, P = 0.048).
ROC curves also provided prognostic insights for P/NR in relation to D-ND and HT post-ischemic stroke following intravenous thrombolysis. Notably, the P/NR offered a 58.4% accurate prognostication for D-ND (AUC = 0.584, 95%CI: 0.504-0.664, P = 0.044), and a 60.7% accurate prognostication for HT (AUC = 0.607, 95%CI: 0.535-0.678, P = 0.004).
These findings, along with further relevant data, are articulated within Figure 1, Tables 1, 2, 3, and 4, and Supplementary Table 1.
| Characteristics | Number |
| Age in yr | 64.5 (53-72) |
| Sex | |
| Male | 249 (59.6) |
| Female | 169 (40.4) |
| Risk factor | |
| Hypertension | 299 (71.5) |
| Dyslipidemia | 276 (66) |
| Diabetes mellitus | 141 (33.7) |
| Atrial fibrillation/atrial flutter | 102 (24.4) |
| Old stroke | 47 (11.2) |
| Current smoking | 73 (17.5) |
| Current alcohol drinking | 42 (10) |
| Etiology | |
| Other determined or undetermined | 177 (42.3) |
| Cardioembolic | 102 (24.4) |
| Small-artery occlusion | 94 (22.5) |
| Large-artery atherosclerosis | 42 (10) |
| Medication before stroke onset | |
| Antihypertensive therapy | 202 (48.3) |
| Antiplatelet therapy | 81 (18.3) |
| Hypoglycemic therapy | 121 (27.3) |
| Time for stroke onset to intravenous rtPA infusion in min | 170.05 (124-218.25) |
| Infarct volume in mL | 3.27 (0.58-24.24) |
| Hemorrhagic transformation | |
| No | 343 (82) |
| Yes | 75 (82) |
| PH1 | 29 (82) |
| PH2 | 27 (6.1) |
| HI1 | 12 (2.7) |
| HI2 | 7 (1.6) |
| Characteristics | Number |
| Baseline blood glucose in mg% | 110 (96-141) |
| Laboratory tests | |
| Hb | 13.3 (12.2-14.4) |
| WBC as 109/L | 8.4 (6.81-10.51) |
| Platelets as 109/L | 227 (192-278) |
| Neutrophil as 109/L | 5.14 (3.8-7.06) |
| Lymphocyte as 109/L | 1.93 (1.36-2.7) |
| P/NR | 43.73 (32.0-59.04) |
| PLR | 115.33 (87.17-170.64) |
| NLR | 2.56 (1.61-4.52) |
| PWR | 27.07 (21.4-33.99) |
| LDL | 114.5 (89-143) |
| NIHSS on admission | 10 (6-16) |
| NIHSS score on discharge date (day 1-7) | 5 (1-10) |
| Outcome events | |
| Increase NIHSS from baseline or death within 7 d after IV rt-PA | |
| Poor outcome (≥ 4 score) | 24 (5.7) |
| Good outcome (< 4 score) | 394 (94.3) |
| mRS on admission | 5 (3.75-5) |
| Poor outcome (3-6) | 348 (83.3) |
| Good outcome (0-2) | 70 (16.7) |
| mRS on discharge date (day 1-7) | 3 (1-4) |
| Poor outcome (3-6) | 236 (56.5) |
| Good outcome (0-2) | 182 (43.5) |
| mRS at 3 mo | 2 (0-4) |
| Poor outcome (3-6) | 168 (40.2) |
| Good outcome (0-2) | 250 (59.8) |
| Variables | Total, n = 418 | No E-ND, n = 394 | E-ND, n = 24 | P value | No HT, n = 342 | HT, n = 75 | P value |
| Age in yr | 64.5 (53-72) | 64 (52-71) | 70 (58.75-77.25) | 0.0451 | 65 (53-72) | 64 (52-74) | 0.9391 |
| Sex | |||||||
| Male | 249 (59.6) | 237 (60.2) | 12 (50) | 0.3252 | 206 (60.2) | 42 (56.0) | 0.4992 |
| Female | 169 (40.4) | 157 (39.8) | 12 (50) | 136 (39.8) | 33 (44.0) | ||
| Risk factor | |||||||
| Hypertension | 299 (71.5) | 279 (71.2) | 20 (83.3) | 0.1982 | 245 (72.1) | 53 (70.7) | 0.8082 |
| Dyslipidemia | 276 (66.0) | 261 (66.9) | 15 (62.5) | 0.6552 | 223 (65.8) | 52 (70.3) | 0.4582 |
| Diabetes mellitus | 141 (33.7) | 126 (32.1) | 15 (62.5) | 0.0022 | 114 (33.4) | 26 (34.7) | 0.8382 |
| Atrial fibrillation/ atrial flutter | 102 (24.4) | 97 (24.6) | 5 (20.8) | 0.6752 | 81 (23.7) | 21 (28.0) | 0.4312 |
| Old stroke | 47 (11.2) | 44 (11.3) | 3 (12.5) | 0.8522 | 44 (13.0) | 3 (4.0) | 0.0272 |
| Current smoking | 73 (17.5) | 70 (82.4) | 3 (100) | 0.4242 | 62 (83.8) | 10 (76.9) | 0.5462 |
| Current alcohol drinking | 42 (10.0) | 42 (91.3) | 0 (0) | 0.0032 | 37 (90.2) | 5 (83.3) | 0.6082 |
| Etiology | |||||||
| Other determined or undetermined | 177 (42.3) | 161 (41.2) | 16 (66.7) | 0.0052 | 143 (42.1) | 34 (45.3) | < 0.0012 |
| Cardioembolic | 102 (24.4) | 100 (25.6) | 2 (8.3) | 73 (21.5) | 29 (38.7) | ||
| Small-artery occlusion | 94 (22.5) | 93 (23.8) | 1 (4.2) | 91 (26.8) | 3 (4.0) | ||
| Large-artery atherosclerosis | 42 (10) | 37 (9.5) | 5 (20.8) | 33 (9.7) | 9 (12.0) | ||
| Medication | |||||||
| Antihypertensive therapy | 202 (45.6) | 75 (19.0) | 6 (25.0) | 0.4732 | 165 (48.2) | 37 (49.3) | 0.8642 |
| Antiplatelet therapy | 81 (18.3) | 191 (48.5) | 11 (45.8) | 0.8012 | 63 (18.4) | 18 (24.0) | 0.2692 |
| Hypoglycemic therapy | 121 (27.3) | 111 (28.2) | 10 (41.7) | 0.162 | 96 (28.2) | 25 (33.3) | 0.3712 |
| Infarct volume in mL | 3.27 (0.58-24.24) | 2.76 (0.45-20.25) | 34.42 (5.57-303.93) | < 0.0011 | 2.67 (0.43-16.88) | 13.99 (1.11-73.07) | 0.3891 |
| Time for stroke onset to intravenous rtPA infusion in min | 170.05 (124-218.25) | 170 (122-217) | 180.5 (147.5-232.25) | 0.1571 | 172 (124.85-218.25) | 160 (124-219) | 0.5161 |
| Variables | Total, n = 418 | No D-ND, n = 182 | D-ND, n = 236 | P value | Good 3-months, n = 250 | Poor 3-months, n = 168 | P value |
| Age in yr | 64.5 (53-72) | 60 (49-67.25) | 67 (58-76.75) | < 0.0011 | 60 (50-68) | 69 (61-75) | < 0.0011 |
| Sex | |||||||
| Male | 249 (59.6) | 120 (65.9) | 129 (54.7) | 0.022 | 162 (64.8) | 87 (51.8) | 0.0082 |
| Female | 169 (40.4) | 62 (34.1) | 107 (45.3) | 88 (35.2) | 81 (48.2) | ||
| Risk factor | |||||||
| Hypertension | 299 (71.5) | 114 (62.6) | 185 (79.1) | < 0.0012 | 163 (65.2) | 136 (81.9) | < 0.0012 |
| Dyslipidemia | 276 (66.0) | 113 (62.8) | 163 (69.7) | 0.1412 | 162 (65.3) | 114 (68.7) | 0.4782 |
| Diabetes Mellitus | 141 (33.7) | 55 (30.4) | 86 (36.4) | 0.1952 | 73 (29.3) | 68 (40.5) | 0.0182 |
| Atrial fibrillation/atrial flutter | 102 (24.4) | 40 (22.0) | 62 (26.3) | 0.3112 | 53 (21.2) | 49 (29.2) | 0.0632 |
| Old stroke | 47 (11.2) | 16 (8.8) | 31 (13.2) | 0.162 | 24 (9.7) | 23 (13.7) | 0.212 |
| Current smoking | 73 (17.5) | 38 (86.4) | 35 (79.5) | 0.3952 | 52 (85.2) | 21 (77.8) | 0.392 |
| Current alcohol drinking | 42 (10.0) | 20 (95.2) | 22 (84.6) | 0.242 | 30 (88.2) | 12 (92.3) | 0.6852 |
| Etiology | |||||||
| Other determined or undetermined | 177 (42.3) | 69 (38.3) | 108 (46.0) | 0.0042 | 93 (37.5) | 84 (50.3) | 0.0022 |
| Cardioembolic | 102 (24.4) | 51 (28.3) | 51 (21.7) | 70 (28.2) | 32 (19.2) | ||
| Small-artery occlusion | 94 (22.5) | 50 (27.8) | 44 (18.7) | 66 (26.6) | 28 (16.8) | ||
| Large-artery atherosclerosis | 42(10) | 10 (5.6) | 32 (13.6) | 19 (7.7) | 23 (13.8) | ||
| Medication | |||||||
| Antihypertensive therapy | 202 (45.6) | 81 (44.5) | 121 (51.3) | 0.172 | 111 (44.4) | 91 (54.2) | 0.052 |
| Antiplatelet therapy | 81 (18.3) | 31 (17.0) | 50 (21.2) | 0.2872 | 44 (17.6) | 37 (22.0) | 0.2622 |
| Hypoglycemic therapy | 121 (27.3) | 53 (29.1) | 68 (28.9) | 0.9672 | 71 (28.5) | 50 (29.8) | 0.7832 |
| Infarct volume in mL | 3.27 (0.58-24.24) | 1.16 (0.05-7.88) | 7.61 (1.53-63.41) | 0.361 | 1.46 (0.13-7.93) | 16.62 (2.51-103.09) | 0.0131 |
| Time for stroke onset to intravenous rtPA infusion in min | 170.05 (124-218.25) | 182 (0-125) | 167.5 (120-219.5) | 0.5281 | 173.5 (125-218) | 166.5 (120-220) | 0.3061 |
The investigation discerned a pertinent association between the P/NR and adverse 3-mo clinical outcomes, HT, and D-ND in patients experiencing AIS post-intravenous administration of rtPA. A diminished P/NR was discernibly correlated with unfavorable outcomes, positioning P/NR as a potential novel prognostic indicator for complications and prognoses in the stated patient demographic.
Despite P/NR being a relatively nascent parameter within stroke research, preliminary studies indicate its potential predictive capabilities for outcomes in AIS. A study conducted by Jin et al[16] posited P/NR as a singularly protective predictor for 90-d outcomes in AIS, also noting that lower P/NR was concomitant with short-term adverse outcomes. In a parallel vein, Wang et al[23] associated post-rtPA P/NR with E-ND, HT, D-ND, and suboptimal 3-mo outcomes, echoing the predictive utility of lower P/NR for worse outcomes. Matsuoka et al[24] suggested P/NR could indicate a hypercoagulable state, potentially inducing ischemic stroke related to gastric cancer. While P/NR is corroborated in several studies as being associated with thrombosis, its relationship with prognoses of patients receiving intravenous rtPA has not been comprehensively explored[25].
Insights from existing research elucidate that platelet-neutrophil interactions play a pivotal role in inflammation and thrombosis, particularly during AIS[26]. The intravascular thrombosis and ensuing inflammatory response precipitate a reduction in platelets and a surge in neutrophils, cumulatively resulting in diminished P/NR levels. Accordingly, a rational deduction can be drawn that low P/NR levels are independently associated with adverse AIS outcomes.
Moreover, considering the thrombolysis combination, symptomatic intracranial hemorrhage potentially exacerbates symptoms. A plethora of studies have demonstrated that a synergy of decreased platelets and elevated neutrophils can contribute to symptomatic intracranial hemorrhage[27-29]. Gensicke et al[30] provided insights into the mechanistic link between poor outcomes and neutrophils, elucidating that the latter disrupts the blood-brain barrier by liberating matrix metalloproteinase-9 and augmenting reactive oxygen and nitrogen species[29,30]. The conglomeration of these findings substantiates the hypothesis that P/NR may serve as a viable prognostic predictor for patient outcomes.
The present study not only benefits from an ample sample size, ensuring enhanced reliability and persuasive power of results, but also distinguishes itself as one of the few concentrating on the correlation between P/NR and prognosis in AIS patients treated with intravenous rtPA. Nonetheless, the implications of the findings should be interpreted considering several limitations, including the retrospective nature of the study and potential unconsidered confounders. Furthermore, the solitary hospital data source may induce selection bias, P/NR levels were measured only at a single time point (upon admission), and no dynamic monitoring was conducted. Also, numerous pre-existing conditions and infections that influence inflammation could potentially impact the P/NR ratio.
In recapitulation, the findings elucidate that P/NR demonstrates an independent association with unfavorable 3-mo outcomes (mRS ≥ 3), HT, and D-ND. A lower P/NR level could potentially serve as a predictor for adverse outcomes, thereby offering a novel parameter that neurologists might employ for prognosticating stroke outcomes in clinical settings. Prospective studies encompassing larger sample sizes and dynamic P/NR monitoring are requisite for further exploration.
The authors wish to thank Prof. Paskorn Sritipsukho from the Center of Excellence in Applied Epidemiology, Thammasat University, for his help with biostatistical analysis.
| 1. | Bundhamcharoen K, Odton P, Phulkerd S, Tangcharoensathien V. Burden of disease in Thailand: changes in health gap between 1999 and 2004. BMC Public Health. 2011;11:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 2. | GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20:795-820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4299] [Cited by in RCA: 3596] [Article Influence: 899.0] [Reference Citation Analysis (0)] |
| 3. | Yamashita T, Abe K. Pathophysiology of Neuronal Cell Death After Stroke. Stroke Revisited: Pathophysiology of Stroke. 2020;. [DOI] [Full Text] |
| 4. | Herpich F, Rincon F. Management of Acute Ischemic Stroke. Crit Care Med. 2020;48:1654-1663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 472] [Article Influence: 118.0] [Reference Citation Analysis (0)] |
| 5. | Muengtaweepongsa S, Prapa-Anantachai P, Dharmasaroja PA. Not only the Sugar, Early infarct sign, hyperDense middle cerebral artery, Age, Neurologic deficit score but also atrial fibrillation is predictive for symptomatic intracranial hemorrhage after intravenous recombinant tissue plasminogen activator. J Neurosci Rural Pract. 2017;8:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | White-Bateman SR, Schumacher HC, Sacco RL, Appelbaum PS. Consent for intravenous thrombolysis in acute stroke: review and future directions. Arch Neurol. 2007;64:785-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Hacke W, Donnan G, Fieschi C, Kaste M, von Kummer R, Broderick JP, Brott T, Frankel M, Grotta JC, Haley EC Jr, Kwiatkowski T, Levine SR, Lewandowski C, Lu M, Lyden P, Marler JR, Patel S, Tilley BC, Albers G, Bluhmki E, Wilhelm M, Hamilton S; ATLANTIS Trials Investigators; ECASS Trials Investigators; NINDS rt-PA Study Group Investigators. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363:768-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1925] [Cited by in RCA: 1753] [Article Influence: 83.5] [Reference Citation Analysis (0)] |
| 8. | Hong KS, Saver JL. Years of disability-adjusted life gained as a result of thrombolytic therapy for acute ischemic stroke. Stroke. 2010;41:471-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Lokeskrawee T, Muengtaweepongsa S, Patumanond J, Tiamkao S, Thamangraksat T, Phankhian P, Pleumpanupatand P, Sribussara P, Kitjavijit T, Supap A, Rattanaphibool W, Prisiri J. Prognostic Parameters for Symptomatic Intracranial Hemorrhage after Intravenous Thrombolysis in Acute Ischemic Stroke in an Asian Population. Curr Neurovasc Res. 2017;14:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Strbian D, Engelter S, Michel P, Meretoja A, Sekoranja L, Ahlhelm FJ, Mustanoja S, Kuzmanovic I, Sairanen T, Forss N, Cordier M, Lyrer P, Kaste M, Tatlisumak T. Symptomatic intracranial hemorrhage after stroke thrombolysis: the SEDAN score. Ann Neurol. 2012;71:634-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 229] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 11. | Lokeskrawee T, Muengtaweepongsa S, Patumanond J, Tiamkao S, Thamangraksat T, Phankhian P, Pleumpanupat P, Sribussara P, Kitjavijit T, Supap A, Rattanaphibool W, Prisiri J. Prediction of Symptomatic Intracranial Hemorrhage after Intravenous Thrombolysis in Acute Ischemic Stroke: The Symptomatic Intracranial Hemorrhage Score. J Stroke Cerebrovasc Dis. 2017;26:2622-2629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Xu XR, Zhang D, Oswald BE, Carrim N, Wang X, Hou Y, Zhang Q, Lavalle C, McKeown T, Marshall AH, Ni H. Platelets are versatile cells: New discoveries in hemostasis, thrombosis, immune responses, tumor metastasis and beyond. Crit Rev Clin Lab Sci. 2016;53:409-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 215] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 13. | Chen Y, Xiao Y, Lin Z, Xiao X, He C, Bihl JC, Zhao B, Ma X, Chen Y. The Role of Circulating Platelets Microparticles and Platelet Parameters in Acute Ischemic Stroke Patients. J Stroke Cerebrovasc Dis. 2015;24:2313-2320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 14. | Easton AS. Neutrophils and stroke - can neutrophils mitigate disease in the central nervous system? Int Immunopharmacol. 2013;17:1218-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Perez-de-Puig I, Miró-Mur F, Ferrer-Ferrer M, Gelpi E, Pedragosa J, Justicia C, Urra X, Chamorro A, Planas AM. Neutrophil recruitment to the brain in mouse and human ischemic stroke. Acta Neuropathol. 2015;129:239-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 326] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 16. | Jin P, Li X, Chen J, Zhang Z, Hu W, Chen L, Feng X, Shao B. Platelet-to-neutrophil ratio is a prognostic marker for 90-days outcome in acute ischemic stroke. J Clin Neurosci. 2019;63:110-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | Gao B, Pan W, Hu X, Huang H, Ren J, Yang C, Zhou X, Zeng T, Hu J, Li S, Gao Y, Zhang S, Chen G. Neutrophil-Related Ratios Predict the 90-Day Outcome in Acute Ischemic Stroke Patients After Intravenous Thrombolysis. Front Physiol. 2021;12:670323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Muengtaweepongsa S, Dharmasaroja P, Kummark U. Outcomes of intravenous thrombolytic therapy for acute ischemic stroke with an integrated acute stroke referral network: initial experience of a community-based hospital in a developing country. J Stroke Cerebrovasc Dis. 2012;21:42-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Brott T, Adams HP Jr, Olinger CP, Marler JR, Barsan WG, Biller J, Spilker J, Holleran R, Eberle R, Hertzberg V. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3282] [Cited by in RCA: 3803] [Article Influence: 105.6] [Reference Citation Analysis (0)] |
| 20. | Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen MR, Tokgozoglu L, Wiklund O; ESC Scientific Document Group. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6024] [Cited by in RCA: 5296] [Article Influence: 1059.2] [Reference Citation Analysis (0)] |
| 21. | Sims JR, Gharai LR, Schaefer PW, Vangel M, Rosenthal ES, Lev MH, Schwamm LH. ABC/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology. 2009;72:2104-2110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 347] [Cited by in RCA: 343] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 22. | von Kummer R, Broderick JP, Campbell BC, Demchuk A, Goyal M, Hill MD, Treurniet KM, Majoie CB, Marquering HA, Mazya MV, San Román L, Saver JL, Strbian D, Whiteley W, Hacke W. The Heidelberg Bleeding Classification: Classification of Bleeding Events After Ischemic Stroke and Reperfusion Therapy. Stroke. 2015;46:2981-2986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 934] [Article Influence: 93.4] [Reference Citation Analysis (0)] |
| 23. | Wang MQ, Sun YY, Wang Y, Yan XL, Jin H, Sun X, Zhang P, Zhu HJ, Guo ZN, Yang Y. Platelet-to-neutrophil Ratio after Intravenous Thrombolysis Predicts Unfavorable Outcomes in Acute Ischemic Stroke. Curr Neurovasc Res. 2020;17:411-419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Matsuoka T, Yashiro M. Biomarkers of gastric cancer: Current topics and future perspective. World J Gastroenterol. 2018;24:2818-2832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 230] [Cited by in RCA: 317] [Article Influence: 45.3] [Reference Citation Analysis (7)] |
| 25. | Ren B, Duan M, Liu Z, Xu D, Liu D, Zhang J, Wang J, Geng X, Yang S, Han D, Du J. Fibrinogen, Neutrophil-to-Lymphocyte Rate and Platelet-to-Neutrophil Rate as Novel Acute Phase Indicators in Patients with Thromboangiitis Obliterans. Ann Vasc Surg. 2020;65:137-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | García-Culebras A, Durán-Laforet V, Peña-Martínez C, Ballesteros I, Pradillo JM, Díaz-Guzmán J, Lizasoain I, Moro MA. Myeloid cells as therapeutic targets in neuroinflammation after stroke: Specific roles of neutrophils and neutrophil-platelet interactions. J Cereb Blood Flow Metab. 2018;38:2150-2164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 27. | Mönch S, Boeckh-Behrens T, Kreiser K, Blüm P, Hedderich D, Maegerlein C, Berndt M, Lehm M, Wunderlich S, Zimmer C, Friedrich B. Thrombocytopenia and declines in platelet counts: predictors of mortality and outcome after mechanical thrombectomy. J Neurol. 2019;266:1588-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Desai SM, Mehta A, Morrison AA, Gross BA, Jankowitz BT, Jovin TG, Jadhav AP. Endovascular Thrombectomy, Platelet Count, and Intracranial Hemorrhage. World Neurosurg. 2019;127:e1039-e1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Maestrini I, Strbian D, Gautier S, Haapaniemi E, Moulin S, Sairanen T, Dequatre-Ponchelle N, Sibolt G, Cordonnier C, Melkas S, Leys D, Tatlisumak T, Bordet R. Higher neutrophil counts before thrombolysis for cerebral ischemia predict worse outcomes. Neurology. 2015;85:1408-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 161] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 30. | Gensicke H, Al Sultan AS, Strbian D, Hametner C, Zinkstok SM, Moulin S, Bill O, Zini A, Padjen V, Kägi G, Pezzini A, Seiffge DJ, Traenka C, Räty S, Amiri H, Zonneveld TP, Lachenmeier R, Polymeris A, Roos YB, Gumbinger C, Jovanovic DR, Curtze S, Sibolt G, Vandelli L, Ringleb PA, Leys D, Cordonnier C, Michel P, Lyrer PA, Peters N, Tatlisumak T, Nederkoorn PJ, Engelter ST; Thrombolysis in Stroke Patients (TRISP) Collaborators. Intravenous thrombolysis and platelet count. Neurology. 2018;90:e690-e697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |