Published online Jun 20, 2024. doi: 10.5493/wjem.v14.i2.92052
Revised: March 15, 2024
Accepted: April 9, 2024
Published online: June 20, 2024
Processing time: 155 Days and 11.4 Hours
Patients with acute pancreatitis (AP) frequently experience hospital readmissions, posing a significant burden to healthcare systems. Acute peripancreatic fluid collection (APFC) may negatively impact the clinical course of AP. It could worsen symptoms and potentially lead to additional complications. However, clinical evidence regarding the specific association between APFC and early readmission in AP remains scarce. Understanding the link between APFC and readmission may help improve clinical care for AP patients and reduce healthcare costs.
To evaluate the association between APFC and 30-day readmission in patients with AP.
This retrospective cohort study is based on the Nationwide Readmission Database for 2016-2019. Patients with a primary diagnosis of AP were identified. Participants were categorized into those with and without APFC. A 1:1 propensity score matching for age, gender, and Elixhauser comorbidities was performed. The primary outcome was early readmission rates. Secondary outcomes included the incidence of inpatient complications and healthcare utilization. Unadjusted analyses used Mann-Whitney U and χ2 tests, while Cox regression models assessed 30-day readmission risks and reported them as adjusted hazard ratios (aHR). Kaplan-Meier curves and log-rank tests verified readmission risks.
A total of 673059 patients with the principal diagnosis of AP were included. Of these, 5.1% had APFC on initial admission. After propensity score matching, each cohort consisted of 33914 patients. Those with APFC showed a higher incidence of inpatient complications, including septic shock (3.1% vs 1.3%, P < 0.001), portal venous thrombosis (4.4% vs 0.8%, P < 0.001), and mechanical ventilation (1.8% vs 0.9%, P < 0.001). The length of stay (LOS) was longer for APFC patients [4 (3-7) vs 3 (2-5) days, P < 0.001], as were hospital charges ($29451 vs $24418, P < 0.001). For 30-day readmissions, APFC patients had a higher rate (15.7% vs 6.5%, P < 0.001) and a longer median readmission LOS (4 vs 3 days, P < 0.001). The APFC group also had higher readmission charges ($28282 vs $22865, P < 0.001). The presence of APFC increased the risk of readmission twofold (aHR 2.52, 95% confidence interval: 2.40-2.65, P < 0.001). The independent risk factors for 30-day readmission included female gender, Elixhauser Comorbidity Index ≥ 3, chronic pulmonary diseases, chronic renal disease, protein-calorie malnutrition, substance use disorder, depression, portal and splenic venous thrombosis, and certain endoscopic procedures.
Developing APFC during index hospitalization for AP is linked to higher readmission rates, more inpatient complications, longer LOS, and increased healthcare costs. Knowing predictors of readmission can help target high-risk patients, reducing healthcare burdens.
Core Tip: The specific association between acute peripancreatic fluid collection (APFC) and early readmission in patients with acute pancreatitis (AP) has not been well characterized. Using a propensity-matched cohort from the Nationwide Readmission Database, this is the first study to reveal that AP patients with APFC have a significantly higher risk of 30-day readmission compared to those without APFC. Patients with APFC also have a higher incidence of inpatient complications, longer hospital stays, and higher healthcare expenditures. Our findings underscore the need for targeted interventions and close monitoring of AP patients with APFC to reduce readmissions and healthcare costs.
- Citation: Ali H, Inayat F, Rasheed W, Afzal A, Chaudhry A, Patel P, Rehman AU, Anwar MS, Nawaz G, Afzal MS, Sohail AH, Subramanium S, Dahiya DS, Budh D, Mohan BP, Adler DG. Association between acute peripancreatic fluid collections and early readmission in acute pancreatitis: A propensity-matched analysis. World J Exp Med 2024; 14(2): 92052
- URL: https://www.wjgnet.com/2220-315x/full/v14/i2/92052.htm
- DOI: https://dx.doi.org/10.5493/wjem.v14.i2.92052
Acute pancreatitis (AP) is an unpredictable and potentially lethal gastrointestinal disease[1]. The annual worldwide incidence of AP is 33.74 cases per 100000 person-years, and it is more than twice as high in some regions[2,3]. The epidemiological trends in AP are showing improvements, but the overall morbidity and mortality still remain high with an aging population[4,5]. It accounts for substantial healthcare utilization and expenditures in the United States, with hospitalization costs of over $30000 per person[6,7]. Hospital readmission is responsible for a considerable AP-related healthcare burden. In a recent nationwide study, Peery et al[8] revealed that 40036 patients had an early readmission documented out of 259284 index AP hospitalizations. In a narrative literature review, Bogan et al[9] described the overall AP-related readmission rate as ranging from 7% to 34%. Therefore, it is crucial to reduce readmission rates in AP[9]. It requires an adequate understanding of the risk factors associated with rehospitalization[9]. Previous research has identified a number of important risk factors, including recurrent AP, discharge to nonhome facilities, a higher Charlson Comorbidity Index, a longer hospital stay, smoldering symptoms, and/or local pancreatic complications[9-12]. Perti
Acute peripancreatic fluid collection (APFC) is a homogeneous collection with fluid density that can form within or around the pancreas following acute interstitial edematous pancreatitis[14]. The revised Atlanta classification defines it as an early local complication that develops within four weeks with no associated peripancreatic necrosis[14]. A retro
To our knowledge, this is the first cohort study conducted in the United States with the aim of evaluating 30-day readmission rates and predictors linked to APFC in patients with AP using a multicenter database. These predictors may help to identify high-risk patients, provide an opportunity to improve the quality of care and discharge planning, reduce morbidity, and save valuable hospital resources by reducing readmissions in AP. Our findings regarding APFC-related readmission risk may also help in refining the selection criteria for a timely treatment for peripancreatic fluid collections.
We utilized data from the publicly available Nationwide Readmission Database (NRD) from 2016 to 2019[22]. NRD was developed by the Agency for Healthcare Research and Quality (AHRQ) as part of the Healthcare Cost and Utilization Project (HCUP)[22]. The database includes samples from 22 state inpatient registries, accounting for approximately 50% of the population and hospitalizations in the United States[22]. Complete information about sample procedures and NRD design can be accessed at: https://www.hcup-us.ahrq.gov/nrdoverview.jsp. NRD 2016 and above uses the International Classification of Diseases, Tenth Revision (ICD-10) codes to identify diagnoses and procedures. It also contains several hospital-specific variables and predefined comorbid conditions (Elixhauser comorbidities)[23]. The NRD uses unique identification numbers to follow the same patient through multiple hospital stays within the same state. However, it does not track patients across different states or over the transition to a new year. In line with previous research, individuals who had been discharged in December were not included in our analysis because their readmissions might have occurred in January of the subsequent year[24]. This retrospective cohort study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines[25].
The ICD-10 codes were used to identify index admissions with a primary diagnosis of AP (I10_DX1) (Supplementary Table 1). These admissions were further classified into: (1) Patients with a secondary diagnosis of APFC on index admission; and (2) those without a secondary diagnosis of APFC on index admission using the ICD-10 code “K86.3”(I10_DX2-40). According to the revised Atlanta classification, a pancreatic pseudocyst takes at least four weeks to form[14]. Therefore, collections developed during index hospitalization should be reported as APFC. Hence, this code is thought to be more indicative of APFC than pancreatic pseudocyst. Participants were excluded if their age was < 18 years or they had concomitant comorbid conditions such as malignant neoplasm, lymphoma, end-stage renal disease, solid organ malignancies, paraplegia, or paresis. These were considered high-risk conditions that could confound the analysis. Patients were also excluded if they had elective or same-day readmissions. The final weighted analytical cohort had a total of 673059 and 67828 patients before and after propensity score matching, respectively.
The major outcomes of interest included 30-day readmission rates, the incidence of inpatient complications, length of stay (LOS), hospital charges, and factors influencing readmission among AP patients with APFC compared to those without APFC.
Propensity score matching was used to create matched cohorts, which reduced the influence of comorbid imbalances between comparative cohorts. Each case was ascribed a propensity score based on a multivariable logistic regression model that considered the baseline demographics of the patient, any Elixhauser comorbidities, and the characteristics of the institution. We then utilized a 1:1 matching algorithm by general caliper matching (without replacement) using a caliper width equal to 0.2 of the standard deviation of the propensity score[26]. In unadjusted analyses, continuous variables were reported as medians with an interquartile range (IQR) and were compared using Mann-Whitney U tests. Categorical variables were presented as frequencies with percentages and were compared using χ2 tests. The discharge weights provided by the HCUP were used to obtain national estimates. All P values were two-sided. A univariate Cox regression model was initially used for readmission risk to report hazard ratios (HR) with a 95% confidence interval (CI) in the matched cohort. A multivariate model was then prepared for final predictors, including variables with P < 0.20 from univariate analysis, and results were reported as adjusted hazard ratios (aHR). The Kaplan-Meier curve was generated to display the overall risk of readmission between cases and controls, and significance was assessed using the log-rank test. The Statistical Software for Data Science (STATA) (StataCorp LLC, College Station, TX, United States), version 16.0, was used for statistical analysis. The ‘pmsampsize’ command in STATA was utilized to calculate the minimum sample size to assess a risk ratio of at least 50% (HR 1.5) between cases and controls for readmission. This computation indicated that a minimum sample size of 800 in each arm was sufficient.
The NRD uses de-identification and anonymization strategies to protect the privacy of patients. The present study did not require institutional review board oversight as it contains de-identified, publicly available observations that cannot be connected to or identified with any specific person. The patient consent for participation and publication of these data was also waived. According to the HCUP Data Use Agreement, any individual table cell counts of ≤ 10 have been masked to ensure privacy and compliance.
Clinical characteristics of patients with a primary diagnosis of AP stratified by APFC on index admission are outlined (Table 1). In the unmatched cohort of 673059 patients, 5.1% had a secondary diagnosis of APFC. The propensity-matched cohort included 33914 in each arm with a satisfactory balance of comorbidities. In the matched cohort, the median (IQR) index LOS was longer among patients with APFC compared to those without APFC [4 (3-7) vs 3 (2-5) days, P < 0.001]. The median (IQR) index hospitalization cost was higher in the APFC cohort than the non-APFC cohort [$29451 ($17292-$56774) vs $24418 ($14865-$42640), P < 0.001]. The Elixhauser comorbidities of index AP hospitalizations before and after matching were also stratified by APFC (Table 2).
| Patient characteristics | Before matching | After matching | ||||
| No acute peripancreatic fluid collections | Acute peripancreatic fluid collections | P value | No acute peripancreatic fluid collections | Acute peripancreatic fluid collections | P value | |
| Total patients | 638801 | 34258 | 33914 | 33914 | ||
| Age in years at admission, median (IQR) | 52.0 (39.0, 63.0) | 49.0 (38.0, 59.0) | < 0.001 | 51.0 (39.0, 61.0) | 49.0 (38.0, 58.0) | < 0.001 |
| Age groups (yr) | < 0.001 | < 0.001 | ||||
| 18-33 | 108146 (16.9) | 5663 (16.5) | 5716 (16.9) | 5641 (16.6) | ||
| 34-49 | 181354 (28.4) | 12056 (35.2) | 10353 (30.5) | 11962 (35.3) | ||
| 50-64 | 200484 (31.4) | 11619 (33.9) | 11169 (32.9) | 11490 (33.9) | ||
| 65-79 | 107936 (16.9) | 4118 (12.0) | 4895 (14.4) | 4033 (11.9) | ||
| ≥ 80 | 40881 (6.4) | 802 (2.3) | 1781 (5.3) | 788 (2.3) | ||
| Length of stay (days), median (IQR) | 3.0 (2.0, 5.0) | 4.0 (3.0, 7.0) | < 0.001 | 3.0 (2.0, 5.0) | 4.0 (3.0, 7.0) | < 0.001 |
| Total charges (USD), median (IQR) | 24238.0 (14623.0, 42043.0) | 29616.5 (17365.0, 57476.0) | < 0.001 | 24418.5 (14865.0, 42640.5) | 29451.0 (17292.0, 56774.0) | < 0.001 |
| 30-day readmission | 37949 (5.9) | 5363 (15.7) | 2215 (6.5) | 5326 (15.7) | < 0.001 | |
| Elixhauser Comorbidity Index score | < 0.001 | 0.093 | ||||
| 0 | 49536 (7.8) | 1366 (4.0) | 1430 (4.2) | 1366 (4.0) | ||
| 1 | 97030 (15.2) | 3875 (11.3) | 3994 (11.8) | 3875 (11.4) | ||
| 2 | 131055 (20.5) | 6353 (18.5) | 6472 (19.1) | 6353 (18.7) | ||
| ≥ 3 | 361180 (56.5) | 22664 (66.2) | 22018 (64.9) | 22320 (65.8) | ||
| Primary payer | < 0.001 | < 0.001 | ||||
| Medicare | 195352 (32.1) | 7702 (23.8) | 9348 (29.1) | 7572 (23.6) | ||
| Medicaid | 140070 (23.0) | 9413 (29.1) | 8332 (25.9) | 9340 (29.2) | ||
| Private | 213873 (35.1) | 11274 (34.8) | 10763 (33.5) | 11163 (34.8) | ||
| Other | 60026 (9.9) | 3980 (12.3) | 3697 (11.5) | 3958 (12.4) | ||
| Median household income national quartile for patient ZIP code | < 0.001 | 0.005 | ||||
| 1st (0-25th) | 203221 (32.2) | 11120 (32.9) | 11169 (33.3) | 11002 (32.8) | ||
| 2nd (26th-50th) | 177998 (28.2) | 9619 (28.4) | 9373 (28.0) | 9535 (28.5) | ||
| 3rd (51st-75th) | 148894 (23.6) | 8093 (23.9) | 7756 (23.2) | 8020 (23.9) | ||
| 4th (76th-100th) | 100757 (16.0) | 5018 (14.8) | 5199 (15.5) | 4955 (14.8) | ||
| Disposition of patient (uniform) | < 0.001 | < 0.001 | ||||
| Routine | 561425 (87.9) | 28082 (82.0) | 29204 (86.1) | 27894 (82.3) | ||
| Transfer to short-term hospital | 4817 (0.8) | 753 (2.2) | 275 (0.8) | 742 (2.2) | ||
| Transfer other: SNF, ICF, another type of facility | 17446 (2.7) | 1392 (4.1) | 1074 (3.2) | 1320 (3.9) | ||
| Home health care | 27211 (4.3) | 2600 (7.6) | 1600 (4.7) | 2540 (7.5) | ||
| Against medical advice | 25350 (4.0) | 1209 (3.5) | 1582 (4.7) | 1207 (3.6) | ||
| Died during hospitalization | 2386 (0.4) | 209 (0.6) | < 0.001 | 169 (0.5) | 198 (0.6) | 0.12 |
| Factors | Before matching | After matching | ||||
| No acute peripancreatic fluid collections | Acute peripancreatic fluid collections | P value | No acute peripancreatic fluid collections | Acute peripancreatic fluid collections | P value | |
| Total patients | 638801 | 34258 | 33914 | 33914 | ||
| Congestive heart failure | 41809 (6.5) | 1922 (5.6) | < 0.001 | 1804 (5.3) | 1804 (5.3) | 1.00 |
| Cardiac arrhythmias | 71251 (11.2) | 3992 (11.7) | 0.004 | 3745 (11.0) | 3909 (11.5) | 0.047 |
| Valvular disease | 13556 (2.1) | 505 (1.5) | < 0.001 | 439 (1.3) | 439 (1.3) | 1.00 |
| Pulmonary circulation | 7130 (1.1) | 482 (1.4) | < 0.001 | 380 (1.1) | 380 (1.1) | 1.00 |
| Peripheral vascular disease | 22966 (3.6) | 1354 (4.0) | < 0.001 | 1248 (3.7) | 1248 (3.7) | 1.00 |
| Uncomplicated hypertension | 293986 (46.0) | 16996 (49.6) | < 0.001 | 16804 (49.5) | 16804 (49.5) | 1.00 |
| Chronic pulmonary diseases | 99431 (15.6) | 5533 (16.2) | 0.004 | 5264 (15.5) | 5440 (16.0) | 0.064 |
| Uncomplicated diabetes | 90054 (14.1) | 4365 (12.7) | < 0.001 | 4261 (12.6) | 4261 (12.6) | 1.00 |
| Complicated diabetes | 91044 (14.3) | 4178 (12.2) | < 0.001 | 4049 (11.9) | 4049 (11.9) | 1.00 |
| Hypothyroidism | 58431 (9.1) | 2132 (6.2) | < 0.001 | 2028 (6.0) | 2028 (6.0) | 1.00 |
| Chronic renal disease | 55947 (8.8) | 2114 (6.2) | < 0.001 | 2458 (7.2) | 2063 (6.1) | 0.088 |
| Liver disease | 130401 (20.4) | 8674 (25.3) | < 0.001 | 8303 (24.5) | 8554 (25.2) | 0.096 |
| PUD excluding bleeding | 10343 (1.6) | 634 (1.9) | < 0.001 | 582 (1.7) | 582 (1.7) | 1.00 |
| HIV/AIDS | 2063 (0.3) | 128 (0.4) | 0.11 | 115 (0.3) | 115 (0.3) | 1.00 |
| Rheumatoid arthritis/CVD | 13799 (2.2) | 569 (1.7) | < 0.001 | 504 (1.5) | 504 (1.5) | 1.00 |
| Coagulopathy | 42399 (6.6) | 2778 (8.1) | < 0.001 | 2667 (7.9) | 2667 (7.9) | 1.00 |
| Obesity | 118599 (18.6) | 4066 (11.9) | < 0.001 | 3946 (11.6) | 3946 (11.6) | 1.00 |
| Weight loss | 34338 (5.4) | 6255 (18.3) | < 0.001 | 6053 (17.8) | 6053 (17.8) | 1.00 |
| Fluid and electrolyte disorder | 251252 (39.3) | 16382 (47.8) | < 0.001 | 16174 (47.7) | 16174 (47.7) | 1.00 |
| Blood loss anemia | 1984 (0.3) | 211 (0.6) | < 0.001 | 119 (0.4) | 206 (0.6) | < 0.001 |
| Iron-deficiency anemia | 23358 (3.7) | 2539 (7.4) | < 0.001 | 1557 (4.6) | 2512 (7.4) | < 0.001 |
| Alcohol abuse | 198085 (31.0) | 17070 (49.8) | < 0.001 | 16908 (49.9) | 16908 (49.9) | 1.00 |
| Substance abuse | 57163 (8.9) | 4181 (12.2) | < 0.001 | 4074 (12.0) | 4074 (12.0) | 1.00 |
| Tobacco use disorder | 96674 (15.1) | 4761 (13.9) | < 0.001 | 4616 (13.6) | 4689 (13.8) | 0.42 |
| Smoking history | 4722 (0.7) | 276 (0.8) | 0.16 | 199 (0.6) | 274 (0.8) | < 0.001 |
| Psychoses | 7357 (1.2) | 471 (1.4) | < 0.001 | 418 (1.2) | 469 (1.4) | 0.085 |
| Depression | 93044 (14.6) | 5899 (17.2) | < 0.001 | 5445 (16.1) | 5804 (17.1) | < 0.001 |
| Complicated hypertension | 72236 (11.3) | 2941 (8.6) | < 0.001 | 3136 (9.2) | 2836 (8.4) | < 0.001 |
In the matched cohort, there was a higher incidence of septic shock (3.1% vs 1.3%, P < 0.001), mechanical ventilation (1.8% vs 0.9%, P < 0.001), portal venous thrombosis (4.4% vs 0.8%, P < 0.001), splenic venous thrombosis (2.4% vs 0.5%, P < 0.001), intensive care unit (ICU) level care (1.5% vs 0.8%, P < 0.001), vasopressor use (0.4% vs 0.2%, P < 0.001), diarrhea (3.2% vs 2.6%, P < 0.001), and jaundice (3.0% vs 1.4%, P < 0.001) in patients with APFC compared to those without APFC (Table 3). Participants in both cohorts also showed a higher predilection for a number of endoscopic diagnostic and therapeutic procedures.
| Clinical outcomes | Before matching | After matching | ||||
| No acute peripancreatic fluid collections | Acute peripancreatic fluid collections | P value | No acute peripancreatic fluid collections | Acute peripancreatic fluid collections | P value | |
| Total patients | 638801 | 34258 | 33914 | 33914 | ||
| Cholangitis | 3814 (0.6) | 164 (0.5) | 0.005 | 190 (0.6) | 160 (0.5) | 0.11 |
| Mechanical ventilation | 3309 (0.5) | 669 (2.0) | < 0.001 | 300 (0.9) | 626 (1.8) | < 0.001 |
| Nausea | 13222 (2.1) | 650 (1.9) | 0.029 | 752 (2.2) | 641 (1.9) | 0.003 |
| Diarrhea | 15337 (2.4) | 1089 (3.2) | < 0.001 | 898 (2.6) | 1074 (3.2) | < 0.001 |
| Septic shock | 5693 (0.9) | 1110 (3.2) | < 0.001 | 451 (1.3) | 1057 (3.1) | < 0.001 |
| Portal venous thrombosis | 4079 (0.6) | 1521 (4.4) | < 0.001 | 265 (0.8) | 1487 (4.4) | < 0.001 |
| Splenic venous thrombosis | 3194 (0.5) | 818 (2.39) | < 0.001 | 154 (0.5) | 811 (2.4) | < 0.001 |
| ICU level admission | 3118 (0.5) | 529 (1.5) | < 0.001 | 284 (0.8) | 496 (1.5) | < 0.001 |
| Vasopressor use | 772 (0.1) | 138 (0.4) | < 0.001 | 70 (0.2) | 130 (0.4) | < 0.001 |
| Acute kidney injury | 68459 (10.7) | 3843 (11.2) | 0.004 | 4038 (11.9) | 3744 (11.0) | < 0.001 |
| New RRT during admission | 8545 (1.3) | 434 (1.3) | 0.27 | 386 (1.1) | 421 (1.2) | 0.22 |
| Abdominal pain | 3070 (0.5) | 124 (0.4) | 0.002 | 159 (0.5) | 120 (0.4) | 0.019 |
| Jaundice | 8149 (1.3) | 1024 (3.0) | < 0.001 | 461 (1.4) | 1013 (3.0) | < 0.001 |
| Obstruction of bile duct | 35968 (5.6) | 2340 (6.8) | < 0.001 | 2259 (6.7) | 2319 (6.8) | 0.36 |
| Endoscopic retrograde cholangiography (no intervention) | 19309 (3.0) | 395 (1.2) | < 0.001 | 740 (2.2) | 390 (1.1) | < 0.001 |
| ERCP biliary with intervention | 31525 (4.9) | 1150 (3.4) | < 0.001 | 1339 (3.9) | 1134 (3.3) | < 0.001 |
| Endoscopic dilation of ampulla and biliary duct | 9456 (1.5) | 253 (0.7) | < 0.001 | 413 (1.2) | 249 (0.7) | < 0.001 |
| Endoscopic insertion of stent (tube) into bile duct | 9784 (1.5) | 751 (2.2) | < 0.001 | 495 (1.5) | 743 (2.2) | < 0.001 |
| Endoscopic removal of stone(s) from biliary tract | 19161 (3.0) | 388 (1.1) | < 0.001 | 759 (2.2) | 384 (1.1) | < 0.001 |
| Endoscopic biopsy of bile duct | 2002 (0.3) | 62 (0.2) | < 0.001 | 99 (0.3) | 62 (0.2) | 0.004 |
| ERCP pancreatic with intervention | 6282 (1.0) | 1107 (3.2) | < 0.001 | 323 (1.0) | 1094 (3.2) | < 0.001 |
| Endoscopic insertion of stent (tube) into pancreatic duct | 5263 (0.8) | 1005 (2.9) | < 0.001 | 278 (0.8) | 993 (2.9) | < 0.001 |
| Endoscopic removal of stone(s) from pancreatic duct | 1045 (0.2) | 152 (0.4) | < 0.001 | 64 (0.2) | 150 (0.4) | < 0.001 |
| Endoscopic dilation of pancreatic duct | 583 (0.1) | 51 (0.1) | < 0.001 | 28 (0.1) | 50 (0.1) | 0.013 |
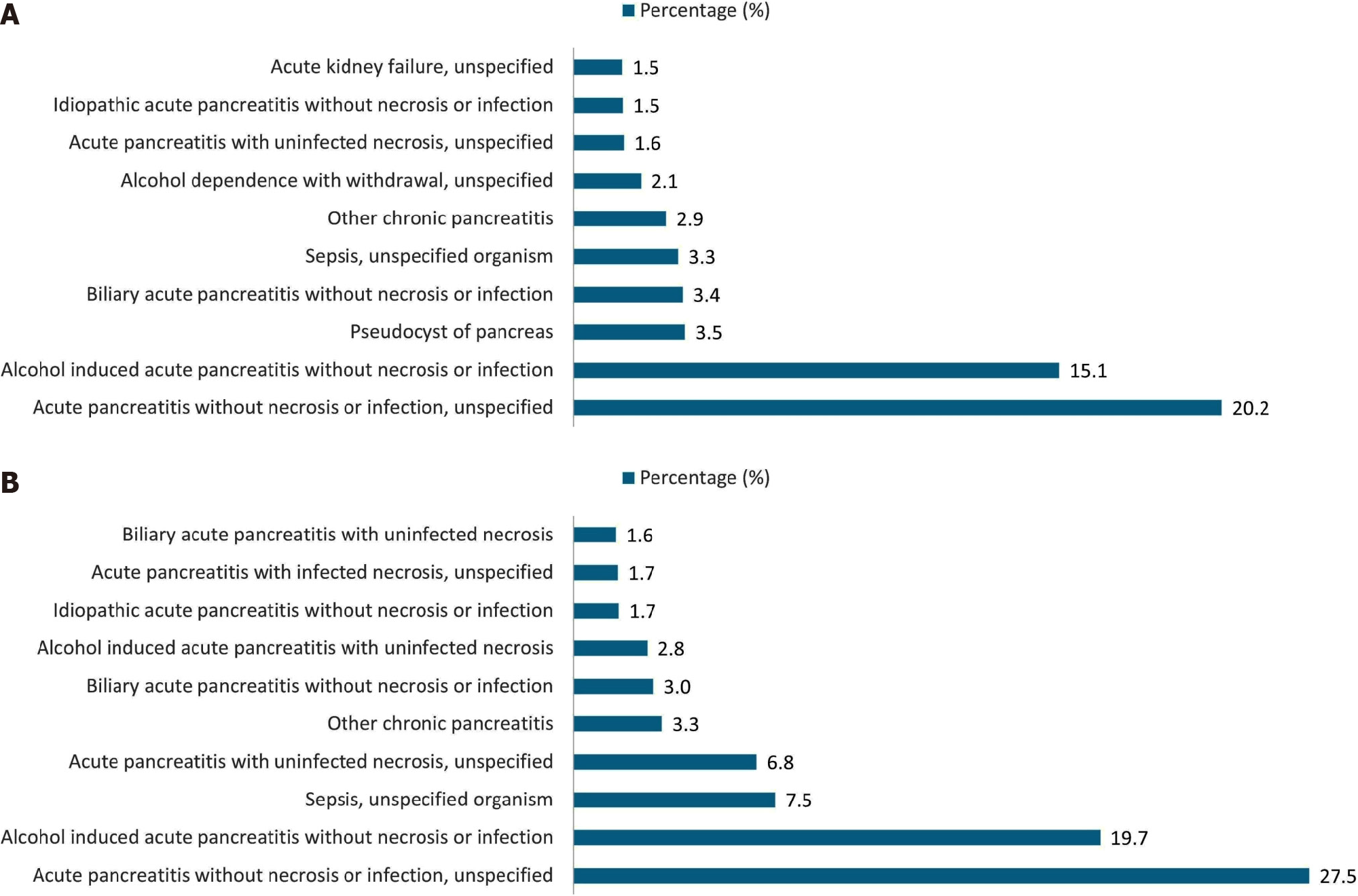
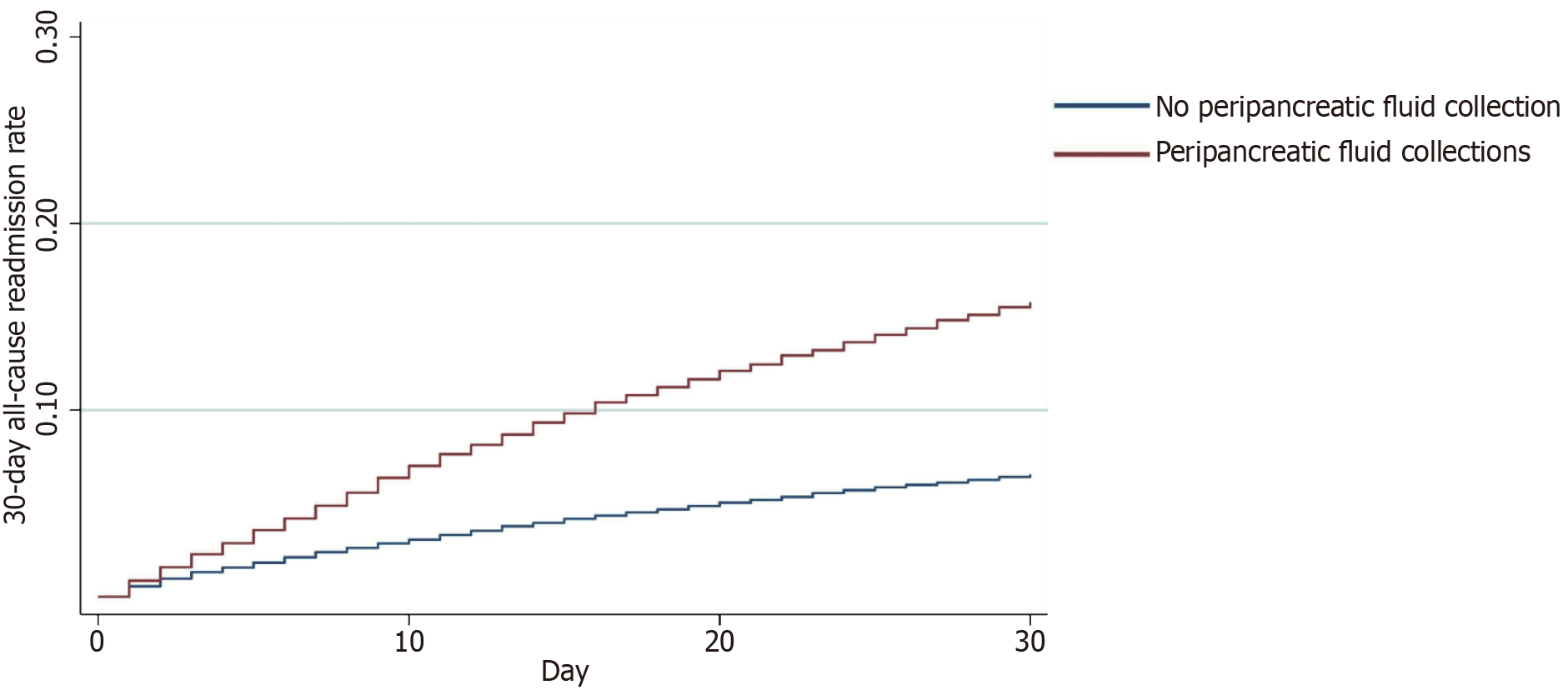
After propensity score matching, 30-day readmissions were higher among AP patients with APFC than non-APFC (15.7% vs 6.5%, P < 0.001). For patients who had an APFC on readmission, the median readmission LOS was longer than patients without an APFC [4 (IQR 3-7) vs 3 (IQR 2-5) days, P < 0.001]. The median readmission costs were also higher among patients who had APFCs on readmission compared to the non-APFC cohort [$28282 ($17012-$50543) vs $22865 ($14131-$39627), P < 0.001]. A plethora of causes were found to be responsible for hospital readmissions in both cohorts (Figure 1). Notably, 3.5% of patients who did not have APFC at their index admission were readmitted due to a new pseudocyst/APFC diagnosis. The presence of an APFC increased the risk of readmission twofold [aHR 2.52 (95%CI: 2.40-2.65), P < 0.001] (Figure 2).
A complete univariate and multivariate analysis was conducted to find independent predictors of 30-day readmission after discharge with a primary diagnosis of AP with APFC (Supplementary Table 2). A number of variables were found to increase the risk of readmission, including female gender [aHR 0.93 (95%CI: 0.89-0.98) P = 0.01], Elixhauser Comorbidity Index ≥ 3 [aHR 1.55 (95%CI: 1.34-1.8), P < 0.001], chronic pulmonary diseases [aHR 1.15 (95%CI: 1.08-1.22), P < 0.001], chronic renal disease (ESRD not included) [aHR 1.17 (95%CI: 1.07-1.41), P = 0.01], protein-calorie malnutrition [aHR 1.19 (95%CI: 1.12-1.26), P < 0.001], alcohol abuse [aHR 1.17 95%CI: 1.11-1.23), P < 0.001], substance abuse [aHR 1.11 (95%CI: 1.03-1.2), P = 0.005], depression [aHR 1.11 (95%CI: 1.04-1.18), P = 0.045], portal venous thrombosis [aHR 1.65 (95%CI: 1.47-1.85), P < 0.001], and splenic venous thrombosis [aHR 1.57 (95%CI: 1.34-1.84), P < 0.001]. Several procedures were also identified as predictors, including endoscopic retrograde cholangiography (no intervention) [aHR 0.66 (95%CI: 0.52-0.85), P = 0.001], endoscopic dilation of the ampulla and biliary duct [aHR 0.65 (95%CI: 0.47-0.9), P = 0.01], and endoscopic removal of stone(s) from the biliary tract [aHR 0.69 (95%CI: 0.54-0.89), P = 0.005].
This population-based study shows that patients diagnosed with an APFC during their initial AP hospitalization have a higher 30-day readmission risk. Patients with APFC also have a higher incidence of inpatient complications, a longer LOS, and higher healthcare costs than those without APFC. A number of readmission predictors were identified to help stratify high-risk AP patients with APFC, which may aid in reducing healthcare burden.
Hospital readmission for AP has been extensively researched[9,27,28]. However, the specific association between APFC and early readmission has not been investigated. Our study revealed a significantly higher 30-day readmission risk among AP patients with APFC compared to those without APFC (aHR 2.52, P < 0.001). While there is a paucity of evidence on gender-specific outcomes of AP, readmission has often been associated with male gender[11,13,29]. However, a prospective study also identified female gender as a significant predictor of AP readmission (odds ratio 2.57, 95%CI: 1.13-5.81, P = 0.024)[30]. Our analysis also revealed female gender as a risk factor for 30-day readmission in AP patients with APFC. Consistent with previous research, an Elixhauser Comorbidity Index score of ≥ 3 was another independent predictor of 30-day readmission in our APFC cohort[9,29]. A retrospective study from the United States revealed chronic pulmonary disease as a readmission predictor after biliary AP (aHR 1.22, P < 0.001)[31]. Previous studies demonstrated that protein-energy malnutrition and chronic kidney disease may also predict readmission in patients with AP[27,32]. Similarly, early systemic anticoagulation in severe AP cases may help in reducing venous thrombosis, which may also help in decreasing readmission risk[33]. In our analysis, chronic pulmonary diseases, protein-calorie malnutrition, chronic kidney disease, and portal and splenic venous thrombosis were also identified as predictors of readmission. Therefore, it is important for clinicians to screen AP patients with APFC for these comorbidities to evaluate the readmission risk.
Alcohol-associated AP was the second most common etiology for 30-day readmissions in our study. It was also associated with worse outcomes in both the APFC and the non-APFC cohorts. Alcohol abuse has been linked in the past to increased rates of AP readmissions. In two retrospective studies from the United States, 30-day readmission rates for alcohol-related AP ranged from 12% to 70%[34,35]. Furthermore, alcoholic etiology is also independently associated with organ failure and pancreatic necrosis in index AP events[36]. Pertinently, Sorrento et al[37] conducted a retrospective study showing AP patients who received alcohol cessation counseling were half as likely to be readmitted after 30 days compared to those who did not get therapy (odds ratio 0.52, P = 0.046). Similarly, a post-hoc data analysis showed that 79% of patients with alcohol-related AP who received brief psychological intervention reported abstinence and no 30-day readmission for recurrent AP[38]. Moreover, the brief intervention effectively decreased gamma-glutamyl transferase levels, correlating this reduction with alcohol abstinence[38]. Notably, depression was also one of the predictors of AP readmission in our APFC cohort. Therefore, psychiatric evaluation and therapy may help to decrease readmissions in AP patients with APFC suspected to have depression or substance use disorder.
Hospital readmissions are a significant problem in the context of healthcare policy and reform[39,40]. The rates of readmission may indicate the quality of care offered by hospitals, which may be independent of patient-level factors[41]. As up to 50% of readmissions are potentially preventable, a decreased complexity of inpatient care may help improve the early readmission rate[42-44]. Our study revealed that the presence of APFC increases the risk of readmission up to twofold. In a retrospective study from the United States, AP patients with 30-day readmissions had a 4.5 times higher one-year mortality risk than those who were not readmitted (HR 4.5, 95%CI: 2.2-9.1)[45]. Therefore, the concurrent occurrence of APFC and any of the aforementioned predictors in index AP hospitalizations merits effective prognostication and clinical vigilance. AP patients with APFC may also require tailored clinical management[46]. Approximately 50% of APFCs produce minimal to no symptoms and undergo spontaneous resolution with supportive medical care[47]. However, persistent symptoms and APFC-related complications necessitate invasive treatments, including percutaneous, surgical, or endoscopic drainage procedures[48].
We also observed a higher rate of inpatient complications among AP patients with APFC compared to those without APFC. These included ICU admission, progression to septic shock, vasopressor use, mechanical ventilation, and portal and splenic venous thrombosis. In two retrospective studies from China, the systemic immune-inflammation index (SII) was considered a severity predictor and a marker of serious complications like acute kidney injury (AKI) in patients with severe AP[49,50]. In a retrospective study from Turkey, Solakoglu et al[51] revealed that the presence of APFC in AP patients was associated with higher values of SII and C-reactive protein. Therefore, the higher rate of inpatient complications may be explained by possible higher levels of SII in the APFC cohort compared to the non-APFC cohort. Sepsis, vasopressor use, and mechanical ventilation in AP patients may also predict other major complications, such as early AKI[52]. A prospective trial from Korea also underscored the clinical importance of close observation for late complications in patients with an early radiological identification of an APFC, especially in moderately severe and severe AP patients[53]. Therefore, APFC detection supplements the need for careful surveillance in moderate and severe AP.
In our study, AP patients with APFC showed higher odds of progression to septic shock compared to those without APFC. A prospective multicenter study from Germany showed colonization of peripancreatic fluid collections in 59% of cultures of the collections[54]. Notably, this study had no clear demarcation of the type of peripancreatic collections (50% of the patients were hospitalized for < 1 month), the positive cultures could have been related to pancreatic seeding of extrapancreatic infections, and it may be a simple colonization rather than a true infection[55]. APFCs have previously been considered low-risk entities for infections. However, our results are concerning due to the higher risk of septic shock among AP patients with APFC, possibly following infected APFCs or extrapancreatic infections. It shows the need for pertinent measures to avoid septic complications in these patients. Clinical practice guidelines from the American College of Gastroenterology, the International Association of Pancreatology (IAP), and the American Pancreatic Association (APA) require a confirmed pancreatic or extrapancreatic infection to start antibiotic treatment[56,57]. Therefore, clinicians should remain vigilant for concomitant infections in AP patients. Early diagnosis and treatment may help to avoid serious complications such as septic shock.
Inpatient complications may also occur following iatrogenic adverse events in index hospitalizations. Our data show that both cohorts underwent a variety of endoscopic diagnostic and therapeutic procedures. Moreover, published literature describes the risk of late complications weeks after the intervention, specifically for peripancreatic fluid collections[58,59]. In our analysis, several procedures were also identified as 30-day readmission predictors in AP patients with APFC, including endoscopic retrograde cholangiography (no intervention), endoscopic dilation of the ampulla and biliary duct, and endoscopic stone removal from the biliary tract. In a retrospective study from the United States, Kim et al[60] showed that surgical or percutaneous drainage of APFC and pancreatic pseudocysts may have a higher burden of illness and an increased local complication risk necessitating intervention compared to endoscopic drainage procedures. Therefore, it is important to opt for appropriate drainage procedures after careful patient selection[60]. Moreover, the American Society of Gastrointestinal Endoscopy recommends bacteremia risk assessment for endoscopic procedures such as drainage of peripancreatic collections[61]. Patients at risk of septic complications may receive antibiotic prophylaxis after this assessment[61]. Notably, the inpatient mortality was similar in both cohorts during index admissions (0.6% vs 0.5%, P = 0.12). However, AP patients with APFC may require targeted clinical treatment due to their higher risk of complications.
The APFC cohort showed higher hospital resource utilization compared to the non-APFC cohort in our analysis. In index hospitalizations, the LOS was longer (4 vs 3 days, P < 0.001) and costlier ($29451 vs $24418, P < 0.001). These trends could be attributed to the higher incidence of inpatient complications in our APFC cohort. Furthermore, these patients were more frequently discharged to short-term hospitals, skilled nursing facilities, and home health care. This trend may have also contributed to additional healthcare costs in the APFC cohort. The readmissions also revealed higher costs in the APFC cohort than the non-APFC cohort ($28282 vs $22865, P < 0.001). The median cost of readmissions was also higher compared to index hospitalizations. In a recent international survey, Nagy et al[62] revealed the efficacy of the AP discharge protocol in significantly reducing median LOS and recurrent AP-related readmission rates. Our findings could enable pancreatologists to devise novel discharge protocols that include index admission APFC and assess their impact in future research.
This retrospective cohort study has several strengths. It has a large sample size, sufficient statistical power to detect meaningful differences in outcomes, and the generalizability of the findings. Our sample population from the NRD also permits the evaluation of real-world healthcare utilization trends, including readmission rates and healthcare costs. Moreover, we used propensity-matching techniques in our analysis. In retrospective studies, these techniques can minimize confounding variables and increase the validity of the findings. Therefore, this study has pertinent implications for highlighting the clinical association between APFC and readmission risk in patients with AP. It also provides crucial insights into healthcare outcomes and utilization patterns in these patients.
There are certain limitations to our study. One major limitation is the lack of data regarding the severity of illnesses and laboratory evaluations in the NRD. Furthermore, coding accuracy in administrative databases may vary, potentially leading to errors in identifying outcomes of interest or misclassification bias. There is also potential for selection bias in propensity score matching, which balances patient characteristics between those with and without APFC. During data extraction for our analysis, efforts were made to include only patients with an APFC diagnosis and exclude those with pancreatic necrosis (K8501, K8502, K8511, K8512, K8521, K8522, K8531, K8532, K8581, and K8582). However, there is a possibility that some misclassification may have occurred. Finally, retrospective studies cannot establish causality as they are observational and cannot account for unmeasured confounding variables.
This study reveals a correlation between the development of an APFC during index AP hospitalization and higher rates of readmission, increased inpatient complications, longer LOS, and higher healthcare costs. The readmission predictors included female gender, Elixhauser Comorbidity Index ≥ 3, chronic pulmonary diseases, chronic renal disease, protein-calorie malnutrition, alcohol abuse, substance abuse, depression, portal and splenic venous thrombosis, and certain procedures. The readmission rate for AP patients with APFC may be reduced by vigilant surveillance of these predictors, efficient infection screening, and safe interventions. Psychological evaluation and counseling strategies can also help AP patients with psychiatric comorbidities. Our analysis may enable pancreatologists and gastroenterologists to improve patient outcomes by including APFC as a factor in AP discharge protocols.
The preliminary form of these data was presented as an abstract at the Digestive Disease Week (DDW), May 19-21, 2024 in Washington, DC, United States.
| 1. | Mederos MA, Reber HA, Girgis MD. Acute Pancreatitis: A Review. JAMA. 2021;325:382-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 491] [Article Influence: 122.8] [Reference Citation Analysis (1)] |
| 2. | Xiao AY, Tan ML, Wu LM, Asrani VM, Windsor JA, Yadav D, Petrov MS. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol. 2016;1:45-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 475] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 3. | Iannuzzi JP, King JA, Leong JH, Quan J, Windsor JW, Tanyingoh D, Coward S, Forbes N, Heitman SJ, Shaheen AA, Swain M, Buie M, Underwood FE, Kaplan GG. Global Incidence of Acute Pancreatitis Is Increasing Over Time: A Systematic Review and Meta-Analysis. Gastroenterology. 2022;162:122-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 359] [Article Influence: 119.7] [Reference Citation Analysis (1)] |
| 4. | Li CL, Jiang M, Pan CQ, Li J, Xu LG. The global, regional, and national burden of acute pancreatitis in 204 countries and territories, 1990-2019. BMC Gastroenterol. 2021;21:332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (1)] |
| 5. | Krishna SG, Kamboj AK, Hart PA, Hinton A, Conwell DL. The Changing Epidemiology of Acute Pancreatitis Hospitalizations: A Decade of Trends and the Impact of Chronic Pancreatitis. Pancreas. 2017;46:482-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 177] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 6. | Petrov MS, Yadav D. Global epidemiology and holistic prevention of pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16:175-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 526] [Article Influence: 87.7] [Reference Citation Analysis (0)] |
| 7. | Wadhwa V, Patwardhan S, Garg SK, Jobanputra Y, Lopez R, Sanaka MR. Health Care Utilization and Costs Associated With Acute Pancreatitis. Pancreas. 2017;46:410-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Peery AF, Crockett SD, Murphy CC, Jensen ET, Kim HP, Egberg MD, Lund JL, Moon AM, Pate V, Barnes EL, Schlusser CL, Baron TH, Shaheen NJ, Sandler RS. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2021. Gastroenterology. 2022;162:621-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 477] [Article Influence: 159.0] [Reference Citation Analysis (1)] |
| 9. | Bogan BD, McGuire SP, Maatman TK. Readmission in acute pancreatitis: Etiology, risk factors, and opportunities for improvement. Surg Open Sci. 2022;10:232-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 10. | Garg SK, Campbell JP, Anugwom C, Wadhwa V, Singh R, Gupta N, Sanaka MR. Incidence and Predictors of Readmissions in Acute Pancreatitis: A Nationwide Analysis. Pancreas. 2018;47:46-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 11. | Melitas C, Sorser SA, Haidar A, Battista N, Turk I, Gardner TB, Adler DG. Factors predicting readmission within 30 days of acute pancreatitis attack: A prospective study. Pancreatology. 2019;19:805-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Whitlock TL, Repas K, Tignor A, Conwell D, Singh V, Banks PA, Wu BU. Early readmission in acute pancreatitis: incidence and risk factors. Am J Gastroenterol. 2010;105:2492-2497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Vipperla K, Papachristou GI, Easler J, Muddana V, Slivka A, Whitcomb DC, Yadav D. Risk of and factors associated with readmission after a sentinel attack of acute pancreatitis. Clin Gastroenterol Hepatol. 2014;12:1911-1919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4328] [Article Influence: 360.7] [Reference Citation Analysis (45)] |
| 15. | Molla NW, Alsergani AH, Alyamani AA, Aljohani MA, Aljohani AA, Alfaiz FA, Alomar MO, BinMayouf MS. Incidence of peripancreatic fluid collections in patients presenting with acute pancreatitis. Saudi Med J. 2022;43:1341-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Cui ML, Kim KH, Kim HG, Han J, Kim H, Cho KB, Jung MK, Cho CM, Kim TN. Incidence, risk factors and clinical course of pancreatic fluid collections in acute pancreatitis. Dig Dis Sci. 2014;59:1055-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | Braha J, Tenner S. Fluid Collections and Pseudocysts as a Complication of Acute Pancreatitis. Gastrointest Endosc Clin N Am. 2018;28:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Oh CH, Song TJ, Lee JK, Park JS, Lee JM, Son JH, Jang DK, Choi M, Byeon JS, Lee IS, Lee ST, Choi HS, Kim HG, Chun HJ, Park CG, Cho JY. Clinical Practice Guidelines for the Endoscopic Management of Peripancreatic Fluid Collections. Gut Liver. 2021;15:677-693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 19. | Zhang HM, Ke HT, Ahmed MR, Li YJ, Nabi G, Li MH, Zhang JY, Liu D, Zhao LX, Liu BR. Endoscopic transgastric fenestration versus percutaneous drainage for management of (peri)pancreatic fluid collections adjacent to gastric wall (with video). World J Gastroenterol. 2023;29:5557-5565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Reference Citation Analysis (2)] |
| 20. | Vanella G, Bronswijk M, Arcidiacono PG, Larghi A, Wanrooij RLJV, de Boer YS, Rimbas M, Khashab M, van der Merwe SW. Current landscape of therapeutic EUS: Changing paradigms in gastroenterology practice. Endosc Ultrasound. 2023;12:16-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 21. | Bhakta D, de Latour R, Khanna L. Management of pancreatic fluid collections. Transl Gastroenterol Hepatol. 2022;7:17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Healthcare Cost and Utilization Project. Overview of the Nationwide Readmissions Database (NRD). Agency for Healthcare Research and Quality. [cited 15 January 2024]. Available from: https://www.hcup-us.ahrq.gov/nrdoverview.jsp. |
| 23. | Moore BJ, White S, Washington R, Coenen N, Elixhauser A. Identifying Increased Risk of Readmission and In-hospital Mortality Using Hospital Administrative Data: The AHRQ Elixhauser Comorbidity Index. Med Care. 2017;55:698-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 626] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 24. | Nieto LM, Salazar M, Kinnucan J, Lukens FJ, Argueta PP. Incidence, Burden, and Predictors of Readmission for Acute Alcoholic Pancreatitis: A National Analysis over 11 Months. Dig Dis Sci. 2023;68:423-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 25. | von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3667] [Cited by in RCA: 6872] [Article Influence: 624.7] [Reference Citation Analysis (0)] |
| 26. | Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10:150-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1784] [Cited by in RCA: 2517] [Article Influence: 193.6] [Reference Citation Analysis (0)] |
| 27. | Ebhohon E, Khoshbin K, Shaka H. Rates and predictors of 30-day hospital readmissions in adults for drug-induced acute pancreatitis: A retrospective study from the United States National Readmission Database. J Gastroenterol Hepatol. 2023;38:1277-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 28. | Ahmed Ali U, Issa Y, Hagenaars JC, Bakker OJ, van Goor H, Nieuwenhuijs VB, Bollen TL, van Ramshorst B, Witteman BJ, Brink MA, Schaapherder AF, Dejong CH, Spanier BW, Heisterkamp J, van der Harst E, van Eijck CH, Besselink MG, Gooszen HG, van Santvoort HC, Boermeester MA; Dutch Pancreatitis Study Group. Risk of Recurrent Pancreatitis and Progression to Chronic Pancreatitis After a First Episode of Acute Pancreatitis. Clin Gastroenterol Hepatol. 2016;14:738-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 213] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 29. | Munigala S, Subramaniam D, Subramaniam DP, Buchanan P, Xian H, Burroughs T, Trikudanathan G. Predictors for early readmission in acute pancreatitis (AP) in the United States (US) - A nationwide population based study. Pancreatology. 2017;17:534-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 30. | Adiamah A, Kushairi A, Tumilty S, Na Y, Crook M, Brooks AJ, Lobo DN; Nottingham University Hospitals Hepatopancreaticobiliary team. Hypertriglyceridaemia as a risk factor for critical care admission in acute pancreatitis: A prospective study. Clin Nutr ESPEN. 2020;39:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 31. | Laswi H, Attar B, Kwei R, Ishaya M, Ojemolon P, Natour B, Darweesh M, Shaka H. Readmissions After Biliary Acute Pancreatitis: Analysis of the Nationwide Readmissions Database. Gastroenterology Res. 2022;15:188-199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 32. | Kichloo A, El-Amir Z, Aucar M, Dahiya DS, Al-Haddad M, Pisipati S, Beiz H, Singh G, Gandhi D, Singh J, Pathappillil P, Mohideen H, Shaka H. Clinical Outcomes and Predictors of Thirty-Day Readmissions of Hypertriglyceridemia-Induced Acute Pancreatitis. Gastroenterology Res. 2022;15:19-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 33. | Mao WJ, Zhou J, Zhang GF, Chen FX, Zhang JZ, Li BQ, Ke L, Li WQ. Early systemic anticoagulation reduces hospital readmission in acute necrotizing pancreatitis patients: A retrospective cohort study. Hepatobiliary Pancreat Dis Int. 2024;23:77-82. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 34. | Argueta PP, Salazar M, Vohra I, Corral JE, Lukens FJ, Vargo JJ, Chahal P, Simons-Linares CR. Thirty-Day Readmission Among Patients with Alcoholic Acute Pancreatitis. Dig Dis Sci. 2021;66:4227-4236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 35. | Kumar V, Dolan RD, Yang AL, Jin DX, Banks PA, McNabb-Baltar J. Characteristics of 30-Day All-Cause Hospital Readmissions Among Patients with Acute Pancreatitis and Substance Use. Dig Dis Sci. 2022;67:5500-5510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Easler JJ, de-Madaria E, Nawaz H, Moya-Hoyo N, Koutroumpakis E, Rey-Riveiro M, Singh VP, Acevedo-Piedra NG, Whitcomb DC, Yadav D, Papachristou GI. Patients With Sentinel Acute Pancreatitis of Alcoholic Etiology Are at Risk for Organ Failure and Pancreatic Necrosis: A Dual-Center Experience. Pancreas. 2016;45:997-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Sorrento C, Shah I, Yakah W, Ahmed A, Tintara S, Kandasamy C, Freedman SD, Kothari DJ, Sheth SG. Inpatient Alcohol Cessation Counseling Is Associated With a Lower 30-Day Hospital Readmission in Acute Alcoholic Pancreatitis. J Clin Gastroenterol. 2022;56:e313-e317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | 38 Nagy R, Ocskay K, Váradi A, Papp M, Vitális Z, Izbéki F, Boros E, Gajdán L, Szentesi A, Erőss B, Hegyi PJ, Vincze Á, Bajor J, Sarlos P, Mikó A, Márta K, Pécsi D, Párniczky A, Hegyi P. In-Hospital Patient Education Markedly Reduces Alcohol Consumption after Alcohol-Induced Acute Pancreatitis. Nutrients. 2022;14:2131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Weinberger M, Oddone EZ, Henderson WG. Does increased access to primary care reduce hospital readmissions? Veterans Affairs Cooperative Study Group on Primary Care and Hospital Readmission. N Engl J Med. 1996;334:1441-1447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 553] [Cited by in RCA: 534] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 40. | Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3684] [Cited by in RCA: 3913] [Article Influence: 244.6] [Reference Citation Analysis (0)] |
| 41. | Krumholz HM, Wang K, Lin Z, Dharmarajan K, Horwitz LI, Ross JS, Drye EE, Bernheim SM, Normand ST. Hospital-Readmission Risk - Isolating Hospital Effects from Patient Effects. N Engl J Med. 2017;377:1055-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 42. | van Walraven C, Bennett C, Jennings A, Austin PC, Forster AJ. Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ. 2011;183:E391-402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 525] [Cited by in RCA: 522] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 43. | Auerbach AD, Kripalani S, Vasilevskis EE, Sehgal N, Lindenauer PK, Metlay JP, Fletcher G, Ruhnke GW, Flanders SA, Kim C, Williams MV, Thomas L, Giang V, Herzig SJ, Patel K, Boscardin WJ, Robinson EJ, Schnipper JL. Preventability and Causes of Readmissions in a National Cohort of General Medicine Patients. JAMA Intern Med. 2016;176:484-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 275] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 44. | Naik H, Murray TM, Khan M, Daly-Grafstein D, Liu G, Kassen BO, Onrot J, Sutherland JM, Staples JA. Population-Based Trends in Complexity of Hospital Inpatients. JAMA Intern Med. 2024;184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 47] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 45. | Lee PJ, Bhatt A, Lopez R, Stevens T. Thirty-Day Readmission Predicts 1-Year Mortality in Acute Pancreatitis. Pancreas. 2016;45:561-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 46. | Bezmarević M, van Dijk SM, Voermans RP, van Santvoort HC, Besselink MG. Management of (Peri)Pancreatic Collections in Acute Pancreatitis. Visc Med. 2019;35:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 47. | Umapathy C, Gajendran M, Mann R, Boregowda U, Theethira T, Elhanafi S, Perisetti A, Goyal H, Saligram S. Pancreatic fluid collections: Clinical manifestations, diagnostic evaluation and management. Dis Mon. 2020;66:100986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 48. | Xiao NJ, Cui TT, Liu F, Li W. Current status of treatments of pancreatic and peripancreatic collections of acute pancreatitis. World J Gastrointest Surg. 2021;13:633-644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (2)] |
| 49. | Lu L, Feng Y, Liu YH, Tan HY, Dai GH, Liu SQ, Li B, Feng HG. The Systemic Immune-Inflammation Index May Be a Novel and Strong Marker for the Accurate Early Prediction of Acute Kidney Injury in Severe Acute Pancreatitis Patients. J Invest Surg. 2022;35:962-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 50. | Liu X, Guan G, Cui X, Liu Y, Liu Y, Luo F. Systemic Immune-Inflammation Index (SII) Can Be an Early Indicator for Predicting the Severity of Acute Pancreatitis: A Retrospective Study. Int J Gen Med. 2021;14:9483-9489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 51. | Solakoglu T, Kucukmetin NT, Akar M, Koseoglu H. Acute peripancreatic fluid collection in acute pancreatitis: Incidence, outcome, and association with inflammatory markers. Saudi J Gastroenterol. 2023;29:225-232. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 52. | Wu S, Zhou Q, Cai Y, Duan X. Development and validation of a prediction model for the early occurrence of acute kidney injury in patients with acute pancreatitis. Ren Fail. 2023;45:2194436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 53. | Lee DW, Kim HG, Cho CM, Jung MK, Heo J, Cho KB, Kim SB, Kim KH, Kim TN, Han J, Kim H. Natural Course of Early Detected Acute Peripancreatic Fluid Collection in Moderately Severe or Severe Acute Pancreatitis. Medicina (Kaunas). 2022;58:1131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 54. | Negm AA, Poos H, Kruck E, Vonberg RP, Domagk D, Madisch A, Voigtländer T, Manns MP, Wedemeyer J, Lankisch TO. Microbiologic analysis of peri-pancreatic fluid collected during EUS in patients with pancreatitis: impact on antibiotic therapy. Gastrointest Endosc. 2013;78:303-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 55. | Afghani E, Singh VK. EUS-guided aspiration of peripancreatic fluid collections for culture: colonization or infection? Gastrointest Endosc. 2014;79:536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 56. | Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400-15; 1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1232] [Cited by in RCA: 1381] [Article Influence: 115.1] [Reference Citation Analysis (3)] |
| 57. | Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13:e1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1080] [Cited by in RCA: 1039] [Article Influence: 86.6] [Reference Citation Analysis (6)] |
| 58. | Rana SS, Shah J, Kang M, Gupta R. Complications of endoscopic ultrasound-guided transmural drainage of pancreatic fluid collections and their management. Ann Gastroenterol. 2019;32:441-450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 59. | Bansal A, Gupta P, Singh AK, Shah J, Samanta J, Mandavdhare HS, Sharma V, Sinha SK, Dutta U, Sandhu MS, Kochhar R. Drainage of pancreatic fluid collections in acute pancreatitis: A comprehensive overview. World J Clin Cases. 2022;10:6769-6783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (5)] |
| 60. | Kim KT, Clark J, Ghneim M, Feliciano DV, Diaz JJ, Harfouche M. Not All Fluid Collections Are Created Equal: Clinical Course and Outcomes of Pancreatic Pseudocysts and Acute Peripancreatic Fluid Collections Requiring Intervention. Am Surg. 2023;89:1774-1780. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 61. | ASGE Standards of Practice Committee; Khashab MA, Chithadi KV, Acosta RD, Bruining DH, Chandrasekhara V, Eloubeidi MA, Fanelli RD, Faulx AL, Fonkalsrud L, Lightdale JR, Muthusamy VR, Pasha SF, Saltzman JR, Shaukat A, Wang A, Cash BD. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2015;81:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 242] [Article Influence: 24.2] [Reference Citation Analysis (2)] |
| 62. | Nagy R, Ocskay K, Sipos Z, Szentesi A, Vincze Á, Czakó L, Izbéki F, Shirinskaya NV, Poluektov VL, Zolotov AN, Zhu Y, Xia L, He W, Sutton R, Szatmary P, Mukherjee R, Burridge IS, Wauchope E, Francisco E, Aparicio D, Pinto B, Gomes A, Nunes V, Tantau VM, Sagau ED, Tantau AI, Suceveanu AI, Tocia C, Dumitru A, Pando E, Alberti P, Cirera A, Molero X, Lee HS, Jung MK, Kim EJ, Lee S, Rebollo MLR, Nistal RB, Santervas SI, Lesko D, Soltes M, Radonak J, Zatorski H, Małecka-Panas E, Fabisiak A, Yaroslav MS, Mykhailo VM, Olekcandr AT, Barauskas G, Simanaitis V, Ignatavicius P, Jinga M, Balaban VD, Patoni C, Gong L, Song K, Li Y, Gonçalves TC, Freitas M, Macedo V, Vornhuelz M, Klauss S, Beyer G, Koksal AS, Tozlu M, Eminler AT, Monclús NT, Comas EP, Oballe JAR, Nawacki Ł, Głuszek S, Rama-Fernández A, Galego M, de la Iglesia D, Aykut UE, Duman DG, Aslan R, Gherbon A, Deng L, Huang W, Xia Q, Poropat G, Radovan A, Vranić L, Ricci C, Ingaldi C, Casadei R, Negoi I, Ciubotaru C, Iordache FM, Constantinescu G, Sandru V, Altintas E, Balci HR, Constantino J, Aveiro D, Pereira J, Gunay S, Misirlioglu Sucan S, Dronov O, Kovalska I, Bush N, Rana SS, Chooklin S, Chuklin S, Saizu IA, Gheorghe C, Göltl P, Hirth M, Mateescu RB, Papuc G, Minkov GA, Enchev ET, Mastrangelo L, Jovine E, Chen W, Zhu Q, Gąsiorowska A, Fabisiak N, Bezmarevic M, Litvin A, Mottes MC, Choi EK, Bánovčin P, Nosáková L, Kovacheva-Slavova MD, Kchaou A, Tlili A, Marino MV, Kusnierz K, Mickevicius A, Hollenbach M, Molcan P, Ioannidis O, Tokarev MV, Ince AT, Semenenko IA, Galeev S, Ramírez-Maldonado E, Sallinen V, Pencik P, Bajor J, Sarlós P, Hágendorn R, Gódi S, Szabó I, Czimmer J, Pár G, Illés A, Faluhelyi N, Kanizsai P, Nagy T, Mikó A, Németh B, Hamvas J, Bod B, Varga M, Török I, Novák J, Patai Á, Sümegi J, Góg C, Papp M, Erőss B, Váncsa S, Teutsch B, Márta K, Hegyi PJ, Tornai T, Lázár B, Hussein T, Tarján D, Lipp M, Kovács B, Urbán O, Fürst E, Tari E, Kocsis I, Maurovich-Horvát P, Tihanyi B, Eperjesi O, Kormos Z, Deák PÁ, Párniczky A, Hegyi P. Discharge protocol in acute pancreatitis: an international survey and cohort analysis. Sci Rep. 2023;13:22109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |