Published online Mar 20, 2024. doi: 10.5493/wjem.v14.i1.89319
Peer-review started: October 27, 2023
First decision: December 7, 2023
Revised: December 20, 2023
Accepted: January 10, 2024
Article in press: January 10, 2024
Published online: March 20, 2024
Processing time: 143 Days and 23.6 Hours
Lung cancer (LC) is a global medical, social and economic problem and is one of the most common cancers and the leading cause of mortality from malignant neoplasms. LC is characterized by an aggressive course, and in the presence of disease recurrence risk factors, patients, even at an early stage, may be indicated for adjuvant therapy to improve survival. However, combined treatment does not always guarantee a favorable prognosis. In this regard, establishing predictors of LC recurrence is highly important both for determining the optimal treatment plan for the patients and for evaluating its effectiveness.
To establish predictors of disease recurrence after radical resection and adjuvant chemotherapy in patients with stage IIb-IIIa lung squamous cell carcinoma (LSCC).
A retrospective case-control cohort study included 69 patients with LSCC who underwent radical surgery at the Orenburg Regional Clinical Oncology Center from 2009 to 2018. Postoperatively, all patients received adjuvant chemotherapy. Histological samples of the resected lung were stained with Mayer's hematoxylin and eosin and examined under a light microscope. Univariate and multivariate analyses were used to identify predictors associated with the risk of disease recurrence. Receiver operating characteristic curves were constructed to discriminate between patients with a high risk of disease recurrence and those with a low risk of disease recurrence. Survival was analyzed using the Kaplan-Meier method. The log-rank test was used to compare survival curves between patient subgroups. Differences were considered to be significant at P < 0.05.
The following predictors of a high risk of disease recurrence in patients with stage IIb-IIa LSCC were established: a low degree of tumor differentiation [odds ratio (OR) = 7.94, 95%CI = 1.08-135.81, P = 0.049]; metastases in regional lymph nodes (OR = 5.67, 95%CI = 1.09-36.54, P = 0.048); the presence of loose, fine-fiber connective tissue in the tumor stroma (OR = 21.70, 95%CI = 4.27-110.38, P = 0.0002); and fragmentation of the tumor solid component (OR = 2.53, 95%CI = 1.01-12.23, P = 0.049). The area under the curve of the predictive model was 0.846 (95%CI = 0.73-0.96, P < 0.0001). The sensitivity, accuracy and specificity of the method were 91.8%, 86.9% and 75.0%, respectively. In the group of patients with a low risk of LSCC recurrence, the 1-, 2- and 5-year disease-free survival (DFS) rates were 84.2%, 84.2% and 75.8%, respectively, while in the group with a high risk of LSCC recurrence the DFS rates were 71.7%, 40.1% and 8.2%, respectively (P < 0.00001). Accordingly, in the first group of patients, the 1-, 2- and 5-year overall survival (OS) rates were 94.7%, 82.5% and 82.5%, respectively, while in the second group of patients, the OS rates were 89.8%, 80.1% and 10.3%, respectively (P < 0.00001).
The developed method allows us to identify a group of patients at high risk of disease recurrence and to adjust to ongoing treatment.
Core Tip: This study identified the following independent predictors of a high risk of disease recurrence in patients with stage IIb-IIIa lung squamous cell carcinoma treated by radical resection and adjuvant chemotherapy: a low degree of tumor differentiation, metastases in regional lymph nodes, the presence of loose, fine-fiber connective tissue in the tumor stroma and fragmentation of the tumor solid component. The area under the curve of the predictive model was 0.846. The sensitivity, accuracy and specificity of the methods were 91.8%, 86.9% and 75.0%, respectively. The developed method allows us to identify a group of patients at high risk of disease relapse and to adjust the treatment.
- Citation: Senchukova MA, Kalinin EA, Volchenko NN. Predictors of disease recurrence after radical resection and adjuvant chemotherapy in patients with stage IIb-IIIa squamous cell lung cancer: A retrospective analysis. World J Exp Med 2024; 14(1): 89319
- URL: https://www.wjgnet.com/2220-315x/full/v14/i1/89319.htm
- DOI: https://dx.doi.org/10.5493/wjem.v14.i1.89319
Lung cancer (LC) is a global medical, social and economic problem and is one of the most common cancers and the leading cause of mortality from malignant neoplasms worldwide[1,2]. Despite advances in the diagnosis and treatment of this pathology, the 5-year survival rate of patients with LC does not exceed 20%[3]. The 5-year survival rate of patients who received radical therapy is higher and is approximately 35% in men and 44% in women[4,5]. Low survival rates are associated with late diagnosis of the disease and the presence of underlying severe disease in elderly patients, which significantly limits the choice of treatment methods, as well as the insufficient effectiveness of systemic therapy in a significant proportion of patients with advanced LC.
It is important to note that LC is a heterogeneous disease. The two major histologic subtypes are non-small-cell lung cancer (NSCLC) and small-cell lung cancer. NSCLC accounts for approximately 85% of all LC cases and includes lung squamous cell carcinoma (LSCC), lung adenocarcinoma and lung large cell carcinoma[6]. The different histological subtypes differ in metabolism, microenvironment, prognosis and treatment regimens[6]. Despite significant differences between subtypes, surgical resection and chemotherapy are the standard treatments for patients with stage IB-IIIA NSCLC[5]. Because NSCLC has an aggressive course, adjuvant therapy is indicated to improve survival even in patients with stage IB disease and risk factors[7,8]. However, combination treatment does not always guarantee a favorable disease prognosis. Despite radical treatment, some patients with NSCLC experience disease recurrence, the frequency of which ranges from 25% to 65%, depending on the stage of the disease[9,10]. Postoperative recurrences in NSCLC can occur even at an early stage, which leads to decreased patient survival. Thus, life expectancy after local relapse averages 12 to 26 months, and in patients with distant metastases, life expectancy is 7 to 8 months[11,12]. In addition, the presence of disease recurrence is associated with higher healthcare costs[12].
Thus, reducing mortality from NSCLC seems to be one of the most important tasks of modern healthcare. To suc
We previously established that tumor microvessels have different morphologies and clinical significance[15-17]. In addition, in squamous cell carcinoma of the cervix, we described the phenomenon of fragmentation of the tumor solid component as the presence of separate fibroblast-like cells in the solid component of the tumor. This study revealed that vessels with weak CD34 expression, located in the loose, fine-fiber connective tissue of the tumor stroma (LFFCT), vessels in the solid component of the tumor, and fragmentation of the tumor solid component were associated with a high risk of relapse of squamous cell carcinoma of the cervix I-IIA stages. The purpose of this study was to establish the prognostic significance of different types of tumor microvessels, as well as the characteristics of the stromal and parenchymal components, in patients with stage IIb-IIIa LSCC who underwent radical resection and adjuvant chemotherapy.
This retrospective case-control cohort study included 69 patients with stage IIb-IIIa LSCC who underwent radical surgery (R0) at the Orenburg Regional Oncology Center from May 20, 2009, to December 14, 2018. The age of the patients was 61.9 ± 6.8 years (median 62 years). The study was conducted in accordance with the Helsinki Declaration and internationally recognized guidelines. Study approval was received from the Ethics Committee of Orenburg State Medical University (Russia, Orenburg). The inclusion criteria for patients were as follows: (1) Patients who underwent radical surgery (R0) in the form of lobectomy, bilobectomy or pneumonectomy; (2) the stage of the disease corresponded to IIb-IIIa; (3) the histological subtype was LSCC; (4) LSCC was the first primary tumor; (5) patients who received at least 4 cycles of adjuvant chemotherapy after surgery (etoposide - 100 mg/m2/d on days 1, 2, and 3 and cisplatin - 80 mg/m2/d on day 1, every 3 wk); and (6) complete follow-up data were obtained.
The exclusion criteria were as follows: (1) Patients who underwent nonradical surgery (R1); (2) patients who received neoadjuvant radiotherapy or chemotherapy; (3) patients who received steroids, nonsteroidal anti-inflammatory drugs, or antihistamines; (4) patients who had significant comorbid pathologies in the decompensation stage; and (5) patients who died within the first three months after surgery. The median follow-up period was 62.4 months.
Sections (4 μm) were cut on a microtome and transferred to glass slides (SuperFrost® Plus, Menzel, Thermo Scientific, United States). Sections were stained with Mayer's hematoxylin and eosin (H&E) and studied via light microscopy (Levenhuk D740T digital microscope connected to a 5.1 MP camera, Russia). The following features of the parenchymal and stromal components of the tumor were estimated: the presence of LFFCT in the tumor stroma, the presence of capillaries in the solid component of the tumor, the presence of fragmentation of the tumor solid component, the presence of peritumoral retraction clefting, and the tumor spreading through the alveolar air spaces (AAS).
Statistical analysis was performed using Statistica 10.0 software. Quantitative data are presented as the mean ± standard deviation (SD), while categorical variables are presented as numbers and percentages (n, %). Clinicopathologic factors were compared between patients with and without disease relapse via the chi-square test. Univariate and multivariate logistic regression analyses were performed to identify potential risk factors for LSCC recurrence. Receiver operating characteristic (ROC) curves were constructed to discriminate between patients with and without recurrence of LSCC. The best threshold (cutoff) values were determined by the largest Youden’s index (J = sensitivity + specificity-1). The effectiveness of the predictive models was assessed by the area under the curve (AUC). Survival was analyzed using the Kaplan-Meier method. The log-rank test was used to compare survival curves between patient subgroups. A value of P < 0.05 was considered to indicate statistical significance.
The baseline patient clinicopathological and treatment information is shown in Table 1.
| Clinical and pathological data | Patients | |
| n | % | |
| Age (yr) | ||
| < 60 | 23 | 33.3 |
| 60-69 | 36 | 52.2 |
| ≥ 70 | 10 | 14.5 |
| Gender | ||
| Male | 67 | 97.1 |
| Female | 2 | 2.9 |
| Tumor location | ||
| Upper lobe | 45 | 65.2 |
| Middle lobe | 1 | 1.5 |
| Lower lobe | 21 | 30.4 |
| Main bronchus | 2 | 2.9 |
| Lateral origin | ||
| Left | 31 | 44.9 |
| Right | 38 | 55.1 |
| Tumor localization | ||
| Central | 57 | 82.6 |
| Peripheral | 12 | 17.4 |
| Histology | ||
| KSCC | 18 | 26.1 |
| NKSCC | 51 | 74.9 |
| Grade | ||
| G1 | 11 | 15.9 |
| G2 | 41 | 59.4 |
| G3 | 17 | 24.7 |
| T stage | ||
| T1b | 7 | 10.1 |
| T2a | 30 | 43.5 |
| T2b | 6 | 8.7 |
| T3a | 26 | 37.7 |
| N stage | ||
| N0 | 10 | 14.5 |
| N1 | 38 | 55.1 |
| N2 | 31 | 30.4 |
| Stage | ||
| IIb | 37 | 53.6 |
| IIIa | 32 | 46.4 |
| Type of surgery | ||
| Lobectomy | 29 | 42.0 |
| Pneumonectomy | 40 | 58.0 |
Nine patients underwent sleeve lobectomies, 6 of whom were older than 70 years. Sixty-five patients received 4 courses of adjuvant chemotherapy, 3 patients received 5 courses of adjuvant chemotherapy, and 1 patient received 6 courses of adjuvant chemotherapy.
Recurrence of LSCC was diagnosed in 49 (71.0%) patients, 43 (62.3%) of whom died from disease progression. Twenty-eight (57.1%) patients with recurrent LC were diagnosed within the first two years after surgery. The one-, two-, three-, four-, and five-year disease-free survival (DFS) rates were 85.7%, 60%, 40.8%, 34.3%, and 30.0%, respectively. The one-, two-, three-, four- and five-year overall survival (OS) rates were 95.7%, 85.7%, 68.6%, 48.6% and 37.1%, respectively.
Local relapse of LSCC was diagnosed in 22 (31.4%) patients, systemic relapse was diagnosed in 22 (31.4%), and local-systemic relapse was diagnosed in 5 (3.9%). In particular, metastases to the lungs and pleura were detected in 14 (20.0%) patients, to the mediastinum in 9 (12.9%), to the liver in 6 (8.6%), to the brain in 3 (4.3%), to the cervical lymph nodes in 1 (1.4%), bones in 4 (5.7%) and multiple disseminations in 12 (17.1%) patients. Due to relapse of LSCC, 31 patients received 1 to 4 courses of mono- or polychemotherapy. Six patients with relapsed LSCC are alive and continue to receive mono
The main condition for including tumor microvessels and features of the parenchymal and stromal components of the tumor in the analysis was the possibility of their detection during routine staining with Mayer's hematoxylin and eosin. In accordance with this, the presence of LFFCTL in the tumor stroma, microvessels in the tumor solid component, fragmentation of the tumor solid component, and the presence of peritumoral retraction clefting were included in the analysis. Additionally, taking into account the literature data, the analysis included indicator such as tumor spreading through the AAS.
LFFCT was most often observed along the invasive edge of the tumor and was rich in cells with large light nuclei (Figure 1). LFFCT was detected in 56 (80%) LSCC samples.
Microvessels in the tumor solid component were represented by capillaries with collapsed walls separated from the tumor cells by empty space (Figure 2A). This type of vessel was noted in 30 (42.9%) LSCC samples.
The fragmentation of the tumor solid component was characterized by the presence of isolated fibroblast-like cells in the tumor parenchyma (Figure 2B). This phenomenon was observed in 54 (77.1%) LSCC samples.
Peritumoral retraction clefting manifested as an empty space located around clusters of tumor cells (Figure 2C). This phenomenon was identified in 43 (61.4%) LSCC samples.
Tumor spread in the AAS was noted in 33 (47.1%) LSCC samples. Clusters of tumor cells were found both in unchanged alveoli (Figure 2D) and in alveoli with thickened, deformed walls. A number of observations revealed fragmentation of the tumor solid component in the alveoli.
The clinicopathological characteristics of patients with and without recurrence of LSCC are presented in Table 2.
| Patients with recurrence of LSCC | Patients without recurrence of LSCC | P value | |||
| n | % | n | % | ||
| Age (yr) | |||||
| < 60 | 15 | 30.6 | 8 | 40.0 | 0.72 |
| 60-69 | 27 | 55.1 | 9 | 45.0 | |
| ≥ 70 | 7 | 14.3 | 3 | 15.0 | |
| Gender | |||||
| Male | 47 | 95.9 | 20 | 100.0 | 0.359 |
| Female | 2 | 4.1 | 0 | 0.0 | |
| Tumor location | |||||
| Upper lobe | 35 | 71.5 | 10 | 50.0 | 0.193 |
| Middle lobe | 0 | 0.0 | 1 | 5.0 | |
| Lower lobe | 13 | 26.5 | 8 | 40.0 | |
| Main bronchus | 1 | 2.0 | 1 | 5.0 | |
| Lateral origin | |||||
| Left | 25 | 51.1 | 6 | 30.0 | 0.111 |
| Right | 24 | 48.9 | 14 | 70.0 | |
| Tumor localization | |||||
| Central | 43 | 87.8 | 14 | 70.0 | 0.08 |
| Peripheral | 6 | 12.2 | 6 | 30.0 | |
| Histology | |||||
| KSCC | 10 | 20.4 | 8 | 40.0 | 0.193 |
| NKSCC | 39 | 79.6 | 12 | 60.0 | |
| Grade | |||||
| G1 | 8 | 16.3 | 3 | 15.0 | 0.040 |
| G2 | 25 | 51.0 | 16 | 80.0 | |
| G3 | 16 | 32.7 | 1 | 5.0 | |
| T stage | |||||
| T1b | 5 | 10.2 | 2 | 10.0 | 0.559 |
| T2a | 24 | 48.9 | 6 | 30.0 | |
| T2b | 4 | 8.2 | 2 | 10.0 | |
| T3a | 16 | 32.7 | 10 | 50.0 | |
| N stage | |||||
| N0 | 4 | 8.2 | 6 | 30.0 | 0.05 |
| N1 | 30 | 61.2 | 8 | 40.0 | |
| N2 | 15 | 30.6 | 6 | 30.0 | |
| Stage | |||||
| IIb | 25 | 51.0 | 12 | 60.0 | 0.497 |
| IIIa | 24 | 49.0 | 8 | 40.0 | |
| Type of surgery | |||||
| Lobectomy | 19 | 38.8 | 10 | 50.0 | 0.618 |
| Pneumonectomy | 30 | 61.2 | 10 | 50.0 | |
According to the data obtained, patients who experienced disease recurrence were significantly more likely to have a low degree of tumor differentiation (P = 0.04) and metastases in regional lymph nodes (P = 0.05). Slightly more often, relapse of the disease was observed when the tumor had a low degree of differentiation (P = 0.08), was localized in the upper lobe (P = 0.19), was in the left lung (P = 0.11) or was nonkeratinizing squamous cell carcinoma (NKSCC) (P = 0.19). The recurrence rate of LSCC did not depend on the age of the patient, T stage, or extent of surgery performed.
The results of univariate and multivariate analyses evaluating the independent factors associated with the risk of LSCC recurrence are presented in Table 3. Thus, we identified 4 independent prognostic factors associated with the risk of LSCC recurrence, namely, tumor grade, N stage, the presence of LFFCT in the tumor stroma and fragmentation of the tumor solid component.
| Characteristic | Univariate analysis OR (95%CI) | P value | Multivariate analysis OR (95%CI) | P value |
| Age (yr) | ||||
| < 60 | 1 | - | ||
| 60-69 | 1.60 (0.51-5.02) | 0.420 | ||
| ≥ 70 | 1.24 (0.25-6.17) | 0.789 | ||
| Tumor localization | ||||
| Peripheral | 1 | - | ||
| Central | 3.07 (0.85-11.07) | 0.089 | ||
| Histology | ||||
| KSCC | 1 | - | ||
| NKSCC | 2.60 (0.84-8.07) | 0.101 | ||
| Grade | ||||
| G1 | 1 | - | 1 | - |
| G2 | 0.59 (0.13-2.54) | 0.590 | 0.91 (0.44-4.56) | 0.345 |
| G3 | 6.00 (1.01-67.28) | 0.0211 | 7.94 (1.08-135.81) | 0.0481 |
| T stage | ||||
| T1b | 1 | - | ||
| T2a | 1.62 (0.25-10.36) | 0.622 | ||
| T2b | 0.80 (0.08-8.47) | 0.853 | ||
| T3 | 0.63 (0.10-3.95) | 0.631 | ||
| N stage | ||||
| N0 | 1 | - | 1 | - |
| N1 | 5.63 (1.27-24.86) | 0.0231 | 5.67 (1.09-36.54) | 0.0481 |
| N2 | 3.75 (0.77-18.21) | 0.101 | 6.30 (0.78-50.78) | 0.08 |
| Stage | ||||
| IIb | 1 | - | ||
| IIIa | 1.44 (0.50-4.14) | 0.498 | ||
| DCs of “contact type” | ||||
| Presence | 1 | - | ||
| Absence | 1.61 (0.53-4.91) | 0.404 | ||
| The capillaries in the tumor solid component | ||||
| Absence | 1 | - | ||
| Presence | 2.24 (0.74-6.79) | 0.154 | ||
| LFFCT in the tumor stroma | ||||
| Absence | 1 | - | 1 | - |
| Presence | 15.33 (3.56-66.04) | 0.00021 | 21.70 (4.27-110.38) | 0.00021 |
| Fragmentation in the tumor solid component | ||||
| Absence | 1 | - | 1 | - |
| Presence | 3.42 (1.06-11.03) | 0.0401 | 2.53 (1.01-12.23) | 0.0491 |
| Peritumoral retraction clefting | ||||
| Absence | 1 | - | ||
| Presence | 0.41 (0.13-1.30) | 0.13 | ||
| Tumor spread in the AAS | ||||
| Absence | 1 | - | ||
| Presence | 1.08 (0.38-3.07) | 0.883 | ||
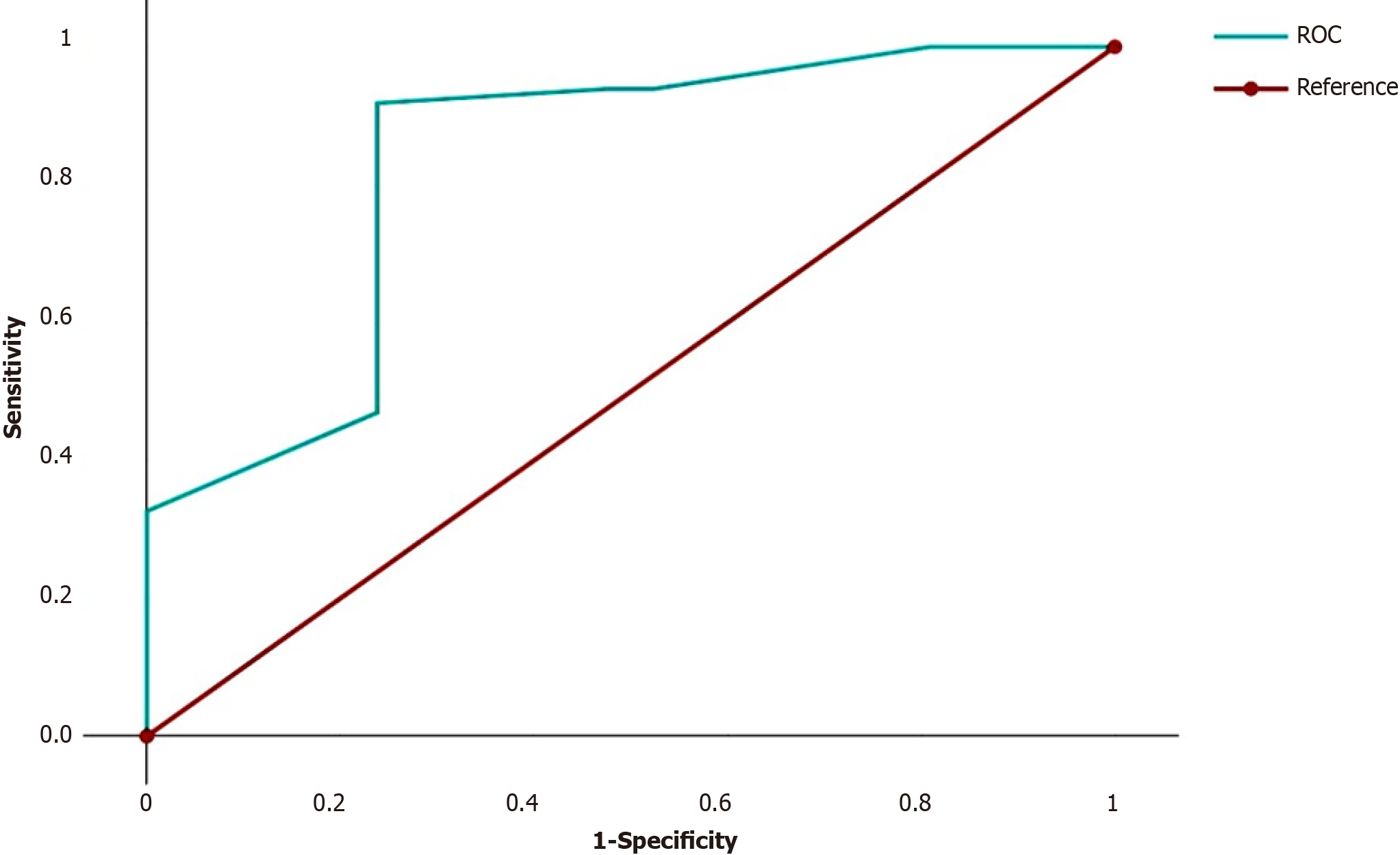
We summarized the odds ratios (ORs) of the independent predictors for each patient. For example, for a patient with G3 (OR = 7.94), N2 (OR = 6.30), fragmentation of the tumor solid component (OR = 2.53) and absence of LFFCT in the tumor stroma (OR = 1), this number was 17.77 (7.94 + 6.30 + 2.53 + 1). On the basis of these results, ROC curves were constructed to discriminate between cases with and without LSCC relapse (Figure 3).
The AUC was 0.846 (95%CI = 0.73-0.96, P < 0.0001). The cutoff for discriminating between cases with and without LSCC relapse, was 26.2. If the sum of the ORs of the four independent predictors of high risk of LSCC recurrence was less than 26.2 (the first group of patients), then recurrence of LSCC was noted in 4 (21.1%) of the 19 patients. If the sum of the ORs was greater than or equal to 26.2 (the second group of patients), then recurrence of LSCC was noted in 45 (90.0%) of the 50 patients. The sensitivity, accuracy and specificity of the method were 91.8%, 86.9% and 75.0%, respectively. In the first group of patients, the 1-, 2- and 5-year DFS rates were 84.2%, 84.2% and 75.8%, respectively, while in the second group of patients, the DFS rates were 71.7%, 40.1% and 8.2%, respectively (P < 0.00001). Accordingly, in the first group of patients, the 1-, 2- and 5-year OS rates were 94.7%, 82.5% and 82.5%, respectively, while in the second group of patients, the OS rates were 89.8%, 80.1% and 10.3%, respectively (P < 0.00001). The relapse-free survival (RFS) and OS curves for these groups are shown in Figure 4.
Establishing independent predictors of the risk of LSCC recurrence is important both for assessing disease prognosis and understanding the mechanisms of LSCC progression, as well as for identifying factors associated with sensitivity to therapy. Currently, assessing the risk of LC recurrence is based on the traditional staging system and other clinicopathological risk factors and their combinations[18,19]. However, it is believed that the current TNM staging system is insufficient for predicting the survival of LC patients[20]. In this regard, the search for reliable predictors of the risk of LC recurrence continues. New methods for assessing the risk of relapse in patients with LC include radiomic signatures[21,22], positron emission tomography[23,24], and machine learning models[25,26]. These methods are highly sensitive but require special equipment and trained personnel.
The inclusion of various tumor markers in the analysis can improve the accuracy of disease prognosis. Thus, in a study by Yu et al[20], a higher C-reactive protein-to-albumin ratio, intrapericardial pulmonary artery ligation, lymph node metastasis, and adjuvant therapy were associated with a high risk of LC recurrence in patients who underwent pneumonectomy. In turn, the independent predictors of OS in this group of patients were intrapericardial pulmonary artery and vein ligation, higher T stage, lymph node metastasis, and no adjuvant therapy. The AUCs for 1-, 3-, and 5-year DFS were 0.655, 0.726, and 0.735, respectively, and those for 1-, 3-, and 5-year OS were 0.741, 0.765, and 0.709, respectively. However, it should be noted that the patient cohort in this study was very heterogeneous and included patients with stage T0-T4 and N0-N2 disease who received both neoadjuvant and adjuvant therapy. Interestingly, the use of adjuvant therapy in this study was associated with a decrease in DFS, while its absence was associated with a decrease in OS[20].
In the study by Jiao et al[27], the independent predictors of a high risk of relapse in NSCLC were disease stage, T stage, N stage, histological tumor type, the presence of radiation therapy and residual tumor, and 4-and-a-half LIM domain protein 2 (FHL2), which is responsible for the proliferation, invasion and metastasis of tumor cells. The authors noted that high FHL2 levels may serve as an independent predictor of DFS in NSCLC patients. However, it is worth noting the insufficient efficiency of the proposed model since the AUCs for 1-, 3- and 5-year OS were 0.56, 0.53 and 0.51, respectively.
In stage IIIA patients receiving adjuvant chemotherapy, the independent predictors associated with decreased OS were adjuvant radiotherapy, targeted therapy, tumor size, N1p, and N2p, whereas the independent predictors associated with decreased OS were only tumor size and N2p[28]. The authors noted moderate agreement between the predicted and actual RFS and OS. The C-index was 0.656 (95%CI: 0.626-0.687) for RFS and 0.651 (95%CI: 0.611-0.691) for OS.
In early NSCLC, the independent predictors of a high risk of disease recurrence were smoking status, total lymph nodes removed, and tumor size[29]. This model was relevant only to patients with stage 1a-1b NSCLC who did not receive adjuvant therapy. It should be noted that data on the role of adjuvant therapy in the treatment of early-stage NSCLC vary widely. A study by Xu et al[30] revealed that in a cohort of patients with stage IB NSCLC, adjuvant che
In another similar study, the authors did not observe any improvement in patient survival with or without adjuvant chemotherapy for stage IB NSCLC[31]. The inclusion of molecular markers (VEGF-C, miR-1, miR-486, miR-499, and miR-30d) in the analysis did not increase the accuracy of predicting the risk of disease relapse in patients with early-stage NSCLC[9].
A number of studies have noted correlations between immune cell levels and DFS. In particular, a study by Wu et al[32] showed that higher levels of CD68 and M1 macrophages are associated with worse DFS (P < 0.0001). Based on the results obtained, a nomogram was constructed that included age and sex, the presence of visceral pleural invasion, the number of lymph nodes removed, clinical stage and the immune-related risk assessment nomogram. The proposed nomogram outperformed the TNM classification and the CD68-based immune-related risk score.
It is worth noting that in most related studies, the authors considered patients with both LSCC and lung adenocarcinomas and patients with different disease stages, and with and without adjuvant therapy[27-29,31]. However, it is known that LSCC and lung adenocarcinoma are malignant tumors that differ in their biological characteristics and sensitivity to special methods of therapy. We believe that the heterogeneity of the groups can significantly affect the effectiveness of prognostic models since some markers may be associated with more aggressive characteristics of LC, for example, a higher stage of the disease and a low degree of tumor differentiation, while other factors may be associated with the sensitivity of tumor cells to systemic therapy. The latter fact is especially significant in locally advanced cancer, in which the sensitivity of tumor cells to treatment significantly affects disease prognosis. Given that lung adenocarcinoma and LSCC have different sensitivities to radiation therapy and chemotherapy, joint evaluation of these two histological types of NSCLC may significantly bias the study results. For example, the effectiveness of a particular prognostic model can be influenced by various factors, including the ratio of subgroups of patients with different histological subtypes of LC.
Unlike most related studies, our study included a fairly homogeneous cohort of patients with stage IIb-IIIa LSCC who underwent radical resection and adjuvant chemotherapy. In addition to the standard clinicopathological characteristics of LC, we also included the following markers in the analysis: The presence of LFFCT in the tumor stroma, microvessels in the tumor solid component and fragmentation of the tumor solid component. Their choice was based on the results of our previous studies. In addition, the presence of peritumoral retraction clefting and tumor spread through the AAS were included in the analysis. The connection of these factors with tumor progression and disease prognosis has been demonstrated in a number of studies. For example, a connection has been established between tumor spread through the AAS and the prognosis of lung adenocarcinoma[33]. In LSCC, tumor spread through the AAS was associated with disease relapse only at stage Ib[34]. In a mixed cohort of patients with stage Ib LC, a decrease in OS and RFS was also noted when the tumor spread through the AAS[35]. However, in this cohort, 67.7% of patients had lung adenocarcinoma, and only 32.3% of patients had LSCC.
The connection between peritumoral retraction clefting and the risk of disease relapse in patients with various malignancies, including breast cancer[36] and oral squamous cell carcinoma[37], has also been described. However, in LC, this phenomenon has practically not been studied. The analysis revealed 4 independent prognostic factors associated with the risk of LSCC recurrence, namely, tumor grade, N stage, the presence of LFFCT in the tumor stroma and fragmentation of the tumor solid component. The associations of tumor grade and N stage with the risk of LC relapse have been confirmed by numerous studies[20,30,38,39]. This dependence can be traced in various malignant neoplasms and is explained by the fact that low differentiation of tumor cells and a tendency to metastasize predominantly characterize the most aggressive subtypes of cancer, which are prone to recurrence. The most significant independent predictor of a high risk of LSCC recurrence was the presence of LFFCT in the tumor stroma (OR = 21.70, 95%CI = 4.27-110.38, P = 0.0002). LFFCT was most often detected in the peritumoral stroma and waswere rich in cells with large pale nuclei and weakly condensed euchromatin. We hypothesized that the described cells may be tumor-associated fibroblasts. It has been suggested that tumor-associated fibroblasts may originate from bone marrow mesenchymal stem cells, hem
Another independent predictor of a high risk of LSCC recurrence was fragmentation of the tumor solid component. We first described this phenomenon in squamous cell carcinoma of the cervix, defined as the presence of separate fibroblast-like cells in the solid component of the tumor. In an immunohistochemical study, the described cells showed nuclear expression of HIF-1α and Shail[17]. In squamous cell carcinoma of the cervix, the described phenomenon was significantly more often observed in the presence of disease relapse than in its absence (P = 0.01). We believe that fragmentation of the tumor solid component can promote the survival of tumor cells under hypoxic conditions through epithelial-mesenchymal transition. Notably, vessels in the tumor solid component, tumor spread through the AAS and peritumoral retraction clefting were not associated with the risk of stage IIb-IIIa LSCC recurrence.
Thus, independent predictors of a high risk of disease relapse in patients with stage IIb-IIIa LSCC after radical resection and adjuvant chemotherapy are tumor grade, N stage, the presence of LFFCT in the tumor stroma and fragmentation in the tumor solid component. The data obtained can be used to clarify the prognosis of the disease and to individualize treatment and observation. The advantages of the developed method for assessing the risk of recurrence of LSCC include its high sensitivity, accuracy and specificity, as well as ease of implementation; moreover, additional research is not needed since the determination of the presence of LFFCT in the tumor stroma and fragmentation in the tumor solid component is possible with routine histological examination by staining histological slides with Mayer hematoxylin and eosin. The main disadvantages of the present study are its single-center nature and the small number of patients. We believe that further research will not only improve the accuracy of LSCC prognosis but also improve our understanding of the mechanisms of tumor progression and drug resistance in this deadly disease.
Establishing predictors of lung cancer (LC) recurrence is highly important both for determining the optimal treatment plan for the patients and for evaluating its effectiveness.
Assessment of different types of tumor microvessels, features of the tumor parenchyma and stroma can improve the accuracy of predicting the risk of lung squamous cell carcinoma (LSCC) recurrence.
This study aimed to establish predictors of disease recurrence after radical resection and adjuvant chemotherapy in patients with stage IIb-IIIa LSCC.
This retrospective analysis of the treatment results of 69 patients with stage IIb-IIa LSCC who underwent radical surgery and received adjuvant chemotherapy. To establish independent predictors of the risk of LSCC recurrence, univariate and multivariate analyzes were performed, which included clinicopathological characteristics of LSCC and the features of tumor parenchyma and stroma.
The following independent predictors of a high risk of disease recurrence in patients with stage IIb-IIa LSCC were established: A low degree of tumor differentiation; metastases in regional lymph nodes; the presence of loose, fine-fiber connective tissue in the tumor stroma; and fragmentation of the tumor solid component.
A method has been developed that allows us to identify a group of patients at high risk of disease recurrence and to adjust to ongoing treatment.
Future studies will contribute to understanding the mechanisms of tumor progression and drug resistance of LSCC.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Russia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Liu Y, China S-Editor: Liu JH L-Editor: A P-Editor: Zhao YQ
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64133] [Article Influence: 16033.3] [Reference Citation Analysis (174)] |
| 2. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12667] [Cited by in RCA: 15282] [Article Influence: 3056.4] [Reference Citation Analysis (4)] |
| 3. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4235] [Cited by in RCA: 11369] [Article Influence: 3789.7] [Reference Citation Analysis (4)] |
| 4. | Howlader N, Forjaz G, Mooradian MJ, Meza R, Kong CY, Cronin KA, Mariotto AB, Lowy DR, Feuer EJ. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N Engl J Med. 2020;383:640-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 734] [Cited by in RCA: 1096] [Article Influence: 219.2] [Reference Citation Analysis (0)] |
| 5. | Pei Q, Luo Y, Chen Y, Li J, Xie D, Ye T. Artificial intelligence in clinical applications for lung cancer: diagnosis, treatment and prognosis. Clin Chem Lab Med. 2022;60:1974-1983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 55] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 6. | Neumann JM, Freitag H, Hartmann JS, Niehaus K, Galanis M, Griesshammer M, Kellner U, Bednarz H. Subtyping non-small cell lung cancer by histology-guided spatial metabolomics. J Cancer Res Clin Oncol. 2022;148:351-360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 7. | Zhang T, Guo Q, Zhang Y, Liu Z, Zhou S, Xu S. Meta-analysis of adjuvant chemotherapy vs surgery alone in T2aN0 stage IB non-small cell lung cancer. J Cancer Res Ther. 2018;14:139-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Pathak R, Goldberg SB, Canavan M, Herrin J, Hoag JR, Salazar MC, Papageorge M, Ermer T, Boffa DJ. Association of Survival With Adjuvant Chemotherapy Among Patients With Early-Stage Non-Small Cell Lung Cancer With vs Without High-Risk Clinicopathologic Features. JAMA Oncol. 2020;6:1741-1750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 9. | Thornblade LW, Mulligan MS, Odem-Davis K, Hwang B, Waworuntu RL, Wolff EM, Kessler L, Wood DE, Farjah F. Challenges in Predicting Recurrence After Resection of Node-Negative Non-Small Cell Lung Cancer. Ann Thorac Surg. 2018;106:1460-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Wei W, Zhou J, Zhang Q, Liao DH, Liu QD, Zhong BL, Liang ZB, Zhang YC, Jiang R, Liu GY, Xu CY, Li Zhou H, Zhu SY, Yang N, Jiang W, Liu ZG. Postoperative intensity-modulated radiation therapy reduces local recurrence and improves overall survival in III-N2 non-small-cell lung cancer: A single-center, retrospective study. Cancer Med. 2020;9:2820-2832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Mizuno T, Arimura T, Kuroda H, Sakakura N, Yatabe Y, Sakao Y. Current outcomes of postrecurrence survival in patients after resection of non-small cell lung cancer. J Thorac Dis. 2018;10:1788-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | West H, Hu X, Zhang S, Song Y, Chirovsky D, Gao C, Lerner A, Jiang A, Signorovitch J, Samkari A. Evaluation of disease-free survival as a predictor of overall survival and assessment of real-world burden of disease recurrence in resected early-stage non-small cell lung cancer. J Manag Care Spec Pharm. 2023;29:749-757. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Rami-Porta R. Future Perspectives on the TNM Staging for Lung Cancer. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Brascia D, De Iaco G, Schiavone M, Panza T, Signore F, Geronimo A, Sampietro D, Montrone M, Galetta D, Marulli G. Resectable IIIA-N2 Non-Small-Cell Lung Cancer (NSCLC): In Search for the Proper Treatment. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Senchukova M, Kiselevsky MV. The "cavitary" type of angiogenesis by gastric cancer. Morphological characteristics and prognostic value. J Cancer. 2014;5:311-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Senchukova MA, Nikitenko NV, Tomchuk ON, Zaitsev NV, Stadnikov AA. Different types of tumor vessels in breast cancer: morphology and clinical value. Springerplus. 2015;4:512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Senchukova MA, Makarova EV, Shurygina EI, Volchenko NN. Morphological Characteristics and Clinical Significance of Different Types of Tumor Vessels in Patients with Stages I-IIA of Squamous Cervical Cancer. J Oncol. 2020;2020:3818051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Huang Y, Liu Z, He L, Chen X, Pan D, Ma Z, Liang C, Tian J. Radiomics Signature: A Potential Biomarker for the Prediction of Disease-Free Survival in Early-Stage (I or II) Non-Small Cell Lung Cancer. Radiology. 2016;281:947-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 552] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 19. | Torrente M, Sousa PA, Guerreiro GR, Franco F, Hernández R, Parejo C, Sousa A, Campo-Cañaveral JL, Pimentão J, Provencio M. Clinical factors influencing long-term survival in a real-life cohort of early stage non-small-cell lung cancer patients in Spain. Front Oncol. 2023;13:1074337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 20. | Yu X, Wang F, Yang L, Ma K, Guo X, Wang L, Du L, Yu X, Lin S, Xiao H, Sui Z, Zhang L, Yu Z. Development and validation of web-based dynamic nomograms predictive of disease-free and overall survival in patients who underwent pneumonectomy for primary lung cancer. PeerJ. 2023;11:e15938. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 21. | Kirienko M, Sollini M, Corbetta M, Voulaz E, Gozzi N, Interlenghi M, Gallivanone F, Castiglioni I, Asselta R, Duga S, Soldà G, Chiti A. Radiomics and gene expression profile to characterise the disease and predict outcome in patients with lung cancer. Eur J Nucl Med Mol Imaging. 2021;48:3643-3655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 22. | Libling WA, Korn R, Weiss GJ. Review of the use of radiomics to assess the risk of recurrence in early-stage non-small cell lung cancer. Transl Lung Cancer Res. 2023;12:1575-1589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 23. | Kwon W, Howard BA, Herndon JE, Patz EF Jr. FDG Uptake on Positron Emission Tomography Correlates with Survival and Time to Recurrence in Patients with Stage I Non-Small-Cell Lung Cancer. J Thorac Oncol. 2015;10:897-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Blumenthaler AN, Hofstetter WL, Mehran RJ, Rajaram R, Rice DC, Roth JA, Sepesi B, Swisher SG, Vaporciyan AA, Walsh GL, Strange CD, Antonoff MB. Preoperative Maximum Standardized Uptake Value Associated With Recurrence Risk in Early Lung Cancer. Ann Thorac Surg. 2022;113:1835-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Zhang Y, Zheng D, Xie J, Li Y, Wang Y, Li C, Xiang J, Zhang Y, Hu H, Sun Y, Chen H. Development and Validation of Web-Based Nomograms to Precisely Predict Conditional Risk of Site-Specific Recurrence for Patients With Completely Resected Non-small Cell Lung Cancer: A Multiinstitutional Study. Chest. 2018;154:501-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 26. | Mohamed SK, Walsh B, Timilsina M, Torrente M, Franco F, Provencio M, Janik A, Costabello L, Minervini P, Stenetorp P, Novácˇek V. On Predicting Recurrence in Early Stage Non-small Cell Lung Cancer. AMIA Annu Symp Proc. 2021;2021:853-862. [PubMed] |
| 27. | Jiao Y, Wei J, Li Z, Zhou J, Liu Y. High FHL2 mRNA expression and its prognostic value in lung cancer. Aging (Albany NY). 2022;14:7986-8000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Zheng D, Wang Y, Li Y, Sun Y, Chen H. Predicting prognosis of post-chemotherapy patients with resected IIIA non-small cell lung cancer. J Thorac Dis. 2018;10:4186-4194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 29. | Merritt RE, Abdel-Rasoul M, Fitzgerald M, D'Souza DM, Kneuertz PJ. Nomograms for Predicting Overall and Recurrence-free Survival From Pathologic Stage IA and IB Lung Cancer After Lobectomy. Clin Lung Cancer. 2021;22:e574-e583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Xu Y, Wan B, Zhu S, Zhang T, Xie J, Liu H, Zhan P, Lv T, Song Y. Effect of Adjuvant Chemotherapy on Survival of Patients With 8th Edition Stage IB Non-Small Cell Lung Cancer. Front Oncol. 2021;11:784289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 31. | Moon Y, Choi SY, Park JK, Lee KY. Prognostic factors in stage IB non-small cell lung cancer according to the 8(th) edition of the TNM staging system after curative resection. J Thorac Dis. 2019;11:5352-5361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Wu XR, Peng HX, He M, Zhong R, Liu J, Wen YK, Li CC, Li JF, Xiong S, Yu T, Zheng HB, Chen YH, He JX, Liang WH, Cai XY. Macrophages-based immune-related risk score model for relapse prediction in stage I-III non-small cell lung cancer assessed by multiplex immunofluorescence. Transl Lung Cancer Res. 2022;11:523-542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Gutierrez-Sainz L, López-Muñoz S, Cruz-Castellanos P, Higuera O, Esteban-Rodríguez MI, Losantos-García I, De Castro-Carpeño J. Retrospective analysis of the prognostic implications of tumor spread through air spaces in lung adenocarcinoma patients treated with surgery. ESMO Open. 2022;7:100568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 34. | Yanagawa N, Shiono S, Endo M, Ogata SY. Tumor spread through air spaces is a useful predictor of recurrence and prognosis in stage I lung squamous cell carcinoma, but not in stage II and III. Lung Cancer. 2018;120:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 35. | Chen Z, Wu X, Fang T, Ge Z, Liu J, Wu Q, Zhou L, Shen J, Zhou C. Prognostic impact of tumor spread through air spaces for T2aN0 stage IB non-small cell lung cancer. Cancer Med. 2023;12:15246-15255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 36. | Li W, Jia H, Wang S, Guo X, Zhang X, Zhang L, Wen HY, Fu L. The presence of retraction clefts correlates with lymphovascular invasion and lymph node metastasis and predicts poor outcome: Analysis of 2497 breast cancer patients. Ann Diagn Pathol. 2022;61:152047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Jain D, Tikku G, Bhadana P, Dravid C, Grover RK. The Impact of Peritumoral Retraction Clefting & Intratumoral Eosinophils on Overall Survival in Oral Squamous Carcinoma Patients. Pathol Oncol Res. 2019;25:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Zhang CC, Hou RP, Feng W, Fu XL. Lymph Node Parameters Predict Adjuvant Chemoradiotherapy Efficacy and Disease-Free Survival in Pathologic N2 Non-Small Cell Lung Cancer. Front Oncol. 2021;11:736892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 39. | Wang W, Zhou J. A Nomogram to Predict the Overall Survival of Patients With Resected T1-2N0-1M0 Non-Small Cell Lung Cancer and to Identify the Optimal Candidates for Adjuvant Chemotherapy in Stage IB or IIA Non-Small Cell Lung Cancer Patients. Cancer Control. 2023;30:10732748231197973. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 40. | Yamamoto Y, Kasashima H, Fukui Y, Tsujio G, Yashiro M, Maeda K. The heterogeneity of cancer-associated fibroblast subpopulations: Their origins, biomarkers, and roles in the tumor microenvironment. Cancer Sci. 2023;114:16-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 74] [Article Influence: 24.7] [Reference Citation Analysis (0)] |