Published online Jun 9, 2025. doi: 10.5492/wjccm.v14.i2.99445
Revised: December 2, 2024
Accepted: December 16, 2024
Published online: June 9, 2025
Processing time: 219 Days and 12.1 Hours
Hematoma expansion (HE) typically portends a poor prognosis in spontaneous intracerebral hemorrhage (ICH). Several radiographic and laboratory values have been proposed as predictive markers of HE.
To perform a systematic review and meta-analysis on the association of neu
Three databases were searched (PubMed, EMBASE, and Cochrane) for studies evaluating the effect of NLR on HE and PHE growth. The inverse variance me
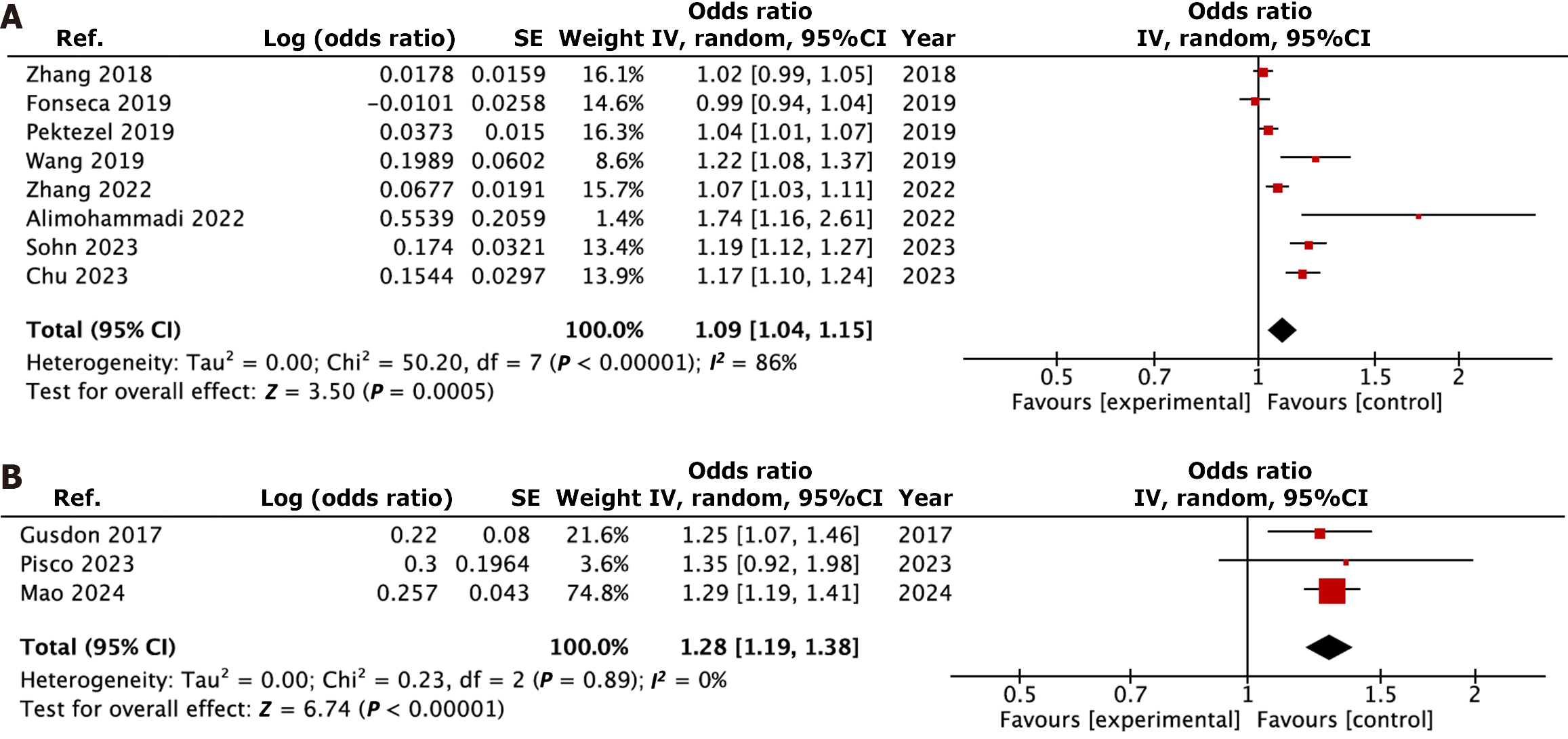
Eleven retrospective cohort studies involving 2953 patients were included in the meta-analysis. Among those, HE was investigated in eight studies, whereas PHE growth was evaluated in three. Blood sample was obtained on admission in ten studies, and at 24 hours in one study. There was no consensus on cut-off value among the studies. NLR was found to be significantly associated with higher odds of HE (OR = 1.09, 95%CI: 1.04-1.15, I2 = 86%, P < 0.01), and PHE growth (OR = 1.28, 95%CI: 1.19-1.38, I2 = 0%, P < 0.01). Qualitative analysis of each outcome revealed overall moderate risk of bias mainly due to lack of control for systemic confounders.
The available literature suggests that a possible association may exist between NLR on admission and HE, and PHE growth. Future studies controlled for systemic confounders should be designed to consolidate this finding. If confirmed, NLR could be added as a readily available and inexpensive biomarker to identify a subgroup of patients at higher risk of developing HE.
Core Tip: In this work, we provide an updated and concise quantitative synthesis of the literature addressing the association between neutrophil-to-lymphocyte ratio and hematoma expansion (HE) in intracerebral hemorrhage. We found an independent association between neutrophil-to-lymphocyte ratio (NLR) on admission and HE, and perihematomal growth. Our findings support the idea that NLR could be added as a readily available and inexpensive biomarker to identify a subgroup of patients at higher risk of developing HE.
- Citation: Loggini A, Hornik J, Henson J, Wesler J, Hornik A. Association between neutrophil-to-lymphocyte ratio and hematoma expansion in spontaneous intracerebral hemorrhage: A systematic review and meta-analysis. World J Crit Care Med 2025; 14(2): 99445
- URL: https://www.wjgnet.com/2220-3141/full/v14/i2/99445.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v14.i2.99445
Spontaneous intracerebral hemorrhage (ICH) is the most devastating type of stroke, and it is associated with high morbidity and mortality[1,2]. Initial hematoma expansion (HE) has been shown to be associated with poor outcome[3]. As such, early predictors of HE, including clinical and radiographic markers, have been extensively investigated[4,5]. Prediction scores for HE have been proposed and compared to each other[6] in an effort to best predict which patients would benefit from enrollment in trials targeted to reducing HE[7,8].
Detection of abnormal systemic inflammatory markers is a frequent encounter in the critically ill patient[9]. In acute ischemic stroke, elevated inflammatory markers have been demonstrated to have a negative prognostic significance given their role in developing secondary neurological injury[10,11]. Neutrophil-to-lymphocyte ratio (NLR) has been reported to be an independent prognostic factor in ICH, being associated with neurological deterioration, short-term mortality, and major disability at 90 days[12–16]. A meta-analysis from 2022 has failed to observe a significant association between NLR and HE[17]. However, the bulk of literature on the topic has significantly grown since then. Here we presented an updated systematic review and meta-analysis aimed at assessing the association between NLR and HE in ICH. The secondary objective was to assess the role of NLR in perihematomal (PHE) growth in ICH.
This systematic review was registered via PROSPERO (No. CRD42024549924) and conducted and presented in accordance with the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) statement[18]. Ethical approval was not required because data was synthetized from previously published studies.
This is a systematic review and meta-analysis aimed at assessing the association of NLR and HE in ICH. Additional outcome investigated was the association between NLR and PHE growth.
Three databases (PubMed, EMBASE, and Cochrane) were searched from inception to May 22, 2024, without linguistic or geographical limitation using the following keywords "neutrophil-to-lymphocyte ratio" or "neutrophil lymphocyte ratio" or "neutrophil-lymphocyte ratio" in conjunction with "hematoma expansion" or "hematoma growth" or "intracerebral hemorrhage" or "ICH". The search strategy is available in supplementary material. Reference lists were searched manually for additional sources.
All articles identified from the literature search were screened by two authors (Loggini A and Hornik J), independently, as per the following inclusion criteria: (1) Adult population with spontaneous ICH; (2) Laboratory value indicating NLR; and (3) Clinical outcome focused on HE and PHE edema growth. Randomized clinical trials and controlled observational cohort studies were included in the review. Detailed information on participants, interventions, comparisons, outcomes and types of studies are provided in Supplementary Table 1. The relevance of the studies was assessed using a hierarchical approach based on title, abstract, and full manuscript.
Risk of bias was computed using the Newcastle-Ottawa Scale (NOS) by two authors (Loggini A and Hornik J), independently[19]. Disagreement between quality of the study was resolved by consensus.
For each included study, the following variables were extracted independently by two authors (Loggini A and Hornik J): (1) Lead author; (2) Publication date; (3) Study design; (4) Number of participants; (5) NLR and its cut-off value; (6) Time of sample collection; (7) Markers of stroke severity; (8) Clinical outcomes of HE and PHE edema; and (9) Corresponding odds ratio (OR) and 95%CI values from multivariable analyses. If any of the above variables were not available in the full text publication, further information was sought by correspondence with the lead author of the study.
The inverse variance method was applied to estimate an overall, unconfounded, effect estimate of NLR for each specific outcome by combining weighted averages of the individual studies’ estimates of the logarithm OR. A Z test was carried out to assess the significance of the OR. The I² was calculated by χ² test to assess variability due to heterogeneity rather than chance. A substantial heterogeneity was assumed with I² > 50%. 95%CI for HE and PHE edema were calculated with the Wilson method and placed in forest plots. A P value < 0.05 was considered statistically significant. Statistical analysis was performed using Review Manager 5.3[20,21].
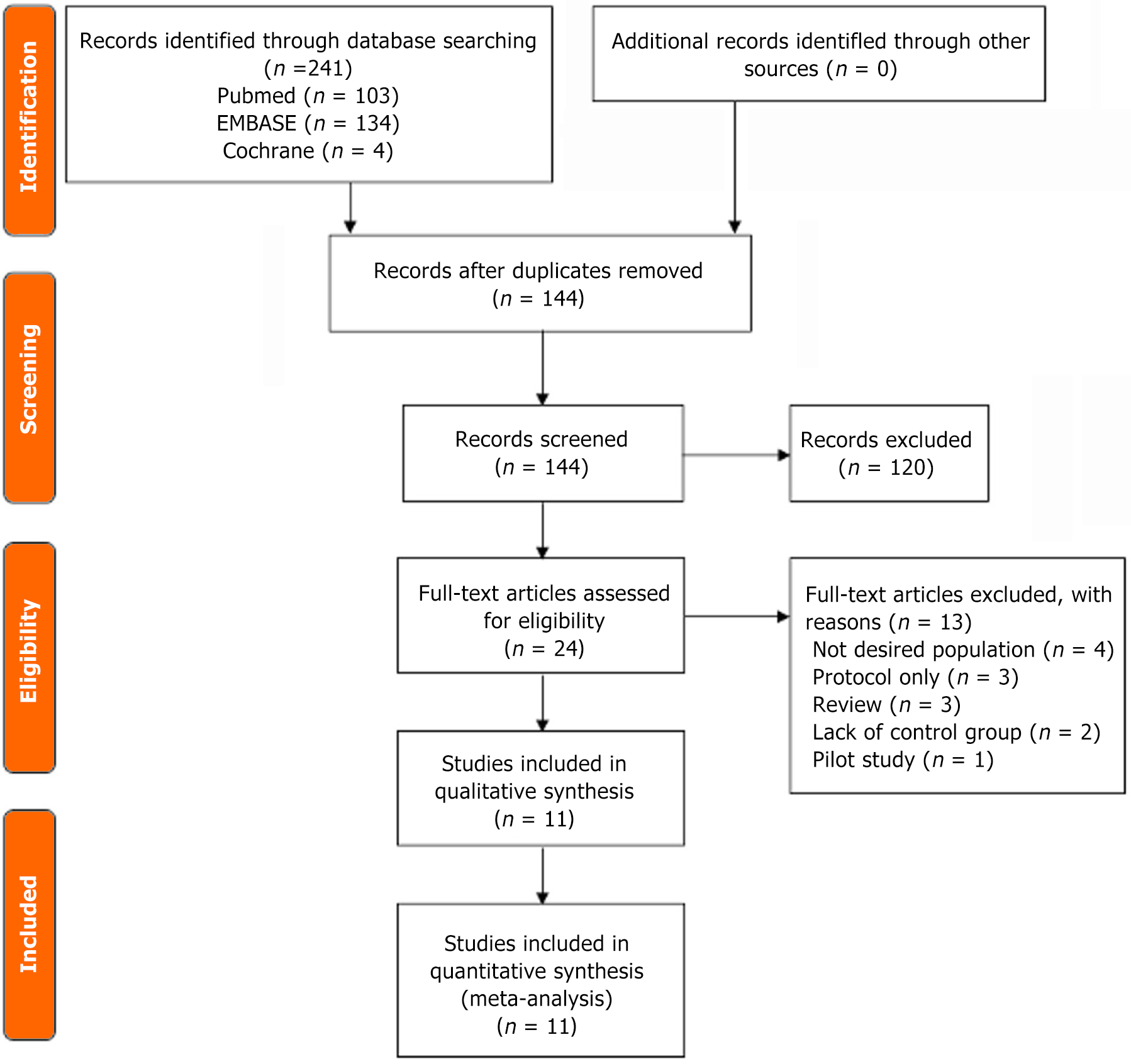
A total of 241 studies were identified through database searching, including PubMed (n = 103), EMBASE (n = 134), and Cochrane (n = 4). After removing 97 duplicates, the remaining studies (n = 144) were screened for eligibility. A total of 120 records were excluded by title and by abstract. As a result, 24 studies were assessed with full-text review. Among those, 13 articles were excluded due to not investigating the desired population (n = 4), protocol only (n = 3), reviews (n = 3), lack of control group (n = 2), and pilot study (n = 1). Finally, this meta-analysis included 11 articles that met the inclusion criteria[22–32]. The detailed PRISMA flow diagram is shown in Figure 1.
The characteristics of the included studies are summarized in Table 1[22-32]. No randomized controlled trials were identified; all studies were single center retrospective cohort analyses. HE was evaluated in eight studies[23,25–29,31,32]. HE was defined as an increase of 33% from baseline hematoma volume or absolute growth of more than 6 mL in five study[25,26,28,31,32], increase of 33% or absolute increase of 12.5 mL from baseline hematoma volume in two study[22,23], whereas it was not defined in one study[27]. PHE growth was evaluated in three studies[24,29,30]. All of the included studies defined PHE growth as the absolute difference in the PHE volume between follow-up and first scan. Blood sample was obtained on admission in ten studies[23–32], and at 24 hours in one study[22]. There was no consensus on cut-off value of NLR among the studies.
| Ref. | Design | N | Sample time | Cut-off value | Outcome | Outcome definition | Factors controlled for |
| Mao et al[30], 2024 | Retrospective | 117 | Admission | - | PHE | Difference between follow up scan and admission PHE | ICH volume, GCS score |
| Pisco et al[29], 2023 | Retrospective | 215 | Admission | - | PHE | Difference between follow up scan and admission PHE | NIHSS, glucose, neurosurgery procedure, ICH volume, ICH location, time to first HCT |
| Kim et al[31], 2023 | Retrospective | 520 | Admission | 5.63 | HE | ICH growth > 6 mL or > 33% of initial volume | Age, hypertension, diabetes, prior anticoagulation, GCS, NIHSS, WBC, glucose, SBP |
| Chu et al[27], 2023 | Retrospective | 301 | Admission | 5 | HE | - | Sex, diabetes, GCS, time to first HCT, ICH volume, WBC |
| Alimohammadi et al[28], 2022 | Retrospective | 221 | Admission | - | HE | ICH growth > 6 mL or > 33% of initial volume | ICH volume, SBP, GCS, WBC |
| Zhang et al[32], 2022 | Retrospective | 506 | Admission | - | HE | ICH growth > 6 mL or > 33% of initial volume | GCS, blend sign, swirl sign, hypodensities on CT |
| Fonseca et al[25], 2019 | Retrospective | 135 | Admission | 7.8 | HE | ICH growth > 6 mL or > 33% of initial volume | Systemic infection, time to fist CT, ICH volume, use of anticoagulant |
| Pektezel et al[22], 2019 | Retrospective | 383 | At 24 hours | - | HE | ICH growth > 12.5 mL or > 33% of initial volume | ICH location, use of anticoagulant |
| Wang et al[26], 2019 | Retrospective | 123 | Admission | 6.49 | HE | ICH growth > 6 mL or > 33% of initial volume | Age, sex, GCS, WBC, ICH volume, midline shift |
| Zhang et al[23], 2018 | Retrospective | 279 | Admission | 14.53 | HE | ICH growth > 12.5 mL or > 33% of initial volume | Time to fist CT, hydrocephalus, IVH, GCS, ICH volume, island sign |
| Gusdon et al[24], 2017 | Retrospective | 153 | Admission | - | PHE | Difference between follow up scan and admission PHE | ICH volume, HE, IVH, external ventricular drain placement, GCS, time to first CT |
The summary of the risk of bias for each of the outcomes is listed in Supplementary Tables 1, 2 and 3[22-32]. Their overall quality was acceptable, ranging on the NOS from 8/9 to 9/9 (9/9 being the highest study quality). Specifically, ten studies had moderate risk of bias for lack of control for systemic factors (systemic infections).
The presence of publication bias was not able to be assessed through visual assessment of asymmetry of the funnel plot as there were less than 10 studies included for each outcome.
Results of study outcomes are presented in Figure 2[22-32]. NLR was associated with significant higher odds of HE (OR = 1.09, 95%CI: 1.04-1.15, I2 = 86%, P < 0.01). A sensitivity analysis on the HE outcome was conducted by excluding studies one by one. After removing the study of Kim et al[30], the heterogeneity was decreased (I2 = 83%) without affecting the result (OR = 1.07, 95%CI: 1.02-1.12, P < 0.01). A subgroup analysis for the HE outcome was performed, including only the studies that uniformly considered HE as growth > 6 mL or > 33% of initial ICH volume. The result of the meta-analysis held true (OR = 1.13, 95%CI: 1.05-1.22, I2 = 86%, P < 0.01). NLR was also found to be associated with PHE growth (OR = 1.28, 95%CI: 1.19-1.38, I2 = 0%, P < 0.01).
This systematic review and meta-analysis included eleven retrospective cohort studies involving 2953 patients with ICH. The increasing volume of published data in recent years testifies to the rapidly developing interest in this topic. Our study showed that in patients with ICH, the NLR is associated with a higher trend of HE and PHE growth.
NLR is a marker of systemic inflammation[33]. In ICH, the local inflammatory response that occurs immediately after hematoma development involves the recruitment of peripheral leukocytes, with neutrophils being the first centrally recruited[34,35]. Additionally, the systemic stress response after ICH promotes the demargination of neutrophils and suppression of lymphocytes, further imbalancing the peripheral NLR[36,37]. Elevation of neutrophils in the brain parenchyma promotes neurotoxicity and alters the permeability of the blood-brain barrier[30,38–40]. The former appears to be the leading mechanism underlying PHE growth, while the latter drives HE.
The association between NLR and HE appears robust. Among the eight studies quantitatively summarized, most were homogeneous in the definition of HE, and a subgroup analysis restricted to a uniformed definition of HE confirmed the results. An association between NLR and PHE growth was also found. However, this association needs to be considered with more caution due to the low number of studies on the topic.
HE is an independent predictor of poor prognosis in ICH patients[3]. Several radiographic and laboratory values have been investigated as predictors of HE[4,5]. Among these, inflammatory markers are inexpensive laboratory tests fre
The association between high NLR and poor outcome has been well-documented in the literature[17]. Several studies have proven a correlation between NLR and mortality, and disability at 30 days and 90 days after ICH[12–16]. Fur
As a nonspecific peripheral inflammatory marker, NLR has been evaluated in several diseases, such as cancer, heart failure, and infectious disease[43–45]. In the hyperacute phase, systemic infections can cause a sudden rise in the innate response alongside suppression of the adaptive response, leading to a rise in NLR[21]. While all included studies were appropriately controlled for known markers of ICH severity, only one study considered systemic infections as a covariable of interest[45].
Finally, it is important to note that the studies included in this meta-analysis were designed as retrospective cohort studies. The association between NLR and HE and PHE appears robust. However, no assumptions can be made about the effect that modulating the NLR could have on ICH outcomes.
A future large multi-center prospective study should be designed to enroll subjects from different geographical areas worldwide, sampling blood of participants at presentation, controlling for markers of ICH severity and systemic confounders, and following the temporal behavior of NLR. This would help to consolidate the available literature and clarify the association and relationship between NLR, HE, PHE, and outcomes in ICH.
The main limitation of this meta-analysis is that all included studies were retrospective cohort analyses, which are inherently subject to selection bias and confounding factors. Additionally, the definition of HE varied among the studies, and the cut-off values for NLR were not consistent. Finally, the relatively small number of studies included in this meta-analysis limited the ability to perform further subgroup analyses and assess consistency across different populations, aside from a subgroup analysis on a homogeneous definition of HE.
The available literature suggests that a possible association may exist between NLR on admission and HE, and PHE growth. Future studies controlled for systemic confounders should be designed to consolidate this finding. If confirmed, NLR could be added as a readily available and inexpensive biomarker to identify a subgroup of patients at higher risk of developing HE.
| 1. | An SJ, Kim TJ, Yoon BW. Epidemiology, Risk Factors, and Clinical Features of Intracerebral Hemorrhage: An Update. J Stroke. 2017;19:3-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 362] [Cited by in RCA: 604] [Article Influence: 75.5] [Reference Citation Analysis (0)] |
| 2. | Zahuranec DB, Lisabeth LD, Sánchez BN, Smith MA, Brown DL, Garcia NM, Skolarus LE, Meurer WJ, Burke JF, Adelman EE, Morgenstern LB. Intracerebral hemorrhage mortality is not changing despite declining incidence. Neurology. 2014;82:2180-2186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 140] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 3. | Dowlatshahi D, Demchuk AM, Flaherty ML, Ali M, Lyden PL, Smith EE; VISTA Collaboration. Defining hematoma expansion in intracerebral hemorrhage: relationship with patient outcomes. Neurology. 2011;76:1238-1244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 512] [Cited by in RCA: 493] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 4. | Liu J, Xu H, Chen Q, Zhang T, Sheng W, Huang Q, Song J, Huang D, Lan L, Li Y, Chen W, Yang Y. Prediction of hematoma expansion in spontaneous intracerebral hemorrhage using support vector machine. EBioMedicine. 2019;43:454-459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 5. | Brouwers HB, Chang Y, Falcone GJ, Cai X, Ayres AM, Battey TW, Vashkevich A, McNamara KA, Valant V, Schwab K, Orzell SC, Bresette LM, Feske SK, Rost NS, Romero JM, Viswanathan A, Chou SH, Greenberg SM, Rosand J, Goldstein JN. Predicting hematoma expansion after primary intracerebral hemorrhage. JAMA Neurol. 2014;71:158-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 264] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 6. | Yogendrakumar V, Moores M, Sikora L, Shamy M, Ramsay T, Fergusson D, Dowlatshahi D. Evaluating Hematoma Expansion Scores in Acute Spontaneous Intracerebral Hemorrhage: A Systematic Scoping Review. Stroke. 2020;51:1305-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Sprigg N, Flaherty K, Appleton JP, Al-Shahi Salman R, Bereczki D, Beridze M, Christensen H, Ciccone A, Collins R, Czlonkowska A, Dineen RA, Duley L, Egea-Guerrero JJ, England TJ, Krishnan K, Laska AC, Law ZK, Ozturk S, Pocock SJ, Roberts I, Robinson TG, Roffe C, Seiffge D, Scutt P, Thanabalan J, Werring D, Whynes D, Bath PM; TICH-2 Investigators. Tranexamic acid for hyperacute primary IntraCerebral Haemorrhage (TICH-2): an international randomised, placebo-controlled, phase 3 superiority trial. Lancet. 2018;391:2107-2115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 291] [Cited by in RCA: 324] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 8. | Law ZK, Ali A, Krishnan K, Bischoff A, Appleton JP, Scutt P, Woodhouse L, Pszczolkowski S, Cala LA, Dineen RA, England TJ, Ozturk S, Roffe C, Bereczki D, Ciccone A, Christensen H, Ovesen C, Bath PM, Sprigg N; TICH-2 Investigators. Noncontrast Computed Tomography Signs as Predictors of Hematoma Expansion, Clinical Outcome, and Response to Tranexamic Acid in Acute Intracerebral Hemorrhage. Stroke. 2020;51:121-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 9. | Yoldas H, Karagoz I, Ogun MN, Velioglu Y, Yildiz I, Bilgi M, Demirhan A. Novel Mortality Markers for Critically Ill Patients. J Intensive Care Med. 2020;35:383-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Mobarra N, Morovatdar N, Di Napoli M, Stranges S, Behrouz R, Amiri A, Farzadfard MT, Hashemy SI, Oskoii R, Khorram B, Azarpazhooh MR. The Association between Inflammatory Markers in the Acute Phase of Stroke and Long-Term Stroke Outcomes: Evidence from a Population-Based Study of Stroke. Neuroepidemiology. 2019;53:20-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Zhang XG, Xue J, Yang WH, Xu XS, Sun HX, Hu L, Liu LY, Yue YH. Inflammatory markers as independent predictors for stroke outcomes. Brain Behav. 2021;11:e01922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 12. | Tao C, Hu X, Wang J, Ma J, Li H, You C. Admission neutrophil count and neutrophil to lymphocyte ratio predict 90-day outcome in intracerebral hemorrhage. Biomark Med. 2017;11:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 13. | Giede-Jeppe A, Bobinger T, Gerner ST, Sembill JA, Sprügel MI, Beuscher VD, Lücking H, Hoelter P, Kuramatsu JB, Huttner HB. Neutrophil-to-Lymphocyte Ratio Is an Independent Predictor for In-Hospital Mortality in Spontaneous Intracerebral Hemorrhage. Cerebrovasc Dis. 2017;44:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 14. | Zhang F, Ren Y, Fu W, Yang Z, Wen D, Hu X, Tao C, Li X, You C, Xin T, Yang M. Predictive Accuracy of Neutrophil-to-Lymphocyte Ratio on Long-Term Outcome in Patients with Spontaneous Intracerebral Hemorrhage. World Neurosurg. 2019;125:e651-e657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Menon G, Johnson SE, Hegde A, Rathod S, Nayak R, Nair R. Neutrophil to lymphocyte ratio - A novel prognostic marker following spontaneous intracerebral haemorrhage. Clin Neurol Neurosurg. 2021;200:106339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Zhang F, Tao C, Hu X, Qian J, Li X, You C, Jiang Y, Yang M. Association of Neutrophil to Lymphocyte Ratio on 90-Day Functional Outcome in Patients with Intracerebral Hemorrhage Undergoing Surgical Treatment. World Neurosurg. 2018;119:e956-e961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Shi M, Li XF, Zhang TB, Tang QW, Peng M, Zhao WY. Prognostic Role of the Neutrophil-to-Lymphocyte Ratio in Intracerebral Hemorrhage: A Systematic Review and Meta-Analysis. Front Neurosci. 2022;16:825859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 18. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11206] [Cited by in RCA: 11017] [Article Influence: 688.6] [Reference Citation Analysis (0)] |
| 19. | Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers' to authors' assessments. BMC Med Res Methodol. 2014;14:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 770] [Cited by in RCA: 1630] [Article Influence: 148.2] [Reference Citation Analysis (0)] |
| 20. | Furlan AD, Malmivaara A, Chou R, Maher CG, Deyo RA, Schoene M, Bronfort G, van Tulder MW; Editorial Board of the Cochrane Back, Neck Group. 2015 Updated Method Guideline for Systematic Reviews in the Cochrane Back and Neck Group. Spine (Phila Pa 1976). 2015;40:1660-1673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 527] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 21. | Gøtzsche PC. [The Nordic Cochrane Center. Status after five years and future perspectives]. Ugeskr Laeger. 1999;161:932-934. [PubMed] |
| 22. | Pektezel MY, Arsava EM, Öge DD, Yildiz OK, Topcuoglu MA. Neutrophil-to-Lymphocyte Ratio and Prognosis of Spontaneous Intracerebral Hemorrhage. Türk Beyin Damar Hast Derg. 2019;25:118-124. [DOI] [Full Text] |
| 23. | Zhang F, Qian J, Tao C, Wang Y, Lin S, You C, Yang M. Neutrophil to lymphocyte ratio predicts island sign in patients with intracranial hemorrhage. Medicine (Baltimore). 2018;97:e13057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Gusdon AM, Gialdini G, Kone G, Baradaran H, Merkler AE, Mangat HS, Navi BB, Iadecola C, Gupta A, Kamel H, Murthy SB. Neutrophil-Lymphocyte Ratio and Perihematomal Edema Growth in Intracerebral Hemorrhage. Stroke. 2017;48:2589-2592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 25. | Fonseca S, Costa F, Seabra M, Dias R, Soares A, Dias C, Azevedo E, Castro P. Systemic inflammation status at admission affects the outcome of intracerebral hemorrhage by increasing perihematomal edema but not the hematoma growth. Acta Neurol Belg. 2021;121:649-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 26. | Wang Z, Gong Q, Guo C, Luo Y, Chen L. Neutrophil-to-lymphocyte ratio predicts hematoma growth in intracerebral hemorrhage. J Int Med Res. 2019;47:2970-2975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Chu H, Huang C, Zhou Z, Tang Y, Dong Q, Guo Q. Inflammatory score predicts early hematoma expansion and poor outcomes in patients with intracerebral hemorrhage. Int J Surg. 2023;109:266-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 28. | Alimohammadi E, Bagheri SR, Mardanpour P, Moradi F, Arjmandnia F, Esmaeili N. Baseline neutrophil-lymphocyte ratio can be associated with hematoma expansion in patients with intracerebral hemorrhage: a retrospective observational study. BMC Neurosci. 2022;23:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Pisco C, Pedro T, Aires A, Fonseca L, Fonseca S, Castro P. The effect of neutrophil-to-lymphocyte ratio and systemic inflammatory response on perihematomal edema after intracerebral hemorrhage. J Clin Neurosci. 2023;115:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 30. | Mao Y, Huang L, Ji G, Wang L, Wang X, Zheng X. Neutrophil-to-lymphocyte ratio on admission predicts early perihematomal edema growth after intracerebral hemorrhage. Medicine (Baltimore). 2024;103:e37585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 31. | Kim Y, Sohn JH, Kim C, Park SY, Lee SH. The Clinical Value of Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio for Predicting Hematoma Expansion and Poor Outcomes in Patients with Acute Intracerebral Hemorrhage. J Clin Med. 2023;12:3004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 32. | Zhang X, Gao Q, Chen K, Wu Q, Chen B, Zeng S, Fang X. A predictive nomogram for intracerebral hematoma expansion based on non-contrast computed tomography and clinical features. Neuroradiology. 2022;64:1547-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 33. | Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2954] [Cited by in RCA: 3832] [Article Influence: 319.3] [Reference Citation Analysis (0)] |
| 34. | Lattanzi S, Brigo F, Trinka E, Cagnetti C, Di Napoli M, Silvestrini M. Neutrophil-to-Lymphocyte Ratio in Acute Cerebral Hemorrhage: a System Review. Transl Stroke Res. 2019;10:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 162] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 35. | Mracsko E, Javidi E, Na SY, Kahn A, Liesz A, Veltkamp R. Leukocyte invasion of the brain after experimental intracerebral hemorrhage in mice. Stroke. 2014;45:2107-2114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 122] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 36. | Askenase MH, Sansing LH. Stages of the Inflammatory Response in Pathology and Tissue Repair after Intracerebral Hemorrhage. Semin Neurol. 2016;36:288-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 37. | Lattanzi S, Cagnetti C, Provinciali L, Silvestrini M. Neutrophil-to-lymphocyte ratio and neurological deterioration following acute cerebral hemorrhage. Oncotarget. 2017;8:57489-57494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 38. | Lee KR, Kawai N, Kim S, Sagher O, Hoff JT. Mechanisms of edema formation after intracerebral hemorrhage: effects of thrombin on cerebral blood flow, blood-brain barrier permeability, and cell survival in a rat model. J Neurosurg. 1997;86:272-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 274] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 39. | Rendevski V, Aleksovski B, Mihajlovska Rendevska A, Hadzi-Petrushev N, Manusheva N, Shuntov B, Gjorgoski I. Inflammatory and oxidative stress markers in intracerebral hemorrhage: Relevance as prognostic markers for quantification of the edema volume. Brain Pathol. 2023;33:e13106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 40. | Chen W, Yang J, Li B, Peng G, Li T, Li L, Wang S. Neutrophil to Lymphocyte Ratio as a Novel Predictor of Outcome in Patients With Severe Traumatic Brain Injury. J Head Trauma Rehabil. 2018;33:E53-E59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 41. | Chen J, Qu X, Li Z, Zhang D, Hou L. Peak Neutrophil-to-Lymphocyte Ratio Correlates with Clinical Outcomes in Patients with Severe Traumatic Brain Injury. Neurocrit Care. 2019;30:334-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 42. | Howard R, Kanetsky PA, Egan KM. Exploring the prognostic value of the neutrophil-to-lymphocyte ratio in cancer. Sci Rep. 2019;9:19673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 187] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 43. | Chen T, Yang M. Platelet-to-lymphocyte ratio is associated with cardiovascular disease in continuous ambulatory peritoneal dialysis patients. Int Immunopharmacol. 2020;78:106063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 44. | Curbelo J, Luquero Bueno S, Galván-Román JM, Ortega-Gómez M, Rajas O, Fernández-Jiménez G, Vega-Piris L, Rodríguez-Salvanes F, Arnalich B, Díaz A, Costa R, de la Fuente H, Lancho Á, Suárez C, Ancochea J, Aspa J. Inflammation biomarkers in blood as mortality predictors in community-acquired pneumonia admitted patients: Importance of comparison with neutrophil count percentage or neutrophil-lymphocyte ratio. PLoS One. 2017;12:e0173947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 99] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 45. | Liesz A, Rüger H, Purrucker J, Zorn M, Dalpke A, Möhlenbruch M, Englert S, Nawroth PP, Veltkamp R. Stress mediators and immune dysfunction in patients with acute cerebrovascular diseases. PLoS One. 2013;8:e74839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |