Published online Mar 9, 2025. doi: 10.5492/wjccm.v14.i1.100486
Revised: October 2, 2024
Accepted: October 30, 2024
Published online: March 9, 2025
Processing time: 115 Days and 13.5 Hours
Coronavirus disease 2019 (COVID-19) is strongly associated with an increased risk of thrombotic events, including severe outcomes such as pulmonary em
To determine the impact of D-dimer levels on COVID-19 severity and their role in guiding clinical decisions.
This retrospective study analyzed COVID-19 patients admitted to two hospitals in Gabon between March 2020 and December 2023. The study included patients with confirmed COVID-19 diagnoses and available D-dimer measurements at admission. Data on demographics, clinical outcomes, D-dimer levels, and healthcare costs were collected. COVID-19 severity was classified as non-severe (outpatients) or severe (inpatients). A multivariable logistic regression model was used to assess the relationship between D-dimer levels and disease severity, with adjusted odds ratios (OR) and 95%CI.
A total of 3004 patients were included, with a mean age of 50.17 years, and the majority were female (53.43%). Elevated D-dimer levels were found in 65.81% of patients, and 57.21% of these experienced severe COVID-19. Univariate analysis showed that patients with elevated D-dimer levels had 3.33 times higher odds of severe COVID-19 (OR = 3.33, 95%CI: 2.84-3.92, P < 0.001), and this association remained significant in the multivariable analysis, adjusted for age, sex, and year of collection. The financial analysis revealed a substantial burden, particularly for uninsured patients.
D-dimer predicts COVID-19 severity and guides treatment, but the high cost of anticoagulant therapy highlights the need for policies ensuring affordable access in resource-limited settings like Gabon.
Core Tip: D-dimer, a key biomarker produced during blood clot breakdown, is widely used to diagnose thrombotic conditions such as deep vein thrombosis and pulmonary embolism. Elevated D-dimer levels have been consistently linked to increased coronavirus disease 2019 (COVID-19) severity and mortality risk. This study highlights the role of D-dimer in predicting COVID-19 outcomes in Gabon. Understanding its role can help optimize healthcare resource allocation, improve patient outcomes, and mitigate the economic burden associated with severe COVID-19 cases in resource-limited settings.
- Citation: Iroungou BA, Nze O A, M Kandet Y H, Longo-Pendy NM, Mezogho-Obame ND, Dikoumba AC, Mangouka GL. Interest of D-dimer level, severity of COVID-19 and cost of management in Gabon. World J Crit Care Med 2025; 14(1): 100486
- URL: https://www.wjgnet.com/2220-3141/full/v14/i1/100486.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v14.i1.100486
At the beginning of the coronavirus disease 2019 (COVID-19) pandemic, several studies suggested that D-dimer levels were elevated in patients with COVID-19. These results were interpreted as an indication of the presence of blood clots in these patients, which could contribute to the severe complications of the disease[1,2].
Since then, many studies have been conducted to investigate the relationship between D-dimer levels and COVID-19. Some of these studies have shown that high D-dimer levels are associated with a higher risk of death in COVID-19 patients, while others have suggested that D-dimer levels could be used as an early indicator of disease severity[3-5].
Despite the expanding body of research, a significant gap remains in understanding the specific implications of D-dimer levels in resource-limited settings like Gabon. This gap is critical due to the challenges these settings face in healthcare infrastructure and resource allocation. Understanding the role of D-dimer as a prognostic biomarker in such contexts is essential for optimizing patient management strategies. This study addresses this gap by examining the relationship between D-dimer levels and COVID-19 severity in Gabon, contributing to the existing literature by providing insights into the economic impact of COVID-19 management in resource-constrained settings.
D-dimer testing has emerged as a key tool in assessing thrombotic risk in COVID-19 patients, offering crucial insights into disease severity. This is particularly relevant in resource-limited settings, where early and accurate prognostic indicators are vital for effective patient management[6]. The primary objective of this study is to investigate the association between D-dimer levels and COVID-19 severity in Gabon. Additionally, the study aims to evaluate the financial burden of managing COVID-19, particularly with regard to anticoagulant therapy costs, which present significant challenges in low-resource environments. By addressing these objectives, this study aims to provide valuable data that can inform healthcare policies and improve clinical outcomes in similar settings.
This retrospective study was conducted at two hospitals in Gabon and included all patients admitted with confirmed COVID-19 infection between March 2020 and December 2023. Eligible participants were those with a confirmed COVID-19 diagnosis via PCR test, available D-dimer measurements upon admission, and flu-like symptoms presenting after April 2022. Exclusion criteria included incomplete medical records regarding D-dimer levels, conditions significantly affecting D-dimer levels (e.g., active malignancies, recent major surgery, pregnancy, or postpartum status), and lack of informed consent.
Data on the participants were retrospectively obtained through an electronic records system. Collected variables included demographic information, laboratory results, clinical characteristics (including D-dimer levels at admission), and clinical outcomes such as COVID-19 severity, treatments administered, and final outcomes. Additionally, cost data were gathered, focusing on direct medical expenses, including diagnostic testing and patient costs for anticoagulant therapy.
COVID-19 severity was classified into two categories: (1) Mild for outpatients; and (2) Severe for inpatients.
Patients underwent a range of laboratory and imaging tests to evaluate their clinical status.
D-dimer testing was recommended for those presenting with flu-like symptoms, positive PCR results, or abnormal computed tomography (CT) scans. The most commonly reported clinical signs were dyspnea, asthenia, fever, and cough[7]. The standard D-dimer threshold was set at less than 500 ng/mL for all patients. For those aged 50 years or older, a D-dimer level was considered negative if it was below the patient’s age multiplied by 10[8].
Resource utilization data included D-dimer testing and anticoagulation treatments. Enoxaparin was the most commonly prescribed anticoagulant for outpatients to prevent deep vein thrombosis and pulmonary embolism. Inpatients received various anticoagulants, including low molecular weight heparin, unfractionated heparin, and oral anticoagulants, based on clinical indications.
The study adopted a patient-centered cost perspective, with all expenses expressed in 2024 United States dollars (USD). Three types of insurance coverage were recognized: (1) "Direct billing" or "third-party payment", where providers are reimbursed directly by insurance companies or government agencies; (2) A co-payment model, where patients paid 20% of the total cost; and (3) Full payment by uninsured patients. The unit cost for D-dimer testing was 10.10 USD, and the cost of anticoagulant therapy was 126.45 USD.
For this population study, continuous variables were summarized using means (SD), medians (interquartile range), and minimum and maximum values, along with the 25th and 75th percentiles. Categorical variables were reported as numbers (percentages).
A bivariate analysis was conducted to describe and compare D-dimer levels. χ² tests were used for categorical variables, and t-tests or Wilcoxon Mann-Whitney tests were applied to continuous variables, depending on their distribution. Variables with a P-value lower or equal to 0.2 in the bivariate analysis were included in the multivariable logistic regression model. To assess whether elevated D-dimer levels could predict COVID-19 severity, multivariable logistic regression analysis was performed, incorporating D-dimer levels (the primary variable of interest), age, sex, and year of collection. Results were reported as odds ratios (OR), with 95%CI and P-values.
All analyses were performed using R software, version 4.4.0.
This study was conducted in accordance with ethical guidelines and received approval from the National Ethics Committee in Gabon. To ensure patient confidentiality, all data were anonymized. Informed consent was obtained from all participants or their legal guardians.
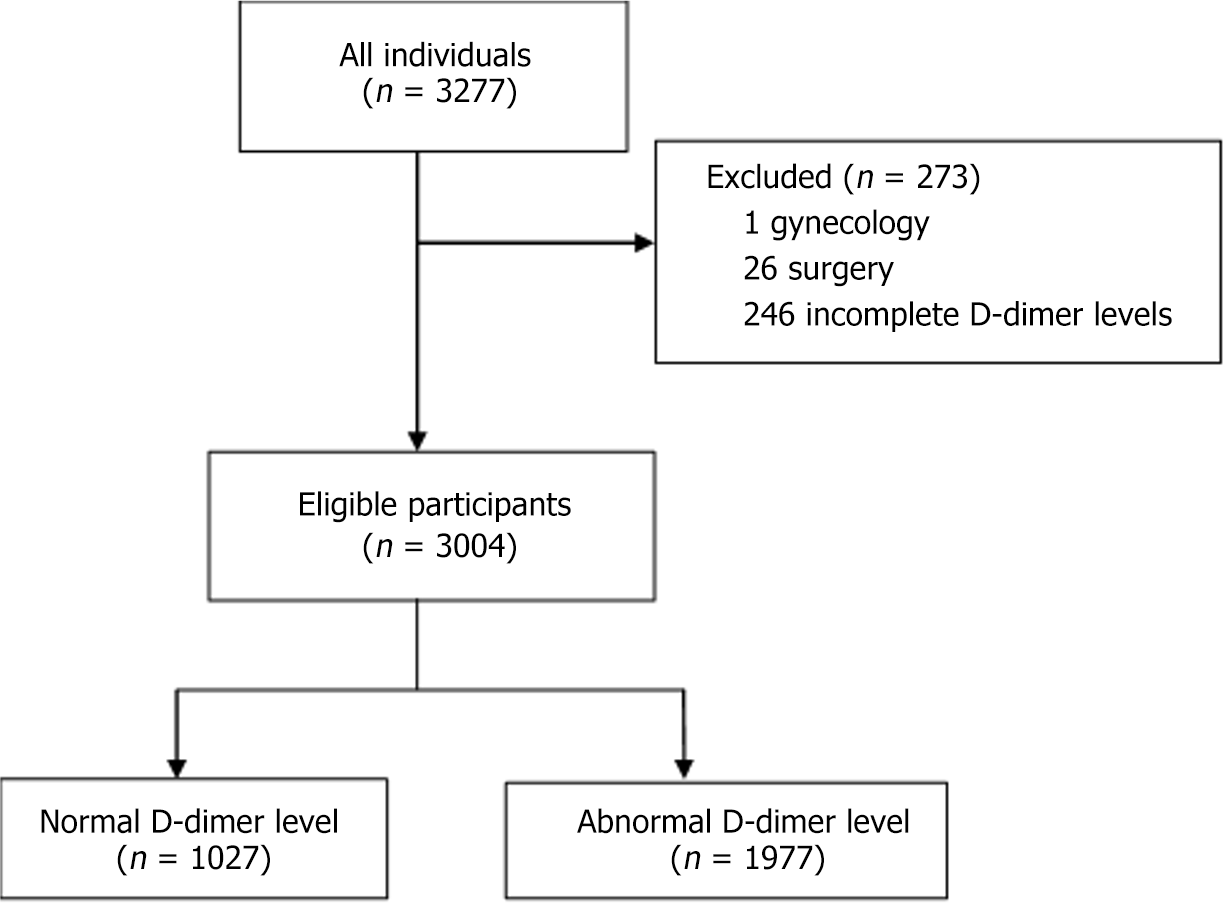
A total of 3004 individuals were enrolled from two hospitals in Gabon between March 2020 and December 2023. All participants presented with flu-like symptoms, had positive PCR results, abnormal CT scans, and underwent D-dimer testing. Of this population, 1027 (34.19%) had normal D-dimer levels, while 1977 (65.81%) had elevated D-dimer levels (Figure 1). Participant characteristics are summarized in Table 1. The average age was 50.17 years ± 16.92 years, and 53.43% of the participants were female. Among the patients, 65.81% had an abnormal D-dimer result.
| Total (n = 3004) | Normal D-dimer level (n = 1027) | Abnormal D-dimer level (n = 1977) | P value | |
| Gender | < 0.001 | |||
| Female | 1605 (53.43) | 504 (49.1) | 1101 (55.7) | |
| Age (year) | < 0.001 | |||
| Mean (SD) | 50.17 (16.92) | 47.19 (15.53) | 51.72 (17.40) | |
| Median (interquartile range) | 48.00 (24.00) | 45.00 (21.00) | 51.00 (26.00) | |
| Quartile 1, quartile 3 | 38.00, 62.00 | 37.00, 58.00 | 38.00, 64.00 | |
| Minimum, maximum | 2.00, 101.00 | 2.00, 99.00 | 2.00, 101.00 | |
| Year | < 0.001 | |||
| 2020 | 612 (20.37) | 222 (21.62) | 390 (19.73) | |
| 2021 | 523 (17.41) | 143 (13.92) | 380 (19.22) | |
| 2022 | 482 (16.05) | 147 (14.31) | 335 (16.94) | |
| 2023 | 1387 (46.17) | 515 (50.15) | 872 (44.11) | |
| Severity of coronavirus disease 2019 | < 0.001 | |||
| Severe | 1425 (47.44) | 294 (28.63) | 1131 (57.21) |
The comparison of patient characteristics based on D-dimer levels is presented in Table 1. Among the 3004 patients with confirmed COVID-19, 294 (28.63%) were clinically diagnosed with severe infection, while 1131 (57.21%) were diagnosed with mild infection. Patients with abnormal D-dimer levels were generally older (51.72 years ± 17.40 years vs 47.19 years ± 15.53 years, P < 0.001) and more likely to be female [1101 (55.7%) vs 504 (49.1%), P < 0.001] compared to those with normal D-dimer levels.
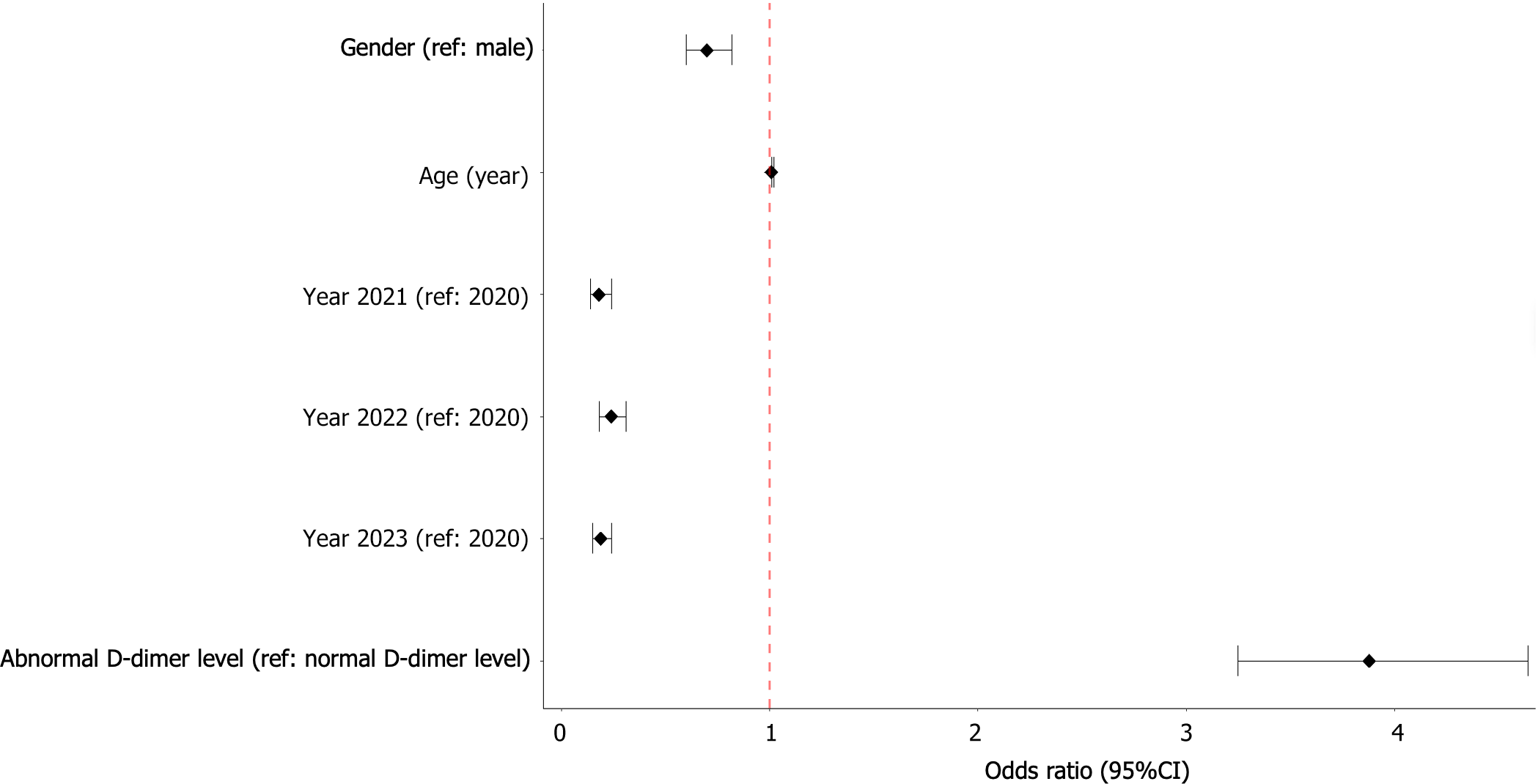
The results of the bivariate and multivariate logistic regression analyses are shown in Table 2 and Figure 2. In the univariate analysis, D-dimer levels, gender, age, and year of collection were associated with COVID-19 severity, with P-values below 0.2. These variables were subsequently included in the multivariate logistic regression model.
| Univariable OR (95%CI) | P value | Multivariable OR (95%CI) | P value | |
| Gender | ||||
| Female vs male | 0.74 (0.64-0.85) | < 0.001 | 0.70 (0.60-0.82) | < 0.001 |
| Age (year) | 1.01 (1.01-1.02) | < 0.001 | 1.01 (1.01-1.01) | < 0.001 |
| Year | ||||
| 2021 vs 2020 | 0.25 (0.19-0.32) | < 0.001 | 0.18 (0.14-0.24) | < 0.001 |
| 2022 vs 2020 | 0.29 (0.22-0.37) | < 0.001 | 0.24 (0.18-0.31) | < 0.001 |
| 2023 vs 2020 | 0.22 (0.18-0.27) | < 0.001 | 0.19 (0.15-0.24) | < 0.001 |
| Abnormal D-dimer level | ||||
| D-dimer level vs normal D-dimer level | 3.33 (2.84-3.92) | < 0.001 | 3.89 (3.27-4.66) | < 0.001 |
In the univariate logistic regression analysis, patients with abnormal D-dimer levels had 3.33 times higher odds of severe COVID-19 (OR = 3.33, 95%CI: 2.84-3.92, P < 0.001) compared to those with normal D-dimer levels. This association remained significantly elevated in the multivariate analysis after adjusting for age, sex, and year of collection (OR = 3.88, 95%CI: 3.25-4.64, P < 0.001).
A total of 3004 D-dimer tests were performed in the study population. Of these, 812 tests were fully funded by the Gabonese state, 1620 were 80% state-funded, and 572 were entirely paid for by the patients. A detailed cost analysis revealed significant disparities based on patients' insurance status. Insured patients benefited from substantial cost reductions, while uninsured patients faced the full expense, resulting in a disproportionately higher financial burden.
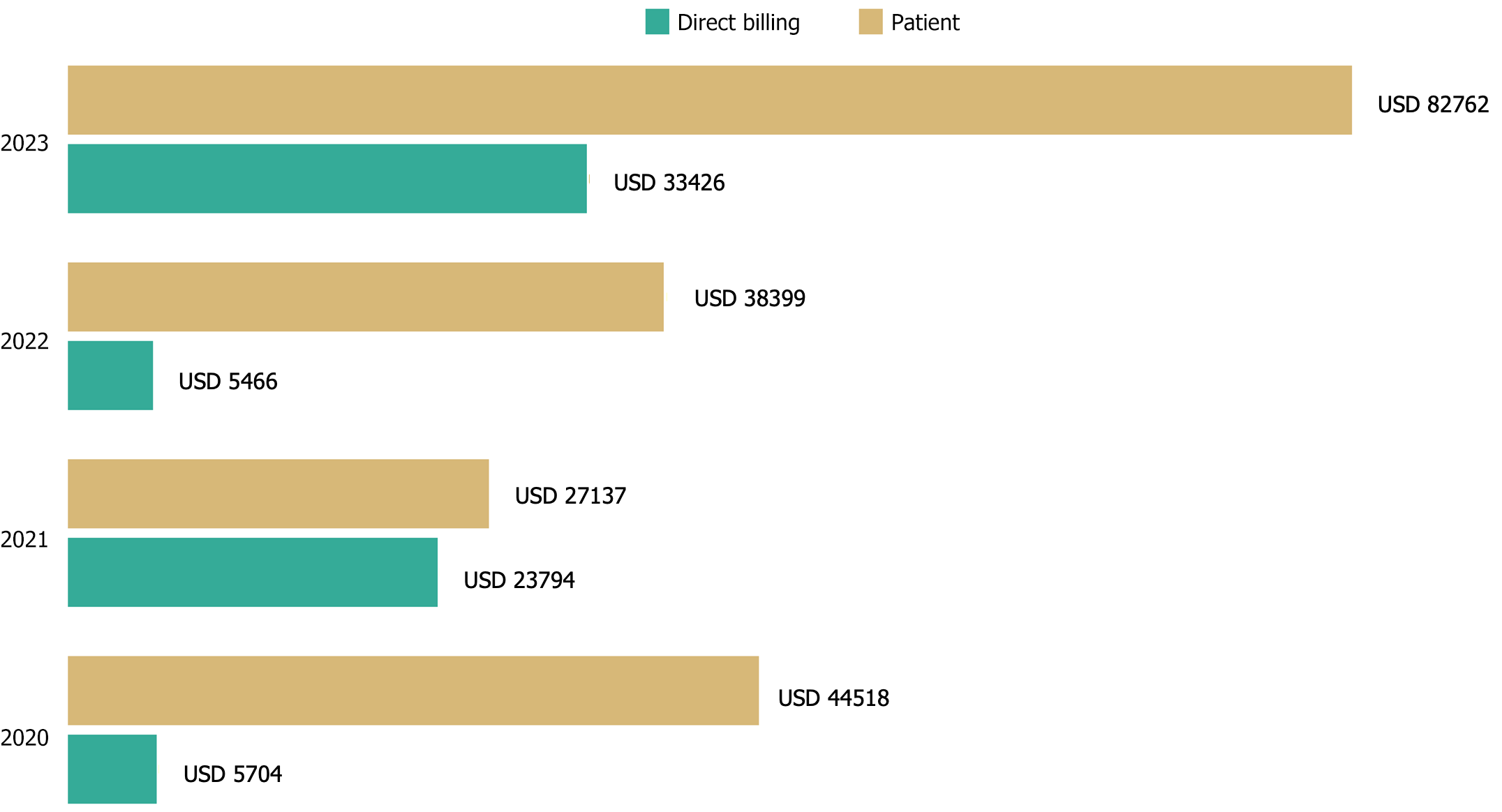
Based on D-dimer test results, 1975 patients were prescribed anticoagulant therapy. The number of D-dimer tests doubled between 2020 and 2023, increasing from 612 tests in 2020 to 1387 in 2023. Despite state efforts to support patient care, the financial responsibility for D-dimer testing and anticoagulant therapy largely falls on patients. This burden was especially pronounced for uninsured patients (range 27137–82762 USD) compared to insured patients (range 5704–33426 USD), underscoring the need for targeted policy interventions to reduce these costs. This financial strain persisted across all study years (Figure 3).
Between January 3, 2020, and April 1, 2022, Gabon experienced four distinct waves of COVID-19, with cases ranging from mild to severe, primarily affecting the respiratory system. During this period, 47584 cases and 303 deaths were reported to the World Health Organization[7]. These figures, though notable, highlight the broader global impact of the pandemic, particularly in regions with limited healthcare infrastructure where resources are often severely constrained.
We conducted a cross-sectional study to assess the impact of D-dimer levels on the severity of COVID-19 in a sample of 3004 patients admitted to two hospitals in Gabon. Additionally, we examined and quantified the financial burden of D-dimer diagnostics and anticoagulant treatment on these patients. The odds of developing severe COVID-19 were 3.33 times higher (95%CI: 2.84–3.92, P < 0.001) in patients with abnormal D-dimer levels compared to those with normal levels. This association remained significantly elevated in multivariable analysis after adjusting for age, sex, and year of collection.
This study has several limitations that should be acknowledged. First, the retrospective design may introduce selection bias, as only patients with recorded D-dimer levels at hospital admission were included, limiting the ability to establish a clear causal relationship between elevated D-dimer levels and COVID-19 severity. Additionally, potential confounding factors, such as variations in treatment protocols, the presence of comorbidities, and differences in healthcare access, were not fully controlled for in this analysis. These limitations suggest that future research should adopt a prospective study design to better account for these variables and validate the findings more rigorously.
The recognition of thromboembolic complications associated with COVID-19 prompted Gabon to incorporate anticoagulant therapy into its national treatment protocol as early as July 2020. This proactive measure was in line with global evidence indicating that coagulopathy is a key factor contributing to severe outcomes, such as pulmonary embolism and other thrombotic events[9-11]. The early adoption of this therapeutic approach in Gabon likely contributed to maintaining a relatively low mortality rate compared to global averages[3,5,12]. This strategy highlights the importance of timely and context-specific public health decisions in mitigating the adverse effects of COVID-19 in resource-limited settings.
D-dimer, a fibrin degradation product, has emerged as a key biomarker for assessing the risk of thromboembolic events in COVID-19 patients. Elevated D-dimer levels have been consistently linked to an increased risk of mortality across numerous studies[3,5,12]. Beyond predicting thrombotic complications, D-dimer levels also serve as a critical indicator of disease severity and progression, helping clinicians make informed decisions regarding patient management[13]. In Gabon, the integration of D-dimer testing into COVID-19 diagnostic and monitoring protocols aligns with a broader global trend of utilizing this biomarker to guide clinical interventions[14]. This shift highlights the broader challenges faced by low-income and middle-income countries (LMICs), where healthcare resources are limited, and the need for cost-effective, scalable alternatives is paramount.
Following the Gabonese government's declaration in April 2022 that the country was COVID-19-free, the reliance on D-dimer testing became more pronounced. The cessation of mass PCR testing and the introduction of fees for CT scans due to economic constraints led to an increased dependence on D-dimer as both a diagnostic and prognostic tool[15]. This shift underscores the broader challenges faced by LMICs, where healthcare resources are limited, emphasizing the need for cost-effective, scalable alternatives[16,17].
Despite the official declaration of the pandemic's end, our study highlights the ongoing risk of thromboembolic events, particularly pulmonary embolism, within the population, as indicated by persistently elevated D-dimer levels (Figure 2C and D). This finding is consistent with research from other regions, which shows that the prothrombotic state in COVID-19 survivors may persist beyond the acute infection phase, warranting continued vigilance[18,19]. The sustained use of D-dimer testing in 2023, along with its partial coverage by health insurance, underscores its continued importance in managing the long-term sequelae of COVID-19, particularly in resource-limited settings.
The elevated D-dimer concentrations observed in hospitalized patients during the pandemic are strongly correlated with disease severity, reinforcing global findings that support the use of D-dimer as a reliable prognostic marker for severe acute respiratory syndrome-coronavirus 2 infection[20-22]. This correlation highlights the importance of incorporating D-dimer testing into routine clinical practice, particularly for patients presenting with severe symptoms or those at increased risk of thromboembolic events.
Economically, the ongoing management of COVID-19 presents significant challenges, particularly in LMICs like Gabon. Our analysis revealed stark disparities in the financial burden between insured and uninsured patients. Insured patients benefited from significant cost reductions due to insurance coverage, paying only a fraction of the total treatment costs. In contrast, uninsured patients were responsible for covering the full costs, resulting in a disproportionately higher financial burden.
The initial government response, which provided widespread access to PCR testing and CT scans, was crucial in controlling the early waves of the pandemic. However, the shift toward increased reliance on D-dimer testing underscores the difficulties in maintaining comprehensive diagnostic coverage when resources are limited[20,23]. This situation emphasizes the need for innovative, cost-effective healthcare strategies that can adapt to the evolving demands of global health emergencies.
Moreover, the financial burden of anticoagulant therapy remains a significant issue, particularly for uninsured patients. With a treatment cost of approximately 126.45 USD per course, this poses a substantial challenge for low-income populations, exacerbating the socio-economic impact of COVID-19 on vulnerable groups[24]. This financial strain highlights the urgent need for targeted policy interventions to reduce barriers to essential treatments, ensuring equitable access to life-saving therapies for all, regardless of economic status[25,26].
D-dimer is a critical prognostic marker for managing COVID-19, especially in assessing thrombotic risk. However, equitable access to anticoagulant therapy remains a major challenge in resource-limited settings like Gabon.
Based on our findings, we recommend prioritizing routine D-dimer testing in COVID-19 protocols to identify severe cases earlier. Additionally, implementing healthcare policies that subsidize the cost of anticoagulant therapy, particularly for low-income and uninsured patients, is essential to reducing financial barriers. Strengthening healthcare infrastructure and training to support the use of diagnostic tools like D-dimer tests will be key to improving patient outcomes and ensuring that all individuals, regardless of economic status, have access to life-saving treatments.
The authors would like to thank Roto ER for his valuable assistance.
| 1. | Gąsecka A, Borovac JA, Guerreiro RA, Giustozzi M, Parker W, Caldeira D, Chiva-Blanch G. Thrombotic Complications in Patients with COVID-19: Pathophysiological Mechanisms, Diagnosis, and Treatment. Cardiovasc Drugs Ther. 2021;35:215-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 98] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 2. | Wang T, Chen R, Liu C, Liang W, Guan W, Tang R, Tang C, Zhang N, Zhong N, Li S. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol. 2020;7:e362-e363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 258] [Cited by in RCA: 273] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 3. | Shah S, Shah K, Patel SB, Patel FS, Osman M, Velagapudi P, Turagam MK, Lakkireddy D, Garg J. Elevated D-Dimer Levels Are Associated With Increased Risk of Mortality in Coronavirus Disease 2019: A Systematic Review and Meta-Analysis. Cardiol Rev. 2020;28:295-302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 4. | Gungor B, Atici A, Baycan OF, Alici G, Ozturk F, Tugrul S, Asoglu R, Cevik E, Sahin I, Barman HA. Elevated D-dimer levels on admission are associated with severity and increased risk of mortality in COVID-19: A systematic review and meta-analysis. Am J Emerg Med. 2021;39:173-179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 5. | Nugroho J, Wardhana A, Maghfirah I, Mulia EPB, Rachmi DA, A'yun MQ, Septianda I. Relationship of D-dimer with severity and mortality in SARS-CoV-2 patients: A meta-analysis. Int J Lab Hematol. 2021;43:110-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Wauthier L, Favresse J, Hardy M, Douxfils J, Le Gal G, Roy PM, van Es N, Ay C, Ten Cate H, Lecompte T, Lippi G, Mullier F. D-dimer testing: A narrative review. Adv Clin Chem. 2023;114:151-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 7. | Sukrisman L, Sinto R. Coagulation profile and correlation between D-dimer, inflammatory markers, and COVID-19 severity in an Indonesian national referral hospital. J Int Med Res. 2021;49:3000605211059939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Lippi G, Mullier F, Favaloro EJ. D-dimer: old dogmas, new (COVID-19) tricks. Clin Chem Lab Med. 2023;61:841-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 9. | Al-Samkari H, Karp Leaf RS, Dzik WH, Carlson JCT, Fogerty AE, Waheed A, Goodarzi K, Bendapudi PK, Bornikova L, Gupta S, Leaf DE, Kuter DJ, Rosovsky RP. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136:489-500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1006] [Cited by in RCA: 926] [Article Influence: 185.2] [Reference Citation Analysis (0)] |
| 10. | Stals M, Kaptein F, Kroft L, Klok FA, Huisman MV. Challenges in the diagnostic approach of suspected pulmonary embolism in COVID-19 patients. Postgrad Med. 2021;133:36-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Rahi MS, Parekh J, Pednekar P, Mudgal M, Jindal V, Gunasekaran K. Role of Therapeutic Anticoagulation in COVID-19: The Current Situation. Hematol Rep. 2023;15:358-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Faucheux L, Bassolli de Oliveira Alves L, Chevret S, Rocha V. Comparison of characteristics and laboratory tests of COVID-19 hematological patients from France and Brazil during the pre-vaccination period: identification of prognostic profiles for survival. Hematol Transfus Cell Ther. 2023;45:306-316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844-847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3992] [Cited by in RCA: 4038] [Article Influence: 807.6] [Reference Citation Analysis (0)] |
| 14. | Cosmi B, Legnani C, Libra A, Palareti G. D-Dimers in diagnosis and prevention of venous thrombosis: recent advances and their practical implications. Pol Arch Intern Med. 2023;133:16604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 15. | Naymagon L, Zubizarreta N, Feld J, van Gerwen M, Alsen M, Thibaud S, Kessler A, Venugopal S, Makki I, Qin Q, Dharmapuri S, Jun T, Bhalla S, Berwick S, Christian K, Mascarenhas J, Dembitzer F, Moshier E, Tremblay D. Admission D-dimer levels, D-dimer trends, and outcomes in COVID-19. Thromb Res. 2020;196:99-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 16. | Zhu J, Bouzid R, Travert B, Géri G, Cohen Y, Picod A, Heming N, Rottman M, Joly-Laffargue B, Veyradier A, Capron C, Coppo P. Combined coagulation and inflammation markers as predictors of venous thrombo-embolism and death in COVID-19. Front Med (Lausanne). 2024;11:1399335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 17. | Goldberg MF, Goldberg MF. Correction to: Response to letter to the editor, "Neuroradiologic Manifestations of COVID-19: What the Emergency Radiologist Needs to Know". Emerg Radiol. 2021;28:441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 18. | Jaffe AS, Lindahl B, Giannitsis E, Mueller C, Cullen L, Hammarsten O, Mockel M, Mair J, Krychtiuk KA, Huber K, Mills NL, Thygesen K. ESC Study Group on Cardiac Biomarkers of the Association for Acute CardioVascular Care: A fond farewell at the retirement of CKMB. Eur Heart J. 2021;42:2260-2264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 19. | Susen S, Tacquard CA, Godon A, Mansour A, Garrigue D, Nguyen P, Godier A, Testa S, Levy JH, Albaladejo P, Gruel Y; GIHP and GFHT. Prevention of thrombotic risk in hospitalized patients with COVID-19 and hemostasis monitoring. Crit Care. 2020;24:364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 20. | Herlo A, Marinescu AR, Cut TG, Laza R, Oancea CI, Manolescu D, Hogea E, Porosnicu TM, Sincaru SV, Dumache R, Ispas S, Nelson Twakor A, Nicolae M, Lazureanu VE. Risk Factors for Pulmonary Embolism in Individuals Infected with SARS-CoV2-A Single-Centre Retrospective Study. Biomedicines. 2024;12:774. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Smilowitz NR, Kunichoff D, Garshick M, Shah B, Pillinger M, Hochman JS, Berger JS. C-reactive protein and clinical outcomes in patients with COVID-19. Eur Heart J. 2021;42:2270-2279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 261] [Article Influence: 65.3] [Reference Citation Analysis (0)] |
| 22. | Scudiero F, Silverio A, Di Maio M, Russo V, Citro R, Personeni D, Cafro A, D'Andrea A, Attena E, Pezzullo S, Canonico ME, Galasso G, Pitì A, Parodi G; Cov-IT Network. Pulmonary embolism in COVID-19 patients: prevalence, predictors and clinical outcome. Thromb Res. 2021;198:34-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 23. | Chassagnon G, El Hajjam M, Boussouar S, Revel MP, Khoury R, Ghaye B, Bommart S, Lederlin M, Tran Ba S, De Margerie-Mellon C, Fournier L, Cassagnes L, Ohana M, Jalaber C, Dournes G, Cazeneuve N, Ferretti G, Talabard P, Donciu V, Canniff E, Debray MP, Crutzen B, Charriot J, Rabeau V, Khafagy P, Chocron R, Leonard Lorant I, Metairy L, Ruez-Lantuejoul L, Beaune S, Hausfater P, Truchot J, Khalil A, Penaloza A, Affole T, Brillet PY, Roy C, Pucheux J, Zbili J, Sanchez O, Porcher R; on the behalf of the French Society of Thoracic Imaging. Strategies to safely rule out pulmonary embolism in COVID-19 outpatients: a multicenter retrospective study. Eur Radiol. 2023;33:5540-5548. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, Bikdeli B, Ahluwalia N, Ausiello JC, Wan EY, Freedberg DE, Kirtane AJ, Parikh SA, Maurer MS, Nordvig AS, Accili D, Bathon JM, Mohan S, Bauer KA, Leon MB, Krumholz HM, Uriel N, Mehra MR, Elkind MSV, Stone GW, Schwartz A, Ho DD, Bilezikian JP, Landry DW. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2419] [Cited by in RCA: 2046] [Article Influence: 409.2] [Reference Citation Analysis (2)] |
| 25. | Arachchillage DJ, Rajakaruna I, Odho Z, Makris M, Laffan M; CA-COVID19 Investigators. Impact of thromboprophylaxis on hospital acquired thrombosis following discharge in patients admitted with COVID-19: Multicentre observational study in the UK. Br J Haematol. 2023;202:485-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Kuderer NM, Choueiri TK, Shah DP, Shyr Y, Rubinstein SM, Rivera DR, Shete S, Hsu CY, Desai A, de Lima Lopes G Jr, Grivas P, Painter CA, Peters S, Thompson MA, Bakouny Z, Batist G, Bekaii-Saab T, Bilen MA, Bouganim N, Larroya MB, Castellano D, Del Prete SA, Doroshow DB, Egan PC, Elkrief A, Farmakiotis D, Flora D, Galsky MD, Glover MJ, Griffiths EA, Gulati AP, Gupta S, Hafez N, Halfdanarson TR, Hawley JE, Hsu E, Kasi A, Khaki AR, Lemmon CA, Lewis C, Logan B, Masters T, McKay RR, Mesa RA, Morgans AK, Mulcahy MF, Panagiotou OA, Peddi P, Pennell NA, Reynolds K, Rosen LR, Rosovsky R, Salazar M, Schmidt A, Shah SA, Shaya JA, Steinharter J, Stockerl-Goldstein KE, Subbiah S, Vinh DC, Wehbe FH, Weissmann LB, Wu JT, Wulff-Burchfield E, Xie Z, Yeh A, Yu PP, Zhou AY, Zubiri L, Mishra S, Lyman GH, Rini BI, Warner JL; COVID-19 and Cancer Consortium. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907-1918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1195] [Cited by in RCA: 1281] [Article Influence: 256.2] [Reference Citation Analysis (0)] |