Published online Jun 9, 2024. doi: 10.5492/wjccm.v13.i2.92585
Revised: April 29, 2024
Accepted: May 21, 2024
Published online: June 9, 2024
Processing time: 125 Days and 0.3 Hours
Pulmonary hypertension (PH) is a serious progressive disorder of the modern world, characterized by endothelial dysfunction and impaired vasoreactivity. Patients with PH usually present exercise intolerance from the very early stages and reduced exercise capacity. Exercise training has been shown to have beneficial effects in patients with cardiovascular comorbidities. However, data regarding the effects of combined exercise training programs in patients with PH still remains limited.
To investigate the effects of combined exercise training programs on exercise capacity and quality of life in patients with PH.
Our search included all available randomized controlled trials (RCTs) regarding combined aerobic, resistance and inspiratory training programs in patients with PH in 4 databases (Pubmed, PEDro, Embase, CINAHL) from 2012 to 2022. Five RCTs were included in the final analysis. Functional capacity, assessed by peak VO2 or 6-min walking test (6MWT), as well as quality of life, assessed by the SF-36 questionnaire, were set as the primary outcomes in our study.
Peak VO2 was measured in 4 out of the 5 RCTs while 6MWT was measured in all RCTs. Both indices of functional capacity were significantly increased in patients with PH who underwent combined exercise training compared to the controls in all of the included RCTs (P < 0.05). Quality of life was measured in 4 out of 5 RCTs. Although patients improved their quality of life in each group, however, only 2 RCTs demonstrated further improvement in patients performing combined training compared to controls.
By this systematic review, we have demonstrated that combined aerobic, resistance and inspiratory exercise training is safe and has beneficial effects on aerobic capacity and quality of life in patients with PH. Such exercise training regimen may be part of the therapeutic strategy of the syndrome.
Core Tip: Pulmonary hypertension (PH) is characterized by endothelial dysfunction and impaired vasoreactivity. Data regarding the effects of combined exercise training programs in patients with PH still remains limited. The effects of combined exercise training programs on exercise capacity and quality of life in patients with PH. We observed that combined exercise training has beneficial effects on functional capacity, assessed by peak VO2 or 6-min walking test, and quality of life in PH. However, further research is required in order to create the maximum beneficial individualized exercise training protocols.
- Citation: Kourek C, Zachariou A, Karatzanos E, Antonopoulos M, Soulele T, Karabinis A, Nanas S, Dimopoulos S. Effects of combined aerobic, resistance and inspiratory training in patients with pulmonary hypertension: A systematic review. World J Crit Care Med 2024; 13(2): 92585
- URL: https://www.wjgnet.com/2220-3141/full/v13/i2/92585.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v13.i2.92585
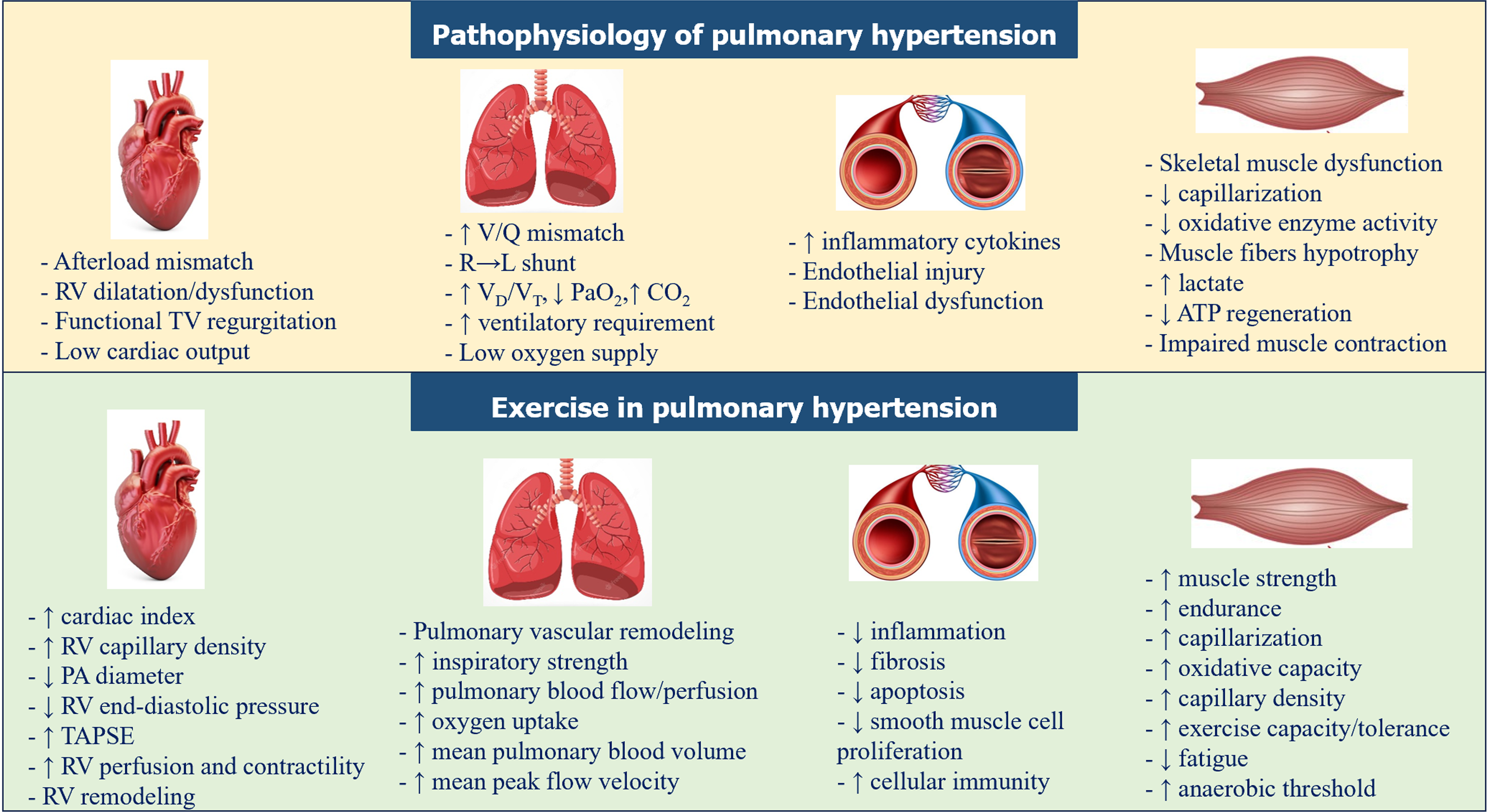
Pulmonary hypertension (PH) is a serious progressive clinical syndrome of the modern world defined as mean pulmonary arterial pressure (PAP) measured by right heart catheterization ≥ 20 mmHg at rest[1]. It has a tremendous social and economic burden in national healthcare systems and a rapid increase in the annual incidence and prevalence during the last years[2]. Prognosis of PH remains low as mortality in these patients is approximately 13.0%, 36.4%, and 62.4%, at 30 d, 1 year, and 5 years, respectively[2]. Pulmonary arterial hypertension (PAH) is the most frequent subgroup of patients with PH. PAH’s prevalence is estimated to be between 15 to 50 persons per million within the United States and Europe[3], with a median survival of 2.8 years[4]. The mean age of PAH patients in the Western world ranges from 45-65 years[2,5]. Although female gender is a risk factor for PH, yet females have higher survival rates than males[2-4,6].
Endothelial cell injury along with impaired vascular regeneration, abnormal vascular remodeling and impaired vasoreactivity are the most characteristic potential mechanisms in the pathogenesis of PH[7-9]. Patients with PH usually present exercise intolerance from the very early stages and reduced exercise capacity, while some patients experience these symptoms for more than 2 years before the establishment of the diagnosis[5]. The impact of PH on functional capacity combined with the psychosocial state adversely affect quality of life in these patients[10]. Specifically, 48% of patients experience mild to extremely severe symptoms of anxiety, 32.6% symptoms of depression, and 27.6% symptoms of stress[11].
Exercise training has been shown to have beneficial effects in patients with cardiovascular comorbidities including heart failure[12], diabetes mellitus[13], arterial hypertension[14] and ischemic disease[15]. Members of the European Respiratory Society tried to develop strategies and increase awareness of exercise training for PH patients[16]. Moreover, European Society of Cardiology guidelines recommend supervised exercise training in patients with PAH under medical therapy[1]. However, data regarding the effects of combined exercise training programs in these patients still remains limited. We hypothesized that a combined exercise training program is safe and improves vascular regeneration, homeostasis, and vasodilation, leading thus, in improvement of the ability of these patients to increase their tolerance and improve their daily quality of life.
The aim of this systematic review was to evaluate the effects of structured combined aerobic, muscle strengthening and respiratory exercise training programs on exercise capacity and quality of life in patients with PH, as well as to assess the safety of combined exercise training programs.
The search was conducted from 2021 until January of 2022 in order to identify 10-year period (from 2012 to 2022) published studies in Pubmed, PEDro, Embase and CINAHL databases that included the most updated data regarding community or outpatient exercise training programs in patients with PH. Terms that were used in our search were (“pulmonary hypertension” OR “pulmonary arterial hypertension” OR “PAH” OR “CTEPH”) AND (“rehabilitation” OR “exercise” OR “training” OR “aerobic exercise”). The initial search results were first screened using only the title and the abstract and then, the full text of the articles was reviewed for eligibility by 2 independent investigators (C.K. and A.Z.). Manual searching of references of all eligible studies was also performed, so that to include any randomized trials that may not have been identified in the original search. The final evaluation of the process, as well as the final decision about the inclusion of a study in case of disagreement between the two investigators, was performed by a third independent investigator (S.D.). PICO approach (patient/population, intervention, comparison and outcomes) was used to define the primary research question of our study. We registered our study at Open Science Framework (https://doi.org/10.17605/OSF.IO/KJD9P).
We included studies that met all the defined inclusion criteria in the beginning of our search. These criteria were: (1) Studies available as full texts in English; (2) published randomized controlled trials (RCTs) in peer-reviewed journals; (3) study groups including patients with specific types of PH (PAH and chronic thromboembolic pulmonary hypertension) and stable medication during the last 2 months; (4) aged ≥ 18 years; (5) combined exercise training programs using aerobic, resistance and respiratory training with duration of ≥ 2 wk; and (6) outcome measures focused on either QOL using SF-36 questionnaire and/or exercise capacity through peak oxygen uptake (peak VO2) or 6-min walking test (6MWT) distance.
Studies with: (1) Additional interventions in study groups except for combined exercise training; (2) control groups including patients not diagnosed with PH; and (3) exercise modalities that were unable to be quantified, were excluded.
Quality of each RCT included in the systematic review was assessed for methodological rigor and risk of bias by 2 reviewers (C.K. and A.Z.) using the Physiotherapy Evidence Database (PEDro). PEDro is an 11-point scale for assessing RCTs for internal validity and control of bias, but with a maximum score of 10 as 1 question does not contribute to total score, where a study with a score of 6-10 is considered as an excellent quality RCT, a study with 4-5 as fair quality, and with a score of 3 or less as a poor-quality study[17]. If the 2 reviewers did not agree for their quality score, then an independent third reviewer (S.D.) made the final decision. PEDro scale is a widely used assessment tool demonstrating a good agreement for most risk of bias items with other tools that evaluate similar constructs[18].
Primary outcome measures were functional capacity indices, including VO2 peak assessed by cardiopulmonary exercise testing (CPET) and 6MWT distance, and quality of life through the 36-Item Short Form Survey (SF-36) questionnaire[19]. The SF-36 questionnaire is an often used, well-researched, self-reported measure of health consisting of 36 questions divided into 8 different aspects of life regarding general health, limitations of activities, physical health problems, emotional health problems, social activities, pain, energy and emotions, and social activities. Secondary outcome measure was safety of combined exercise training programs in PH patients, assessed by major adverse effects such as severe arrythmias, acute coronary syndrome and ischemic stroke.
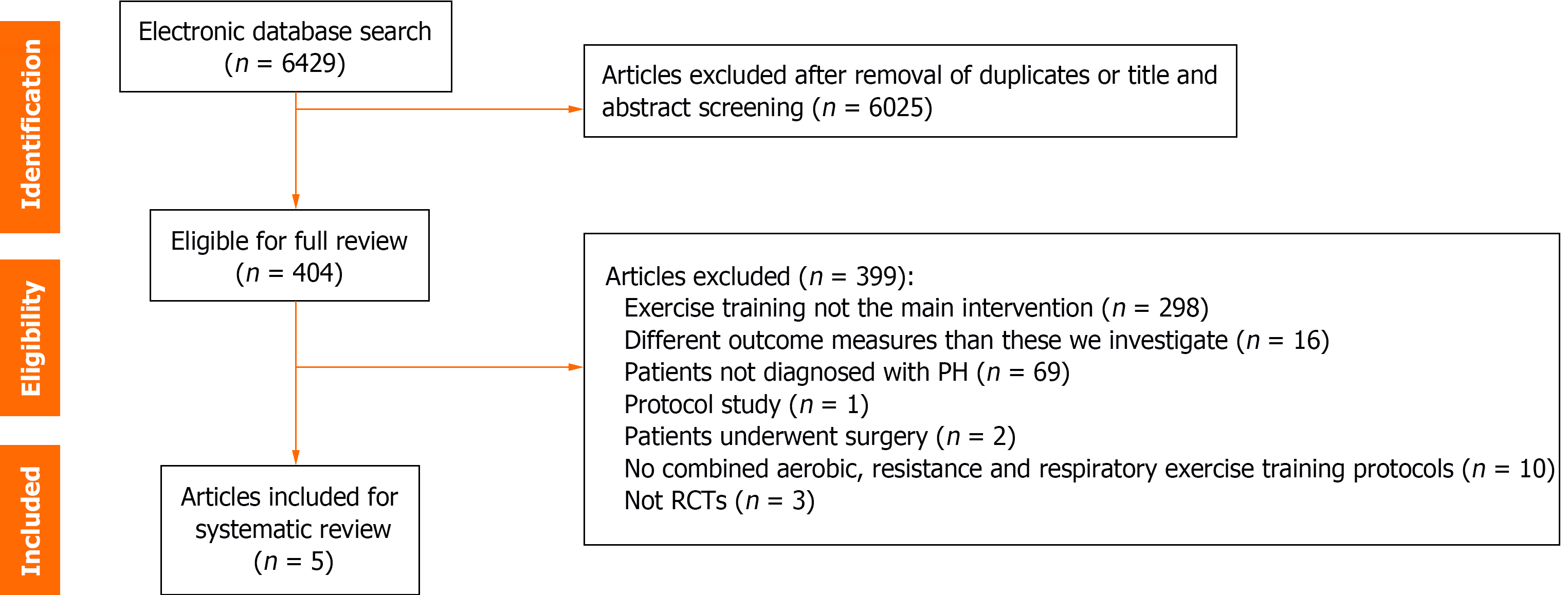
PRISMA flowchart was used to describe the search and screening results (Figure 1). The initial search strategy identified 6429 articles from Pubmed, PEDro, Embase and CINAHL databases. Among these articles, only five met the inclusion criteria and were finally included in our systematic review[20-24].
Table 1 shows the details of the study samples of the included RCTs. The five studies resulted in 288 participants in total, 148 of them belonging to the intervention group and the rest 140 to the control group. The mean age of the participants ranged from 46 to 56 years, while the majority of PH patients were females (190 vs 98 males). The main reason of PH was PAH in most studies, either idiopathic or heritable, and chronic thromboembolic PH was the second one. Mean PAP, mean pulmonary vascular resistance and mean cardiac index of intervention and control group in each study are described in Table 1.
| Ref. | Sample size (n) | Sex (Male/Femail) | Mean age (yr) | Type of PH, n (%) | Mean PAP (mmHg) | Mean PVR (dyn × s /cm5) | Cardiac index (L/min/m2) | Peak VO2 (mL/min/kg) | 6MWT (m) |
| Mereles et al[20] | I: 15 | 5/10 | 47 ± 12 | PAH: 13 (86.6); CT: 2 (13.3) | 49.5 ± 17.6 | 968.7 ± 444.1 | 2.3 ± 0.5 | 13.2 ± 3.1 | 411 ± 86 |
| C: 15 | 5/10 | 53 ± 14 | PAH: 11 (73.3); CT: 4 (26.7) | 49.6 ± 12.3 | 901.8 ± 358.0 | 2.1 ± 0.5 | 11.9 ± 3.1 | 439 ± 82 | |
| Ley et al[21] | I: 10 | 2/8 | 47 ± 8 | PAH: 7 (70); CT: 1 (10); Other1: 2 (20) | 48 ± 19 | 731 ± 256 | 2.78 ± 0.57 | NA | 449 ± 80 |
| C: 10 | 4/6 | 54 ± 14 | PAH: 5 (50); CT: 3 (30); Other1: 2 (20) | 50 ± 15 | 909 ± 374 | 2.2 ± 0.51 | NA | 423 ± 101 | |
| Ehlken et al[22] | I: 46 | 20/26 | 55 + 15 | PAH: 35 (76.1); CT: 11 (23.9) | 41.0 + 11.7 | 540 + 267 | 2.68 + 0.73 | 13.3 ± 3.6 | 453 ± 91 |
| C: 41 | 20/21 | 57 + 15 | PAH: 26 (63.4); CT: 15 (36.5) | 37.6 + 11.8 | 512 + 338 | 2.69 + 0.89 | 12.7 ± 4.0 | 413 ± 95 | |
| González-Saiz et al[23] | I: 20 | 8/12 | 46 ± 11 | PAH: 7 (35); CT: 2 (10); Other2: 11 (55) | 47 ± 15 | 11 ± 6 (WU) | Cardiac output: 4414 ± 91 (ml/min) | 15.7 ± 3.3 | 500 ± 70 |
| C: 20 | 8/12 | 45 ± 12 | PAH: 11 (55); CT: 2 (10); Other2: 7 (35) | 47 ± 14 | 9 ± 5 (WU) | Cardiac output: 4827 ± 135 (ml/min) | 19.8 ± 6.5 | 546 ± 99 | |
| Grünig et al[24] | I: 58 | 18/40 | 52.3 ± 12.4 | PAH: 39 (67.2); CT: 7 (12.1); Other3: 12 (20.7) | 46.5 ± 15.5 | 8.6 ± 5.5 (WU) | 2.7 ± 0.7 | 14.2 ± 5.2 | 447.2 ± 117.7 |
| C: 58 | 13/45 | 55.0 ± 12.7 | PAH: 34 (58.6); CT: 11 (19.0); Other3: 13 (22.4) | 46.7 ± 14.9 | 7.7 ± 4.5 (WU) | 2.8 ± 0.7 | 15.3 ± 4.3 | 447.4 ± 120.0 |
A comprehensive analysis of intervention characteristics, outcomes and main results of the studies included in the systematic review are demonstrated in details in Table 2. Exercise training protocols in all studies included combined aerobic, resistance and inspiratory training with differences in intensity, sets and duration among studies.
| Ref. | Intervention by study group | Duration | Outcomes | Main results |
| Mereles et al[20] | I: First 3 wk: (1) Interval bicycle ergometer training with a lower workload (10-60 W) for 1⁄2 min and a higher workload for 1 min (20 to 35 W) for 10 to 25 min/d, corresponding to 60% to 80% of the heart rate and 60 min of walking was performed 5 d/w (flat-ground and uphill walking); (2) 30 min of dumbbell training of single muscle groups with low weights (500 to 1000 g) 5d/w; and (3) 30 min of respiratory training, including stretching, breathing techniques such as pursed lip breathing, body perception, Yoga, and strengthening of respiratory muscles | 15 wk (7 d per week for 3 wk in-hospital training and 5 d per week for 12 wk training at home) | Primary (after 3w and 15w): 6MWT, SF-36. Secondary (after 3w and 15w): WHO functional class, Borg scale, echocardiographic indices, CPET indices (peak VO2, VO2@AT, peak workload, etc.) | Intervention group: ↑ in 6MWT and ↓ in SF-36 after 15 wk. Also, ↑ in max workload, max heart rate, peak VO2, predicted peak VO2, workload and VO2@AT after 15 wk. Control group: ↓ in 6MWT and SF-36 after 15 wk. ↑ in Borg scale after 15 wk. Intervention vs control group: Intervention group improved 6MWT and SF-36, as well as max workload, max heart rate, peak VO2, workload and VO2@AT and PASP at rest compared to controls after 15 wk. Improvements within each group and between groups in the same indices after 3 wk |
| 12 wk: (1) Bicycle exercise training close to their target heart rate once daily for a total of 15 to 30 min for 5 d/w; (2) respiratory exercise; and (3) dumbbell training for 15 to 30 min every other day, iv. walking 2 d/w | ||||
| C: Common rehabilitation program based on healthy nutrition, physical therapy such as massages, inhalation, counseling, and muscular relaxation without exercise and respiratory training. Allowed to perform daily activity as usual | ||||
| Ley et al[21] | I: Specialized respiratory and exercise training program at home similar to Mereles et al[18] | 3 wk | After 3 wk: 6MWD, MR perfusion (time to peak, pulmonary blood flow, pulmonary blood volume and mean transit time) and flow measurements (peak velocity, time to peak velocity, mean velocity and average blood flow per minute) | Intervention group: ↑ in 6MWT, pulmonary blood volume and ↓ in peak velocity after 3 wk. Control group: ↑ in mean transit time after 3 wk. Intervention vs control group: Intervention group improved 6MWT and peak velocity compared to controls after 3 wk |
| C: Program without specific exercise training | ||||
| Ehlken et al[22] | I: Similar to Mereles et al[18] | 15 wk (7 d per week for 3 wk in-hospital training and 5 d per week for 12 wk training at home) | Primary (after 15 wk): Peak VO2. Secondary (after 15 wk): Hemodynamics at rest and during CPET (oxygen pulse, heart rate at rest and max, systolic and diastolic BP at rest and max, max workload), RHC indices (mPAP, CO, CI, PAWP, PVR), 6MWT, SF-36, WHO functional class and NT-proBNP | Intervention group: ↑ in peak VO2 and 6MWT after 15 wk. Also, improvement in most hemodynamics measurements and RHC indices at rest and during exercise after 15 wk. Control group: No change in peak VO2 and 6MWT after 15 wk. Also, most hemodynamics measurements and RHC indices at rest and during exercise remained unchanged after 15 wk. Intervention vs control group: Intervention group improved peak VO2, 6MWT, hemodynamics measurements at rest and during exercise compared to controls after 15 wk. Quality of life (improvement in most aspects of SF-36) improved in all patients compared to the general population |
| 3 wk in-hospital: 1.5 h/d exercise training consisting of: (1) Interval cycle ergometer training at low workloads 7 d/w; (2) walking; (3) dumbbell training of single muscle groups using low weights (500–1000 g); and (4) respiratory training 5 d/w | ||||
| 12 wk at home: 15 min/d for 5 d/w, mental training | ||||
| C: Did not receive any advice on exercise training. Psychological support was offered to all participants | ||||
| González-Saiz et al[23] | I: Aerobic training→ cycle ergometer training for 20-40 min/session for 5 d/w with a gradually increased duration/intensity in each session, with exercise-rest intervals at a 1:1 ratio and at 50% of the power output eliciting the AT. Resistance training→ 3-time circuit of exercises involving large muscle groups in the following order: Leg and bench press, leg extension, lateral pulldown and abdominal crunches, following aerobic sessions 3 times per week. Inspiratory training→ 30 inspirations through a specific pressure-load device against 40% of PImax, total session duration approximately 5 min, 2 times daily for 6 sessions/w | 8 wk [Aerobic: 5 sessions per week from Monday to Friday, 40 sessions in total, 20–40 min each session duration; Resistance: 3 sessions per week (Monday, Wednesday and Friday), 24 sessions in total, following the aerobic sessions; Inspiratory: 6 sessions per week from Monday to Saturday, 2 times daily] | Primary (after 8 wk): Upper/lower body muscle power (leg press, bench press). Secondary (after 8 wk): NT-proBNP, 6MWT, peak VO2, VE/VO2@AT, PETO2@AT, PETCO2@AT, VE/VCO2@AT, SF-36, adverse episodes (syncopal/pre-syncopal episodes, severe dyspnea, arrhythmias, asthma, signs of poor peripheral perfusion, ataxia, tremors), muscle mass | Intervention group: ↑ in leg press and bench press after 8 wk. Also, improve |
| C: Standard care, regularly scheduled visits with their clinicians | ||||
| Grünig et al[24] | I: Respiratory therapy, cycle ergometer training, dumbbell training, guided walks, and mental training 5-7 d per week for 10-30 d in-house training and 3-7 d per week for 11-12 wk training at home, similar to Mereles et al[18] and Ehlken et al[20] protocols. Training intensity was 40%–60% of the patients’ achieved max workload during ergometer test | 15 wk (5-7 d per week for 10-30 d in-house training and 3-7 d per week for 11-12 wk training at home) | Primary (after 15 wk): 6MWT. Secondary (after 15 wk): Peak VO2, WHO functional class, NT-proBNP, CPET indices (HR at rest, SaO2 at rest, peak HR, peak SaO2, peak VO2, predicted peak VO2, predicted workload, VE/VCO2 slope), echocardiographic indices (sPAP, TAPSE, RA area, RV area), SF-36, adverse events | Intervention group: ↑ 6MWT after 8 wk. Also, improvement in peak VO2, predicted peak VO2, and most aspects of SF-36 after 15 wk. Control group: Slight but not statistically significant ↓ in 6MWT after 15 wk. Also, improvement in sPAP and some aspects of SF-36 after 15 wk. Intervention vs control group: Intervention group improved 6MWT, SF-36, WHO functional class, peak VO2, sPAP, compared to controls after 15 wk. No difference in other parameters were observed |
| C: Usual daily activity at home |
PEDro scores for the included RCTs ranged from 5 to 7, with 3 out of 5 studies being scored with 6 points, 1 study with 5 points and 1 study with 7 points (Table 3). The weakest field of scoring was blindness of therapists and participants. According to the PEDro scale, all RCTs were assessed as high-quality studies.
| Mereles et al[20], 2006 | Ley et al[21], 2013 | Ehlken et al[22], 2016 | González-Saiz et al[23], 2017 | Grünig et al[24], 2021 | |
| Eligibility Criteria | √ | √ | √ | ||
| Random allocation | √ | √ | √ | √ | √ |
| Concealed allocation | √ | ||||
| Baseline comparability | √ | √ | √ | √ | √ |
| Blinded subjects | |||||
| Blinded therapists | |||||
| Blinded assessors | √ | √ | √ | √ | |
| Adequate follow-up | √ | √ | √ | √ | |
| Intention-to-treat | √ | ||||
| Between-group-analysis | √ | √ | √ | √ | √ |
| Point estimates and variability | √ | √ | √ | √ | √ |
| Total score | 6/10 | 5/10 | 6/10 | 7/10 | 6/10 |
The effects of combined aerobic, resistance and respiratory exercise training on functional capacity were assessed by peak VO2 and 6MWT (Table 2).
Peak VO2 was measured in 4 out of the 5 included RCTs[20,22-24] and it was set as a primary endpoint in one single study[22], while in the other studies as a secondary endpoint[20,23,24]. In all of these studies, peak VO2 was significantly increased in PH patients who underwent the combined compared to the controls. Specifically, peak VO2 was found to significantly improve in the training group compared to the control group in the study of Ehlken et al[22] [training group (TG): +3.1 + 2.7 mL/min/kg (mean increase relative to baseline +24.3%) vs control group (CG): -0.2 + 2.3 mL/min/kg (mean increase relative to baseline +0.9%), P < 0.001], in the study of Mereles et al[20] (after 3 wk TG: from 13.2 ± 3.1 to 14.5 ± 3.5 ml/kg/min vs CG: From 11.9 ± 3.1 to 11.6 ± 3.4 mL/kg/min, P < 0.05; after 15 wk TG: From 13.2 ± 3.1 to 15.4 ± 3.7 mL/kg/min vs CG: From 11.4 ± 3.3 mL/kg/min after 15 wk, P < 0.05), in the study of González-Saiz et al[23] (TG: From 15.7 ± 3.3 to 18.3 ± 3.2 mL/kg/min vs CG: From 19.8 ± 6.5 to 19.4 ± 6.8 mL/kg/min, P < 0.001) and in the study of Grünig et al24] study (TG: Improvement equals 8.5 ± 17.6% vs CG: 1.0 ± 13.8%, P = 0.015).
On the other hand, 6MWT was measured as a primary endpoint in 3 out of 5 RCTs[20,21,24], and as a secondary endpoint in the other studies[22,23]. In 4 out of these 5 studies[20-22,24], 6MWT was significantly increased in the intervention group compared to the controls. Specifically, a statistically significant increase in the 6MWT in the training compared to the control group was demonstrated in Ehlken’s et al study[22] (TG: increase of 29 + 53 m vs CG: Decrease of 212 + 46 m, P = 0.001), in Mereles’s et al study[20] (after 3 wk TG: Increase of 85 ± 56 m vs CG: Increase of 12 ± 37 m, P = 0.0003; after 15 wk TG: Increase of +96 ± 61 m vs CG: Decrease of 15 ± 54 m, P < 0.0001), in Ley’s et al study[21] [TG: From 449 ± 80 to 540 ± 68 m (mean change: +91.4 ± 66.2 m) vs CG: From 423 ± 101 to 440 ± 104 m (mean change:16.9 ± 39.8 m), P = 0.004] and in Grünig’s et al study[24] (TG: Increase of 30.7 ± 57.9 m vs CG: Decrease of 3.4 ± 25.9 m, P < 0.0001). On the other hand, González-Saiz et al[23], showed no significant difference between the 2 groups but only a trend towards a training-induced improvement (P = 0.015 for the interaction effect).
Other CPET and echocardiographic indices were also investigated in these studies. Most parameters of functional capacity were improved in patients who underwent exercise training compared to those who received the usual care.
Quality of life was assessed through the SF-36 questionnaire in 4 out of the 5 included RCTs[20,22-24]. Although patients improved their quality of life in each group, however, only 2 RCTs demonstrated further improvement in patients performing combined training compared to controls[20,24]. Specifically, Mereles et al[20] a further improvement in 7 scales of the SF-36 [physical component scale (P = 0.013), mental component scale (P = 0.027), physical functioning (P = 0.018), role limitations due to physical limitation (P = 0.003), social functioning (P = 0.002), mental health (P = 0.017), and vitality (P = 0.001)] in the training group compared to the controls while Grünig et al[24] demonstrated a statistically significant improvement in mental health (P = 0.004) and a trend for physical functioning (P = 0.07) and social functioning (P = 0.09) in the training group over the control group.
As far as safety of exercise training in patients with PH is concerned, in most studies, no significant adverse effects occurred during exercise. In the study of Mereles et al[20] there were no adverse effects although patients had severe PH. González-Saiz et al[23], noticed only 1 episode of atrioventricular nodal reentrant tachycardia during CPET in one patient and dizziness, without syncope, during an aerobic training session in another patient, due to hypoglycemia. Other frequent adverse effects in Grünig et al[24] study were arrhythmias and respiratory infections, as well as more serious including decompensated diabetes. However, none of them were evaluated to be related to exercise training[24].
The present study is a systematic review investigating the effect of combined aerobic, resistance and respiratory exercise training protocols on functional capacity and quality of life in patients with PH. By the findings of the present systematic review emerged that combined exercise training is safe and has beneficial effects on exercise capacity and quality of life in patients with stable PH. More specifically, a significant improvement in peak VO2 and 6MWT, both independent, powerful prognostic indices[25-28] and in quality of life (through the SF-36 questionnaire) was demonstrated in PH.
The 6MWT has been used as the primary end point in most randomized clinical trials involving patients with PH[20,21,24]. Physical activity, and especially the distance of 6MWT, is a strong and independent predictor of prognosis and highly correlated with daily activity and haemodynamics in PH[28,29]. The threshold of 6MWT improvement for clinical importance is set at 33 m in PH[30]. In some cases[20,24], exercise training-related increase in walking distance may be higher than the use of medical therapy alone, including sildenafil[31], intravenous epoprostenol[32], inhaled iloprost[33], and oral bosentan[34]. Both 6MWT and peak VO2 are significant indices which assess functional capacity of patients with cardiopulmonary diseases, providing global analysis of the respiratory, cardiac, and metabolic system[35,36]. Peak VO2 is considered the best available index for assessment of exercise capacity[37] and is also a strong predictor of outcomes in PH[38-40]. Studies have shown that a peak VO2 cutoff < 12.5 mL/kg/min identifies patients at high risk, whereas values > 12.5 mL/kg/min categorize patients with a better midterm prognosis[1,41], but future studies are required in order to establish the best cut-off points for this variable. In addition, peak VO2 independently predicts prognosis and has predictive capacity regarding mortality and the risk of transplantation in patients with PH[42]. Specifically, PH is associated with hyperventilation at rest and at exercise, an increase in physiologic dead space, dynamic arterial O2 desaturation, as well as reduced maximal cardiac output due to right ventricular dysfunction[43]. As a result, low peak VO2 refers to poor prognosis in PH patients[25]. Improvement in 6MWT after exercise is also shown to be supported by significant improvement of other parameters such as WHO functional class and peak VO2[24]. As a result, a combination between 6MWT and indices such as peak VO2 could be interpreted for risk stratification models in PH and quality of life[1,24,44].
In this systematic review, most studies showed that combined exercise training program significantly improves quality of life, as measured by the SF-36 questionnaire, in PH compared to controls[20,24]. Only 1 study showed that there was no difference in SF-36 scores although the physical component of SF-36 improved within the training group[23]. Patients with PH usually present poor quality of life, mainly in the physical functioning domain[45]. In addition, due to functional limitations, dyspnea, adverse effects of therapy and social isolation, they tend to suffer from neurological disorders such as depression, anxiety, stress, or sleep disorders, which are all significantly correlated with poorer quality of life[45]. 6MWT is also correlated with quality of life[11,46]. Baseline psychological assessment before the initiation of exercise training and timely referral for mental health services are suggested[47]. Moreover, social and psychological support during the participation in an exercise training program may further benefit patients with PH and prevent stress and anxiety[47].
Patients with PH present substantial impairments of peripheral muscle microcirculation, decreased tissue O2 saturation, slower reactive hyperemia time due to endothelium dysfunction, and peripheral systemic vasoconstriction, as assessed by dynamic near-infrared spectroscopy[48,49]. Acute hyperoxic breathing improves resting tissue O2 saturation and decreases the oxygen consumption rate and reactive hyperemia time during reperfusion, possibly due to increased oxidative stress and evoked vasoconstriction[48]. The precise mechanisms of exercise training beneficial effects in patients with PH still remain unclear. Possible hypotheses might be the improvement of muscular gas exchange, ventilatory efficiency and the reversal of skeletal muscle atrophy and endothelial dysfunction as in other chronic cardiovascular diseases[50-55]. Indeed, endurance exercise training causes a significant increase in skeletal muscle capillarization, characterized by an elevated capillary density and capillary-to-fibre ratio[56,57]. This mechanism could explain the improve
Combined exercise training programs proved to be safe in patients with PH as no significant adverse effects occurred during exercise. An important condition of safety is that patients should be stable for at least 2 months before the initiation of exercise training, and for severe PH, advanced medical therapy should be administered.
Patients with severe chronic PH usually present restricted physical capacity, impaired quality of life, and poor prognosis because of the syndrome. The present systematic review is the first to evaluate the effects of combined aerobic, resistance and respiratory exercise training programs on prognostic functional capacity indices such as peak VO2 and 6MWT, as well as quality-of-life-assessment in stable patients with PH under medical therapy. Other studies should also assess the potential beneficial effects of other exercise regimens such as interval exercise training and/or neuromuscular stimulation in these patients. It still remains unknown, whether home derived rehabilitation or telerehabilitation are safe and efficient. More studies are required to evaluate the effects of exercise training (type, duration, dose, intensity) for its PH severity stage. For safety reasons, initial screening assessment and exercise training should be performed in hospital in patients with severe PH and then, in an outpatient setting under close monitoring either at home or rehabilitation center. Due to the disease severity a multidisciplinary team approach is necessary prior to participation at exercise training programs. Larger multicenter RCTs are required in order to better understand the potential mechanisms of exercise in PH and its therapeutic targets and confirm its beneficial outcome.
Some limitations exist in our systematic review. Firstly, the number of RCTs included in our review is limited to only five studies; however, results were consistent in all studies supporting final conclusions. Moreover, study samples of the included studies may present heterogeneity in some point, due to different mean age, different functional capacity at baseline and probably different percentages of each PH type within the groups. Some studies had an exploratory analysis and no adjustment for multiple comparisons was performed. A meta-analysis, additionally to the systematic review, would possibly increase the quality of the manuscript and help to extract safer conclusions. We did not perform a meta-analysis as we did not have access to data of all the included RCTs. Finally, we could not exclude a potential inclusion bias whereby patients who undertook an exercise intervention may have been more motivated than controls since all studies were not blinded.
By this systematic review we have demonstrated that combined aerobic, resistance and inspiratory exercise training is safe and seems to have beneficial effects on aerobic capacity and quality of life in patients with PH. Such exercise training regimen may be part of the therapeutic strategy of the syndrome.
| 1. | Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, Carlsen J, Coats AJS, Escribano-Subias P, Ferrari P, Ferreira DS, Ghofrani HA, Giannakoulas G, Kiely DG, Mayer E, Meszaros G, Nagavci B, Olsson KM, Pepke-Zaba J, Quint JK, Rådegran G, Simonneau G, Sitbon O, Tonia T, Toshner M, Vachiery JL, Vonk Noordegraaf A, Delcroix M, Rosenkranz S; ESC/ERS Scientific Document Group. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43:3618-3731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 1868] [Article Influence: 622.7] [Reference Citation Analysis (35)] |
| 2. | Wijeratne DT, Lajkosz K, Brogly SB, Lougheed MD, Jiang L, Housin A, Barber D, Johnson A, Doliszny KM, Archer SL. Increasing Incidence and Prevalence of World Health Organization Groups 1 to 4 Pulmonary Hypertension: A Population-Based Cohort Study in Ontario, Canada. Circ Cardiovasc Qual Outcomes. 2018;11:e003973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 195] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 3. | Beshay S, Sahay S, Humbert M. Evaluation and management of pulmonary arterial hypertension. Respir Med. 2020;171:106099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 4. | Prins KW, Thenappan T. World Health Organization Group I Pulmonary Hypertension: Epidemiology and Pathophysiology. Cardiol Clin. 2016;34:363-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 120] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 5. | Levine DJ. Pulmonary arterial hypertension: updates in epidemiology and evaluation of patients. Am J Manag Care. 2021;27:S35-S41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 6. | Leber L, Beaudet A, Muller A. Epidemiology of pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: identification of the most accurate estimates from a systematic literature review. Pulm Circ. 2021;11:2045894020977300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 128] [Article Influence: 32.0] [Reference Citation Analysis (35)] |
| 7. | Bourgeois A, Omura J, Habbout K, Bonnet S, Boucherat O. Pulmonary arterial hypertension: New pathophysiological insights and emerging therapeutic targets. Int J Biochem Cell Biol. 2018;104:9-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Ranchoux B, Harvey LD, Ayon RJ, Babicheva A, Bonnet S, Chan SY, Yuan JX, Perez VJ. Endothelial dysfunction in pulmonary arterial hypertension: an evolving landscape (2017 Grover Conference Series). Pulm Circ. 2018;8:2045893217752912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 105] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 9. | Yuan JX, Rubin LJ. Pathogenesis of pulmonary arterial hypertension: the need for multiple hits. Circulation. 2005;111:534-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 133] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 10. | Delcroix M, Howard L. Pulmonary arterial hypertension: the burden of disease and impact on quality of life. Eur Respir Rev. 2015;24:621-629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 136] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 11. | M M Vanhoof J, Delcroix M, Vandevelde E, Denhaerynck K, Wuyts W, Belge C, Dobbels F. Emotional symptoms and quality of life in patients with pulmonary arterial hypertension. J Heart Lung Transplant. 2014;33:800-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Kourek C, Alshamari M, Mitsiou G, Psarra K, Delis D, Linardatou V, Pittaras T, Ntalianis A, Papadopoulos C, Panagopoulou N, Vasileiadis I, Nanas S, Karatzanos E. The acute and long-term effects of a cardiac rehabilitation program on endothelial progenitor cells in chronic heart failure patients: Comparing two different exercise training protocols. Int J Cardiol Heart Vasc. 2021;32:100702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Reddy R, Wittenberg A, Castle JR, El Youssef J, Winters-Stone K, Gillingham M, Jacobs PG. Effect of Aerobic and Resistance Exercise on Glycemic Control in Adults With Type 1 Diabetes. Can J Diabetes. 2019;43:406-414.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 14. | Lopes S, Mesquita-Bastos J, Garcia C, Bertoquini S, Ribau V, Teixeira M, Ribeiro IP, Melo JB, Oliveira J, Figueiredo D, Guimarães GV, Pescatello LS, Polonia J, Alves AJ, Ribeiro F. Effect of Exercise Training on Ambulatory Blood Pressure Among Patients With Resistant Hypertension: A Randomized Clinical Trial. JAMA Cardiol. 2021;6:1317-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 15. | Taylor JL, Holland DJ, Keating SE, Leveritt MD, Gomersall SR, Rowlands AV, Bailey TG, Coombes JS. Short-term and Long-term Feasibility, Safety, and Efficacy of High-Intensity Interval Training in Cardiac Rehabilitation: The FITR Heart Study Randomized Clinical Trial. JAMA Cardiol. 2020;5:1382-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 16. | Grünig E, Eichstaedt C, Barberà JA, Benjamin N, Blanco I, Bossone E, Cittadini A, Coghlan G, Corris P, D'Alto M, D'Andrea A, Delcroix M, de Man F, Gaine S, Ghio S, Gibbs S, Gumbiene L, Howard LS, Johnson M, Jurevičienė E, Kiely DG, Kovacs G, MacKenzie A, Marra AM, McCaffrey N, McCaughey P, Naeije R, Olschewski H, Pepke-Zaba J, Reis A, Santos M, Saxer S, Tulloh RM, Ulrich S, Vonk Noordegraaf A, Peacock AJ. ERS statement on exercise training and rehabilitation in patients with severe chronic pulmonary hypertension. Eur Respir J. 2019;53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 112] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 17. | Recovery CPfS. PEDro score. 2017. Accessed August 1, 2022. Available from: https://www.strokengine.ca/glossary/pedro-score/. |
| 18. | Moseley AM, Rahman P, Wells GA, Zadro JR, Sherrington C, Toupin-April K, Brosseau L. Agreement between the Cochrane risk of bias tool and Physiotherapy Evidence Database (PEDro) scale: A meta-epidemiological study of randomized controlled trials of physical therapy interventions. PLoS One. 2019;14:e0222770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 109] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 19. | Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473-483. [PubMed] |
| 20. | Mereles D, Ehlken N, Kreuscher S, Ghofrani S, Hoeper MM, Halank M, Meyer FJ, Karger G, Buss J, Juenger J, Holzapfel N, Opitz C, Winkler J, Herth FF, Wilkens H, Katus HA, Olschewski H, Grünig E. Exercise and respiratory training improve exercise capacity and quality of life in patients with severe chronic pulmonary hypertension. Circulation. 2006;114:1482-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 406] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 21. | Ley S, Fink C, Risse F, Ehlken N, Fischer C, Ley-Zaporozhan J, Kauczor HU, Klose H, Gruenig E. Magnetic resonance imaging to assess the effect of exercise training on pulmonary perfusion and blood flow in patients with pulmonary hypertension. Eur Radiol. 2013;23:324-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 22. | Ehlken N, Lichtblau M, Klose H, Weidenhammer J, Fischer C, Nechwatal R, Uiker S, Halank M, Olsson K, Seeger W, Gall H, Rosenkranz S, Wilkens H, Mertens D, Seyfarth HJ, Opitz C, Ulrich S, Egenlauf B, Grünig E. Exercise training improves peak oxygen consumption and haemodynamics in patients with severe pulmonary arterial hypertension and inoperable chronic thrombo-embolic pulmonary hypertension: a prospective, randomized, controlled trial. Eur Heart J. 2016;37:35-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 167] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 23. | González-Saiz L, Fiuza-Luces C, Sanchis-Gomar F, Santos-Lozano A, Quezada-Loaiza CA, Flox-Camacho A, Munguía-Izquierdo D, Ara I, Santalla A, Morán M, Sanz-Ayan P, Escribano-Subías P, Lucia A. Benefits of skeletal-muscle exercise training in pulmonary arterial hypertension: The WHOLEi+12 trial. Int J Cardiol. 2017;231:277-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 24. | Grünig E, MacKenzie A, Peacock AJ, Eichstaedt CA, Benjamin N, Nechwatal R, Ulrich S, Saxer S, Bussotti M, Sommaruga M, Ghio S, Gumbiene L, Palevičiūtė E, Jurevičienė E, Cittadini A, Stanziola AA, Marra AM, Kovacs G, Olschewski H, Barberà JA, Blanco I, Spruit MA, Franssen FME, Vonk Noordegraaf A, Reis A, Santos M, Viamonte SG, Demeyer H, Delcroix M, Bossone E, Johnson M. Standardized exercise training is feasible, safe, and effective in pulmonary arterial and chronic thromboembolic pulmonary hypertension: results from a large European multicentre randomized controlled trial. Eur Heart J. 2021;42:2284-2295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 25. | Wensel R, Francis DP, Meyer FJ, Opitz CF, Bruch L, Halank M, Winkler J, Seyfarth HJ, Gläser S, Blumberg F, Obst A, Dandel M, Hetzer R, Ewert R. Incremental prognostic value of cardiopulmonary exercise testing and resting haemodynamics in pulmonary arterial hypertension. Int J Cardiol. 2013;167:1193-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 26. | Schwaiblmair M, Faul C, von Scheidt W, Berghaus TM. Ventilatory efficiency testing as prognostic value in patients with pulmonary hypertension. BMC Pulm Med. 2012;12:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Zhong XJ, Jiang R, Yang L, Yuan P, Gong SG, Zhao QH, Luo CJ, Qiu HL, Li HT, Zhang R, He J, Wang L, Tang J, Liu JM. Peak oxygen uptake is a strong prognostic predictor for pulmonary hypertension due to left heart disease. BMC Cardiovasc Disord. 2022;22:137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 28. | Farber HW, Miller DP, McGoon MD, Frost AE, Benton WW, Benza RL. Predicting outcomes in pulmonary arterial hypertension based on the 6-minute walk distance. J Heart Lung Transplant. 2015;34:362-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 29. | Sitbon O, Gomberg-Maitland M, Granton J, Lewis MI, Mathai SC, Rainisio M, Stockbridge NL, Wilkins MR, Zamanian RT, Rubin LJ. Clinical trial design and new therapies for pulmonary arterial hypertension. Eur Respir J. 2019;53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 145] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 30. | Mathai SC, Puhan MA, Lam D, Wise RA. The minimal important difference in the 6-minute walk test for patients with pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186:428-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 213] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 31. | Galiè N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, Fleming T, Parpia T, Burgess G, Branzi A, Grimminger F, Kurzyna M, Simonneau G; Sildenafil Use in Pulmonary Arterial Hypertension (SUPER) Study Group. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353:2148-2157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1739] [Cited by in RCA: 1612] [Article Influence: 80.6] [Reference Citation Analysis (0)] |
| 32. | Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB, Groves BM, Tapson VF, Bourge RC, Brundage BH, Koerner SK, Langleben D, Keller CA, Murali S, Uretsky BF, Clayton LM, Jöbsis MM, Blackburn SD, Shortino D, Crow JW; Primary Pulmonary Hypertension Study Group. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med. 1996;334:296-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1913] [Cited by in RCA: 1768] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 33. | Olschewski H, Simonneau G, Galiè N, Higenbottam T, Naeije R, Rubin LJ, Nikkho S, Speich R, Hoeper MM, Behr J, Winkler J, Sitbon O, Popov W, Ghofrani HA, Manes A, Kiely DG, Ewert R, Meyer A, Corris PA, Delcroix M, Gomez-Sanchez M, Siedentop H, Seeger W; Aerosolized Iloprost Randomized Study Group. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med. 2002;347:322-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1191] [Cited by in RCA: 1105] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 34. | Rubin LJ, Badesch DB, Barst RJ, Galie N, Black CM, Keogh A, Pulido T, Frost A, Roux S, Leconte I, Landzberg M, Simonneau G. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346:896-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1937] [Cited by in RCA: 1767] [Article Influence: 76.8] [Reference Citation Analysis (0)] |
| 35. | Karanth MS, Awad NT. Six Minute Walk Test: A Tool for Predicting Mortality in Chronic Pulmonary Diseases. J Clin Diagn Res. 2017;11:OC34-OC38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Kaminsky DA, Knyazhitskiy A, Sadeghi A, Irvin CG. Assessing maximal exercise capacity: peak work or peak oxygen consumption? Respir Care. 2014;59:90-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Ahmadian HR, Sclafani JJ, Emmons EE, Morris MJ, Leclerc KM, Slim AM. Comparison of Predicted Exercise Capacity Equations and the Effect of Actual vs Ideal Body Weight among Subjects Undergoing Cardiopulmonary Exercise Testing. Cardiol Res Pract. 2013;2013:940170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | ERS Task Force, Palange P, Ward SA, Carlsen KH, Casaburi R, Gallagher CG, Gosselink R, O'Donnell DE, Puente-Maestu L, Schols AM, Singh S, Whipp BJ. Recommendations on the use of exercise testing in clinical practice. Eur Respir J. 2007;29:185-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 365] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 39. | Ferrazza AM, Martolini D, Valli G, Palange P. Cardiopulmonary exercise testing in the functional and prognostic evaluation of patients with pulmonary diseases. Respiration. 2009;77:3-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 123] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 40. | Pichurko BM. Exercising your patient: which test(s) and when? Respir Care. 2012;57:100-10; discussion 110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 41. | Guimaraes GV, d'Avila VM, Silva MS, Ferreira SA, Ciolac EG, Carvalho VO, Bocchi EA. A cutoff point for peak oxygen consumption in the prognosis of heart failure patients with β-blocker therapy. Int J Cardiol. 2010;145:75-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 42. | Barbagelata L, Masson W, Bluro I, Lobo M, Iglesias D, Molinero G. Prognostic role of cardiopulmonary exercise testing in pulmonary hypertension: a systematic review and meta-analysis. Adv Respir Med. 2022;90:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 43. | Systrom D, Warren A, Naeije R. The Role of Exercise Testing in Pulmonary Vascular Disease: Diagnosis and Management. Clin Chest Med. 2021;42:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 44. | Halank M, Einsle F, Lehman S, Bremer H, Ewert R, Wilkens H, Meyer FJ, Grünig E, Seyfarth HJ, Kolditz M, Wieder G, Höffken G, Köllner V. Exercise capacity affects quality of life in patients with pulmonary hypertension. Lung. 2013;191:337-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 45. | Sarzyńska K, Świątoniowska-Lonc N, Dudek K, Jonas K, Kopeć G, Gajek J, Jankowska-Polańska B. Quality of life of patients with pulmonary arterial hypertension: a meta-analysis. Eur Rev Med Pharmacol Sci. 2021;25:4983-4998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 46. | Halimi L, Marin G, Molinari N, Gamez AS, Boissin C, Suehs CM, Vachier I, Bourdin A. Impact of psychological factors on the health-related quality of life of patients treated for pulmonary arterial hypertension. J Psychosom Res. 2018;105:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 47. | Von Visger TT, Kuntz KK, Phillips GS, Yildiz VO, Sood N. Quality of life and psychological symptoms in patients with pulmonary hypertension. Heart Lung. 2018;47:115-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 48. | Dimopoulos S, Tzanis G, Manetos C, Tasoulis A, Mpouchla A, Tseliou E, Vasileiadis I, Diakos N, Terrovitis J, Nanas S. Peripheral muscle microcirculatory alterations in patients with pulmonary arterial hypertension: a pilot study. Respir Care. 2013;58:2134-2141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 49. | Dimopoulos S, Tzanis G, Karabinis A, Nanas S. Dynamic near-infrared spectroscopy assessment as an important tool to explore pulmonary arterial hypertension pathophysiology. Eur Respir J. 2017;49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 50. | Coats AJ, Adamopoulos S, Radaelli A, McCance A, Meyer TE, Bernardi L, Solda PL, Davey P, Ormerod O, Forfar C. Controlled trial of physical training in chronic heart failure. Exercise performance, hemodynamics, ventilation, and autonomic function. Circulation. 1992;85:2119-2131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 633] [Cited by in RCA: 622] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 51. | Drexler H, Riede U, Münzel T, König H, Funke E, Just H. Alterations of skeletal muscle in chronic heart failure. Circulation. 1992;85:1751-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 520] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 52. | Piepoli MF, Davos C, Francis DP, Coats AJ; ExTraMATCH Collaborative. Exercise training meta-analysis of trials in patients with chronic heart failure (ExTraMATCH). BMJ. 2004;328:189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 422] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 53. | Tzanis G, Philippou A, Karatzanos E, Dimopoulos S, Kaldara E, Nana E, Pitsolis T, Rontogianni D, Koutsilieris M, Nanas S. Effects of High-Intensity Interval Exercise Training on Skeletal Myopathy of Chronic Heart Failure. J Card Fail. 2017;23:36-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 54. | Tryfonos A, Tzanis G, Pitsolis T, Karatzanos E, Koutsilieris M, Nanas S, Philippou A. Exercise Training Enhances Angiogenesis-Related Gene Responses in Skeletal Muscle of Patients with Chronic Heart Failure. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 55. | Hirai DM, Musch TI, Poole DC. Exercise training in chronic heart failure: improving skeletal muscle O2 transport and utilization. Am J Physiol Heart Circ Physiol. 2015;309:H1419-H1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 128] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 56. | Brodal P, Ingjer F, Hermansen L. Capillary supply of skeletal muscle fibers in untrained and endurance-trained men. Am J Physiol. 1977;232:H705-H712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 78] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 57. | Hudlicka O, Brown M, Egginton S. Angiogenesis in skeletal and cardiac muscle. Physiol Rev. 1992;72:369-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 418] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 58. | Kourek C, Karatzanos E, Psarra K, Ntalianis A, Mitsiou G, Delis D, Linardatou V, Pittaras T, Vasileiadis I, Dimopoulos S, Nanas S. Endothelial progenitor cells mobilization after maximal exercise in patients with chronic heart failure. Hellenic J Cardiol. 2021;62:70-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 59. | Kourek C, Karatzanos E, Psarra K, Georgiopoulos G, Delis D, Linardatou V, Gavrielatos G, Papadopoulos C, Nanas S, Dimopoulos S. Endothelial progenitor cells mobilization after maximal exercise according to heart failure severity. World J Cardiol. 2020;12:526-539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 60. | Kitzman DW, Whellan DJ, Duncan P, Pastva AM, Mentz RJ, Reeves GR, Nelson MB, Chen H, Upadhya B, Reed SD, Espeland MA, Hewston L, O'Connor CM. Physical Rehabilitation for Older Patients Hospitalized for Heart Failure. N Engl J Med. 2021;385:203-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 350] [Article Influence: 87.5] [Reference Citation Analysis (0)] |