Published online Jun 9, 2024. doi: 10.5492/wjccm.v13.i2.92458
Revised: February 17, 2024
Accepted: March 26, 2024
Published online: June 9, 2024
Processing time: 128 Days and 23.2 Hours
Extracorporeal organ support (ECOS) has made remarkable progress over the last few years. Renal replacement therapy, introduced a few decades ago, was the first available application of ECOS. The subsequent evolution of ECOS enabled the enhanced support to many other organs, including the heart [veno-arterial extracorporeal membrane oxygenation (ECMO), slow continuous ultrafiltration], the lungs (veno-venous ECMO, extracorporeal carbon dioxide removal), and the liver (blood purification techniques for the detoxification of liver toxins). Moreover, additional indications of these methods, including the suppression of excessive inflammatory response occurring in severe disorders such as sepsis, coronavirus disease 2019, pancreatitis, and trauma (blood purification techniques for the removal of exotoxins, endotoxins, or cytokines), have arisen. Multiple organ support therapy is crucial since a vast majority of critically ill patients present not with a single but with multiple organ failure (MOF), whereas, traditional therapeutic approaches (mechanical ventilation for acute respiratory failure, antibiotics for sepsis, and inotropes for cardiac dysfunction) have reached the maximum efficacy and cannot be improved further. However, several issues remain to be clarified, such as the complexity and cost of ECOS systems, standar
Core Tip: Supportive therapy remains the cornerstone of care for critically ill patients. Nowadays, extracorporeal organ support (ECOS) systems have made remarkable technological progress and have become widely available in almost every intensive care unit around the world. Long-lasting multiple organ support therapy is feasible for the kidneys, liver, heart, and lungs, while the use of ECOS systems for suppression of various hyperinflammatory conditions, such as sepsis and coronavirus disease 2019, during cardiac surgery, and after cardiac arrest, is an emerging and rapidly recognized indication. Nowadays, combinations of supportive strategies have been developed tailored to the needs of each patient, leading to new ways of understanding and managing multiple organ failure. Moreover, the crosstalk between native and artificial organs is a novel concept that must be further studied, while further research is needed to clarify the indications, therapeutic protocols, and groups of patients suitable for such therapies.
- Citation: Papamichalis P, Oikonomou KG, Xanthoudaki M, Valsamaki A, Skoura AL, Papathanasiou SK, Chovas A. Extracorporeal organ support for critically ill patients: Overcoming the past, achieving the maximum at present, and redefining the future. World J Crit Care Med 2024; 13(2): 92458
- URL: https://www.wjgnet.com/2220-3141/full/v13/i2/92458.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v13.i2.92458
Besides the primary insult that affects a critically ill patient [a pathogen causing sepsis or a critical condition (e.g., trauma, pancreatitis)], secondary interactions induced by humoral and cellular processes occur, resulting in secondary impacts to multiple organs. Even several distant organs become involved in a cascade of interactions triggered by the initial precipitating factor. Although the intensity of the initial insult is of utmost importance, in several cases, it is the host’s response, i.e., the dysregulation of the immune system, which results in catastrophic organ dysfunction. Pharmacological approaches to treat this dysregulation remain unsuccessful in resolving multiple organ failure (MOF). Blood purification and extracorporeal organ support (ECOS) therapy have evolved greatly and attracted attention during the last few decades[1,2]. This strategy is implemented by systems, devices, and circuits that offer organ support by removing and then purifying - processing a patient’s blood[3,4]. The main indications for this strategy are outlined in Table 1.
| Modality | Indications | Remarks |
| MARS | Liver support in ALF-ACLF/hepatic encephalopathy/hepatorenal syndrome/removal of toxins | Spares exogenous albumin by using a secondary circuit system, can lead to hemorrhagic complications, due to consumption of platelets and coagulation factors |
| Prometheus | Liver support in ALF-ACLF/hepatic encephalopathy/hepatorenal syndrome/removal of toxins | Recirculates patient’s endogenous albumin |
| SPAD | Liver support in ALF-ACLF/hepatic encephalopathy/hepatorenal syndrome/removal of toxins | Lack of cost-effectiveness due to albumin waste |
| Bioartificial systems | Liver support in ALF-ACLF/hepatic encephalopathy/hepatorenal syndrome/removal of toxins/enhancement of liver synthetic function | Research in progress, regulatory are safety issues need to be addressed, not yet widely available |
| (VV-) ECMO | Refractory respiratory failure – severe ARDS/severe respiratory acidosis Circulatory – cardiac failure/cardiac arrest/respiratory failure | Provides immediate and effective support for cardiac function and oxygenation, needs to be applied early during disease course when other supportive measures are insufficient or fail |
| (VA-) ECMO | ||
| SCUF | Acute or chronic heart failure/fluid overload | Controls balance of fluids, decreases preload or right heart chambers |
| LVAD | End stage heart failure | Assists left ventricle to deliver sufficient blood volume and maintain organ perfusion, serves as bridge to transplant |
| IABP | Cardiogenic shock | Increases cardiac output by reducing afterload, improves myocardial oxygen perfusion |
| ECCO2R | Hypercapnic respiratory failure at exacerbations of COPD/severe hypercapnia in setting of protective ventilation | Uses lower blood flows in comparison to ECMO |
| Hemoperfusion | Severe sepsis/septic shock/liver support | Acts as immunomodulator by restoring balance of pro- and anti-inflammatory molecules in sepsis/ALF/ACLF |
| Plasmapheresis/plasma exchange | Elimination of autoantibodies, endotoxins and other substances/liver support | Potential immunomodulatory effect on CRS. Replaces coagulation factors and other diminished plasma proteins in patients with ALF or ACLF and improves outcome |
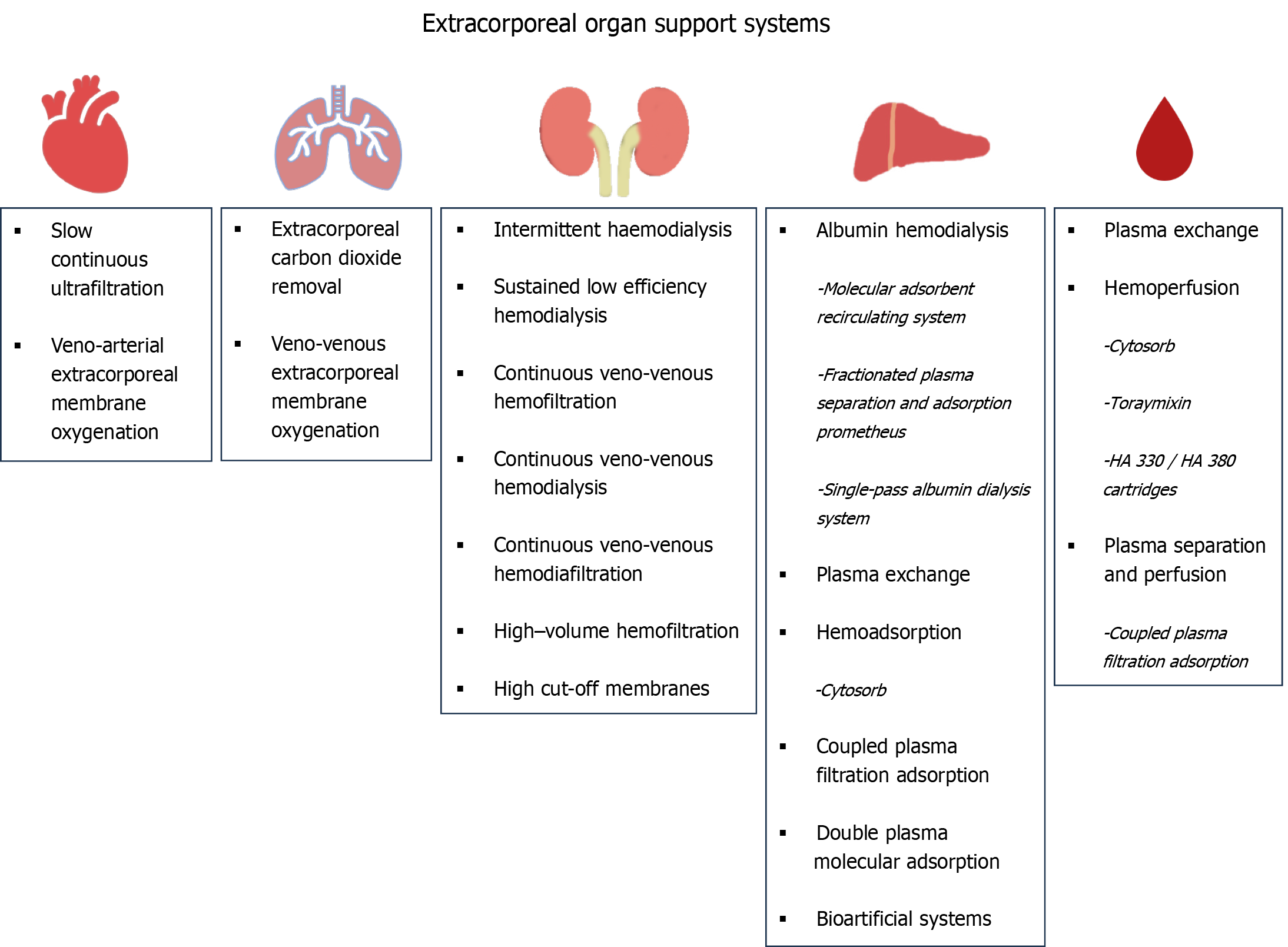
Nowadays, these techniques offer complex, multiple organ support therapy (MOST), which can provide sequential or simultaneous ECOS to any affected organ, including the kidneys, heart, lungs, and liver (Figure 1).
Therapies for the support of impaired renal function have been in use for over three decades. They represent the first type of ECOS systems to be introduced in clinical practice in intensive care units (ICUs). Their evolution was remarkable; renal replacement therapy (RRT) was made feasible for critically ill patients for whom peritoneal dialysis or intermittent hemodialysis are contraindicated mainly due to hemodynamic reasons. Following the first therapy of this kind, namely continuous arterio-venous hemofiltration, technological, and medical evolution have yielded four generations of continuous RRT (CRRT) machines. This has also led to the introduction of vital prescription details, such as the quantification of CRRT dose (35 mL/kg/h)[5]. Nowadays, CRRT machines, equipped with advanced membranes and complex circuits, are central components of MOST and can perform several other interventions, such as extracorporeal carbon dioxide removal (ECCO2R), liver support, and hemoperfusion/blood purification therapies, besides RRT.
Continuous veno-venous hemodialysis (CVVHD) (diffusive method), continuous veno-venous hemofiltration (CVVHF) (convective method), and continuous veno-venous hemodiafiltration (CVVHDF) (convective and diffusive method) are used in routine clinical practice in ICUs. While the removal of hydrophilic, low-molecular-weight uremic toxins is feasible with simple membranes (e.g., low-flux) and techniques (e.g., CVVHD, CVVHF, and CVVHDF), the need to step forward and extract medium-molecular-weight toxins and cytokines has led to the introduction of new membranes (high-flux, medium cut-off, high cut-off) and techniques [high-volume hemofiltration (HVHF), adsorption]. The rationale behind this is to improve the outcome of patients with renal failure and hyperinflammatory conditions by preventing the accumulation of medium-molecular-weight uremic toxins and cytokines. Advances in hemocompatibility (new synthetic and polymeric membranes) and anticoagulation (regional citrate anticoagulation) have facilitated the evolution and applicability of these techniques and methods.
HVHF is a convective, resource-light therapy with markedly elevated effluent rates, which is either delivered conti
High cut-off membranes differ from conventional ones in larger pore sizes of about 0.01 µm, with a molecular cut-off of 60 kDa, which allows the removal of medium-molecular-weight molecules and cytokines. Either convection or diffusion can be used for toxin removal. However, no additional benefit from the use of these filters in septic patients with acute kidney injury (AKI) has been proven[7].
As none of the above-mentioned methods can achieve sufficient cytokine removal and immunomodulation, the application of more efficient techniques is necessary in sepsis and other hyperinflammatory conditions. One such option for blood purification is adsorption, which has growing evidence and has been widely used over the past few decades. It uses natural or synthetic sorbents such as activated charcoal or synthetic resins, respectively, for drug poisoning or removal of cytokines or endotoxins. It can be performed either by direct hemoperfusion or by plasma separation and perfusion and involves either the selective adsorption of certain molecules such as endotoxin (Toraymixin) or the non-selective removal of various inflammatory molecules such as cytokines [Cytosorb, HA 330/HA 380 cartridges, coupled plasma filtration adsorption (CPFA)].
Cytosorb is perhaps the most widely used adsorption filter, with several case reports and case series reporting a favorable outcome of its use for sepsis and during the Coronavirus disease 2019 pandemic, especially when combined with extracorporeal membrane oxygenation (ECMO)[8]. Its principal target molecule is interleukin (IL)-6, with other biomarkers for its use, including C-reactive protein and ferritin. It also eliminates myoglobulin and albumin-bound toxins. It has been used in several hyperinflammatory conditions, such as in septic patients, patients with acute pancreatitis, or during cardiac surgery[9,10]. Despite these encouraging preliminary data, results from a recent meta-analysis did not support the widespread use of Cytosorb in ICUs[11]. Moreover, concerns about the cost, side effects, and undesirable elimination of antibiotics and nutrients have been raised[12]. Further research and randomized controlled trials (RCTs) are needed to clarify the role of Cytosorb in sepsis and hyperinflammatory conditions.
Toraymixin has also shown encouraging results, especially when its use is directed by a biomarker[13,14]. However, data from the use of HA 330 and HA 380 cartridges are preliminary, while CPFA is currently not indicated in patients with septic shock due to potential harm[15].
The difficulty in estimating possible survival benefits from these methods is a general problem for therapies for sepsis in critically ill patients. Due to the syndrome’s diversity, complexity, and severity, the design of trials leading to definite results is demanding and complex. New, well-designed, homogenous, statistically powerful RCTs, with the inclusion of measures of outcome besides mortality, and using biomarkers or genetic markers, are needed to draw reliable conclusions.
Liver failure is associated with high morbidity and mortality rates. In multiple-organ dysfunction syndrome, critically ill patients develop either acute liver failure (ALF) or acute-on-chronic liver failure (ACLF). An impaired liver function may lead to renal and brain pathology, hepatorenal syndrome, and hepatic encephalopathy, respectively, due to the changes in systemic circulation and the accumulation of toxic molecules such as ammonia and bilirubin[3,16].
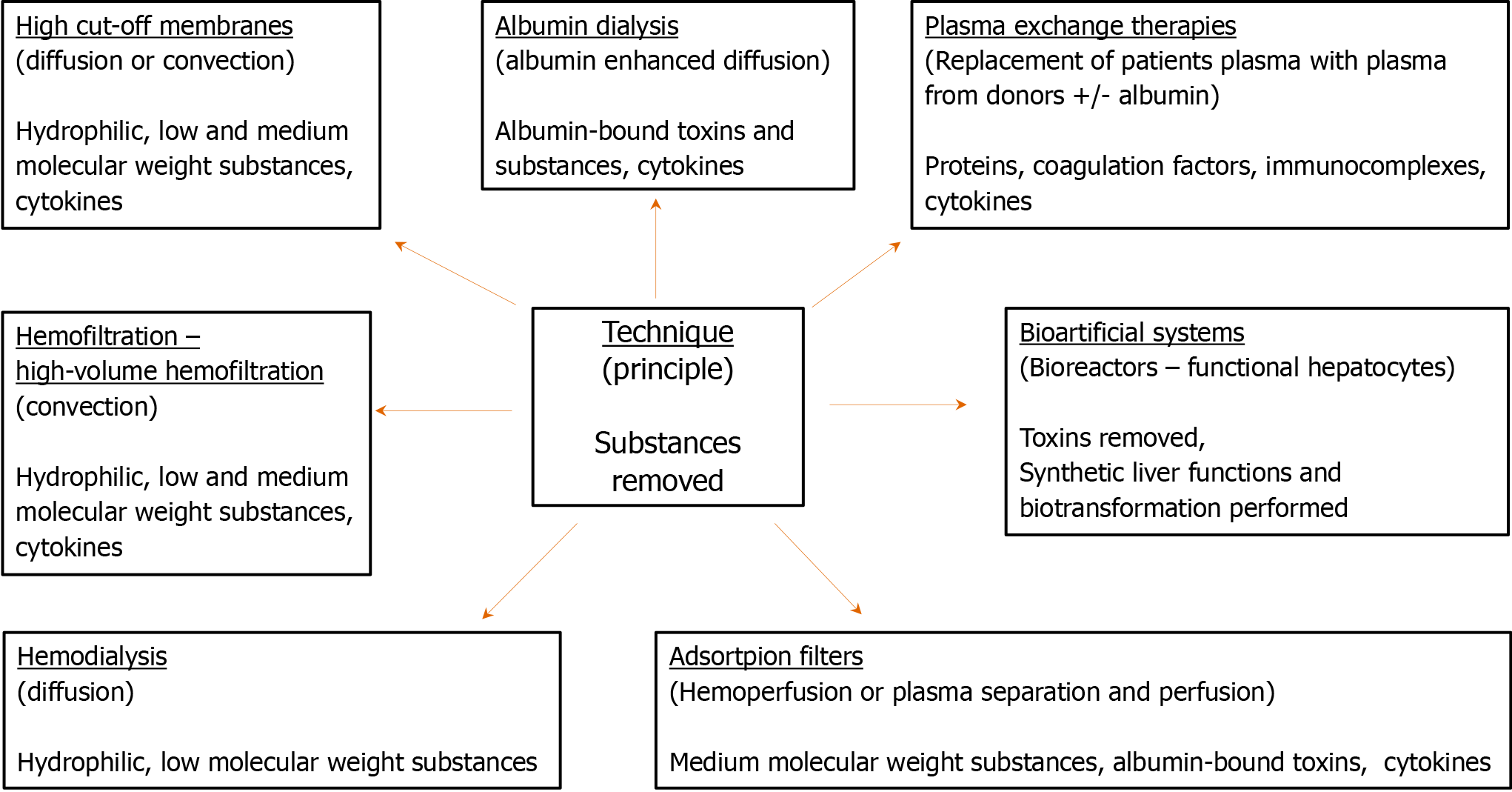
Extracorporeal liver support techniques (Figure 2) have been developed during the last few decades. Liver replacement therapy can be achieved through several methods of artificial liver support, which use membrane separation processes, resins, columns, or bioreactors. Protein-bound toxins, albumin, and ammonia are the main molecules removed through this process. Protein-bound toxins require special detoxification methods, such as albumin dialysis, plasma exchange (PEX) therapy, adsorption filters, and bioartificial systems. Ammonia, the removal of which is especially crucial for patients with ALF, can be removed by simple diffusive (hemodialysis) or convective (hemofiltration) methods, and more efficiently by HVHF[3,17,18].
In albumin dialysis, the clearance of albumin-bound toxins from patients’ blood is achieved by diffusion through a membrane to an albumin-containing solution[19]. It is performed by three main systems, the molecular adsorbent-recirculating system (MARS), the fractionated plasma separation and adsorption [FPSA (Prometheus)], and the single-pass albumin dialysis (SPAD). SPAD is used in conjunction with dialysis for the removal of protein-bound toxins[18]. It is simple, resource-light, and effective in the removal of bilirubin and copper. However, it remains an expensive option due to the albumin waste, particularly when repeated sessions are required[18]. To overcome this limitation, the MARS spares albumin using a secondary circuit while it effectively removes bilirubin, ammonia, and creatinine. By improving portal hypertension and hepatic circulation, it also decreases hyperdynamic circulation and increases the systemic vascular resistive index and mean arterial pressure. Loss of antibiotics and consumption of coagulation factors and platelets, leading to bleeding disorders, have been associated with the use of MARS. However, it is considered a relatively safe method with rarely occurring adverse events[18,20]. Prometheus removes albumin-bound toxins using resins and absorbers. It is different from MARS in the fact that MARS utilizes exogenous albumin, whereas Prometheus recirculates the patient’s albumin, thus leading to minor reductions in the patient’s albumin concentration. Prometheus has been proven to be a safe and efficient method[18,21].
The PEX therapy uses macro-porous filters with pore sizes of approximately 0.2 µm and a molecular cut-off of 1000 kDa, which can efficiently remove immunocomplexes, lipoproteins, antibodies, paraproteins, and cytokines while replacing diminished plasma proteins. Its immunomodulatory properties, along with a correction of the deficiency in coagulation factors often observed in ACF and ACLF patients, have been proven beneficial. Therefore, this therapy is recommended by guidelines, reviews, and RCTs[22-24].
Adsorption filters are an interesting alternative to the more expensive, complex, and resource-intensive albumin dialysis systems. They use resins to remove cytokines and albumin-bound toxins, offering both suppression of the immunological dysregulation observed in acute liver dysfunction and detoxification[25]. The most popular adsorption systems available include Cytosorb, double-plasma molecular adsorption system (BS 330 + HA 330-II), and CPFA (Figure 1). Although adsorption is a promising method for liver detoxification, some issues still need clarification and resolution, such as their cost-effectiveness, the undesirable loss of substances (necessitating therapeutic drug monitoring of antibiotics), their application protocol, and the need for more evidence-based data regarding their use.
Bioartificial liver systems use bioreactors and aim at both toxin removal and the enhancement of synthetic functions and biotransformation. Although these systems are promising because they offer the perspective of complete replacement of liver function besides detoxification (which is solely performed by artificial liver support systems), they remain controversial. Their principle is based on the use of liver cells, which are isolated from animals or tumors and connected to a substrate that preserves the metabolism and morphology of the cells. Currently, four systems are under clinical investigation, although the cost, safety during cell preparation and preservation from bench to bedside, and several other regulatory issues need to be addressed before introducing these advanced methods of liver support in everyday clinical practice. Thus, additional research and trials are needed to address these issues[16,18].
Some of the most common indications of liver replacement therapies include hepatorenal syndrome, ALF, ACLF, portal hypertension, and hepatic encephalopathy. Although no clear data describe the optimal time to initiate artificial liver support or its optimal duration, the above methods of liver replacement may serve as “salvage therapy” in patients with severe liver dysfunction and/or liver failure, and as a bridge to either recovery or transplantation[17,18].
Severe hypoxemic respiratory failure, occurring mainly due to primary and secondary acute respiratory distress syndrome (ARDS), remains a major problem in ICUs worldwide and is associated with high mortality. Despite the advancements in mechanical ventilation strategies and the implementation of protective ventilation, mortality remains high, attributed to the syndrome itself, along with the side effects of mechanical ventilation, such as ventilator-induced lung injury (VILI). Veno-venous-ECMO (VV-ECMO) was described in 1972 by Zapol et al[26] as a membrane lung that could “buy us time” by providing extracorporeal oxygenation. In recent years, there has been a noticeable increase in the utilization of VV-ECMO, especially in patients with severe and potentially reversible refractory hypoxemia [partial pressure of oxygen (pO2)/fraction of inspired oxygen (FiO2) < 80] mainly due to ARDS, after a short course of optimal mechanical ventilation and the application of prone position. Additional indications for VV-ECMO include its use in severe respiratory acidosis and as a bridging therapy for lung transplantation[27].
During VV-ECMO, blood is pumped from the inferior or superior vena cava (through the femoral or jugular veins) via large-bore cannulas through a membrane oxygenator and returned to the right atrium, where it is mixed with native venous return and is led to the patient’s circulation. Cannulation can be achieved with ultrasound-guided placement of two single-lumen catheters, although double-lumen single-catheter cannulas have also been utilized recently[27,28]. The former gravity-dependent pumps are replaced with centrifugal pumps to drive the patient’s blood to the oxygenator. In the oxygenator, O2 is transferred through the hollow fibers of a membrane, most commonly consisting of polymethylpentene, which has replaced the silicone membranes previously used. This allows gas exchange while simultaneously restricting the fluid exchange[29,30]. To achieve optimal oxygenation, high blood flow (up to and sometimes more than 4000 mL/min) is required through the oxygenator. Furthermore, sufficient fluid volume and hemoglobulin levels should be maintained to achieve adequate oxygen delivery. Despite advances in technology, the process of ECMO cannulation, the requirement of high blood flow, and the necessity for anticoagulation can induce major complications such as bleeding and thrombosis, hemolysis, pulmonary edema and/or hemodynamic instability during the initiation of therapy, hypoxemia, and VILI[27,29,31]. Nevertheless, in the modern era, VV-ECMO is more feasible, and its application has been proven to be related to favorable outcomes among patients with severe ARDS. Future research is crucial for the clarification of issues regarding the role of ECMO in pharmacokinetics, the limitation of adverse events, and optimal mechanical ventilation settings during ECMO sessions.
ECCO2R therapy, a technique similar to VV-ECMO, aims to eliminate CO2, reverse respiratory acidosis, and allow ultra-protective ventilation strategies. Contrary to VV-ECMO, ECCO2R requires a lower blood flow (500 mL/min or less), which is sufficient to eliminate CO2 but has no impact on oxygenation due to the different diffusion and solubility properties of O2 and CO2. Although an arteriovenous technique has also been used, veno-venous cannulation using a double-lumen single catheter or a dialysis catheter and blood drainage via pumping is most commonly preferred[32]. Moreover, modern low-flow polymethylpentene membranes can be applied using the RRT platform, with the advantage of enhanced feasibility[18,33]. Although ECCO2R has shown promising results and fewer complications than VV-ECMO in ARDS patients, hypercapnic respiratory failure due to the acute exacerbation of chronic obstructive pulmonary disease remains the main indication for ECCO2R therapy[29,34].
Notably, present-day bioengineering is working towards pumpless extracorporeal membranes and intravascular lung assists[35].
Despite recent significant advances regarding the treatment of cardiogenic shock, it remains a serious health problem that directly threatens the lives of patients. Annually, over 100000 patients with this diagnosis are admitted to hospitals in the United States, causing unacceptably high morbidity and mortality[36]. For a significant proportion of these patients who are critically ill, the support of cardiac muscle function by conventional pharmacological methods is inadequate. Nowadays, Veno-arterial-ECMO (VA-ECMO) is widely used in patients with circulatory or cardiac failure to provide gas exchange support, restore the function of the organs of the human body by ensuring satisfactory end-organ perfusion and timely become the bridge to either recovery or to durable mechanical circulatory support of the cardiac muscle [for example, through percutaneous and surgical ventricular assist devices (VADs)], or to the ultimate solution of heart transplantation, for severe, persistent heart failure[3].
Besides the VA-ECMO, another ECOS system available to assist cardiac function is slow-continuous ultrafiltration (SCUF), which uses a central venous catheter for hemodialysis. It aims to control the balance of fluids and decrease fluid overload in acute or chronic heart failure. When the fluid overload is not treated promptly in patients with heart failure, it results in either acute renal failure or cardiorenal syndrome, which ultimately require the application of RRT[37]. SCUF can reduce central hyperhydration through a decrease in the right heart chamber preload. In several cases, renal recovery is achieved, which is characterized by an increase in the diuresis and a simultaneous improvement of cardiac function.
In patients with end-stage heart failure, a left-ventricular assist device (LVAD) is implanted to assist the left ventricle in the delivery of sufficient blood volume to the rest of the body. Another option for patients with cardiogenic shock is the intra-aortic balloon pump (IABP), which increases myocardial oxygen perfusion and indirectly cardiac output through the reduction of the afterload. Both LVAD and IABP are suitable for assisting the compromised heart function, serving as parts of MOST. However, by definition, they cannot be characterized as ECOS systems[3,4].
Lorusso et al[38] published the interim guidelines of the Extracorporeal Life Support Organization regarding the use of VA-ECMO in critically ill adult cardiac patients, with the following key points:
Ideally, VA-ECMO should be applied within the first six hours after the onset of cardiogenic shock and if drug therapy and fluid restoration approaches fail.
Aortic valve regurgitation is one of the main potential contraindications of the method.
The main exclusion criteria are acute brain injury, liver failure, severe vascular disease, serious immune system disorders, and poor life expectancy.
Advanced age alone is not an exclusion criterion.
Prognostic scores can assist treating physicians in deciding on whether to apply the method.
The outcome depends not only on the severity and the possibility of recovery of the underlying disease but also on the physical and neuro-cognitive status of the patient. The main recommendations[38] for critical care monitoring during VA-ECMO therapy include the following: (1) Continuous assessment of O2 delivery and venous saturation; (2) Continuous assessment of right-sided tissue perfusion; (3) Monitoring of pulse pressure to assess cardiac function; (4) Assessment of lung ventilation; and (5) Continuous monitoring of blood cells and free hemoglobin, D-dimer, and lactic acid levels.
Therefore, it is possible to use a wide range of mechanical cardiac support devices for the treatment of cardiogenic shock, especially in critically ill patients. These devices include SCUF, LVAD (both pulsative and non-pulsative), biventricular assist device, IABP, and ECMO. Recent literature data prove that ECMO, particularly VA-ECMO, can provide immediate and effective support for the patient’s cardiac function and oxygenation. However, in cases where prolonged support is required, conversion to a more long-lasting device such as LVAD is recommended as a bridge to subsequent heart transplantation due to the occurrence of significant complications[39].
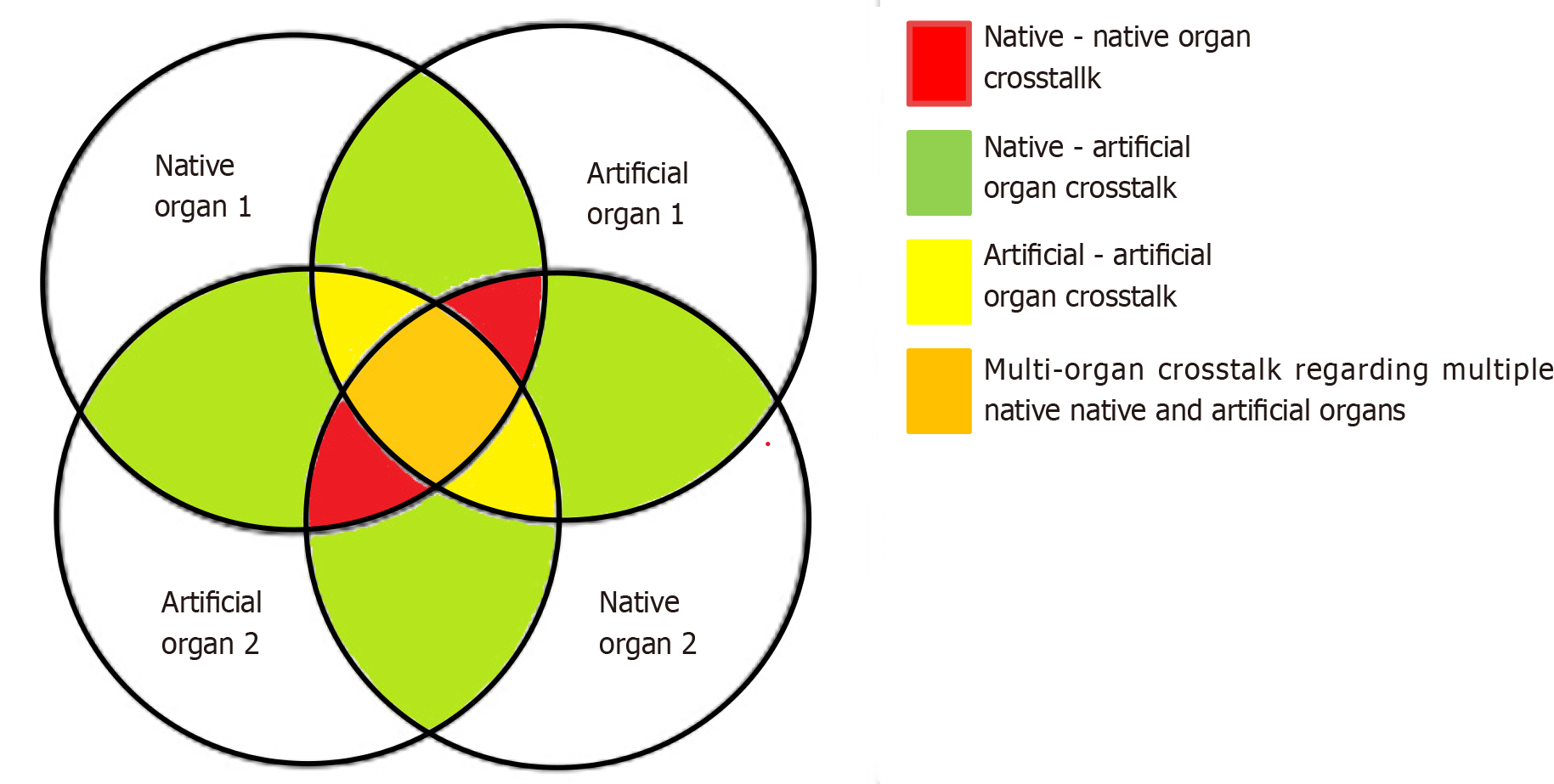
The burden of MOF in critically ill patients has highlighted the need for the application of MOST. Multiple complicated interactions among affected native organs and between the applied artificial organ support systems take place in such patients, which are mediated by cellular, molecular, biochemical, and hemodynamic processes[17]. These multilateral interactions are termed crosstalk and can be divided into four subgroups (Figure 3): (1) The crosstalk between two or more native organs when the dysfunction of one organ triggers and precipitates the failure of other organs. Cardiorenal syndromes, sepsis-induced AKI, and hepatorenal syndrome are characteristic examples of this type; (2) The crosstalk between native and artificial organs. This category mainly involves side effects occurring in native organs due to procedures involving artificial organs. Characteristic examples include bleeding complications due to anticoagulation in the circuits of ECOS systems; hemodynamic compromise during ECOS therapy; AKI induced by mechanical ventilation due to positive-pressure ventilation and its effect on the cardiac output or due to biotrauma (VILI); AKI induced by ECMO due to ischemia-reperfusion injury, increased incidence of septic episodes, diminished renal cortical blood flow resulting from the non-pulsatile nature of ECMO, augmented exposure to artificial surfaces inducing inflammation, platelet dysfunction, hypercoagulability, microvascular obstruction, and hemolysis; and AKI induced by liver replacement therapies due to the loss of antibiotics and nutrients, predisposing the patient to antibiotic failure and malnutrition; (3) The crosstalk between two or more artificial organs due to interactions arising from their concurrent use, with special precautions that must be taken to achieve the correct interconnection and proper overall function. An example of this crosstalk is the connection of RRT with ECMO or ECCO2R, which can be done with several available setups. If not performed properly, it can result in oxygenator clotting, pulmonary embolism, or arterial embolism; and (4) Multiple native and artificial organ crosstalk, which involves concomitant interactions among several native and artificial organs. This is the usual clinical scenario in ICUs today, as MOF frequently occurs and the availability of ECOS systems increases[3,17,20].
Understanding these different types of crosstalk will help us amplify the expected benefits of ECOS therapy while minimizing its undesirable effects. Due to the complexity of the resulting interactions, this is feasible only through multidisciplinary collaborations between well-trained critical care physicians and physicians from other specialties (cardiologists, cardiothoracic and vascular surgeons, nephrologists, pulmonologists), with the incorporation of an integrated multifaceted monitoring approach including close evaluation of mechanical parameters and of the vital signs, coagulation and biochemistry parameters of the patients. The next step will probably include a system that will gather all this information and provide feedback regarding modifications to the parameters to optimize homeostasis in patients. Artificial intelligence could assist this process, especially when the legal and ethical issues are resolved and technology reaches the required level of expertise needed[3,40,41].
The history of ECOS evolution has reached tremendous technological and medical progress. The rapid development of ECOS was closely related to improvements in the management of critically ill patients. Nowadays, it is difficult to provide advanced, integrated support to patients without the use of ECOS. CRRT machines are in every list of basic equipment in ICUs, while experts in these therapies encourage physicians working in ICUs to get familiar with new concepts such as the sequential extracorporeal therapy and modify everyday clinical practice using new techniques and ECOS systems[42,43]. In accordance with these suggestions and with recognition of these therapies’ utility and prospect, therapeutic approach in our ICU incorporates CVVHDF for RRT, Cytosorb for sepsis, rhabdomyolysis and in selected cases of acute pancreatitis, CPFA and SPAD as liver replacement therapies and IABP for support of cardiac function. Simultaneous application of IABP, CVVHDF and Cytosorb has given remarkable results after cardiac arrest in a patient with AKI and sepsis. Based on our experience, we recognize that it is difficult to introduce new, complex techniques and equipment that aim to treat complex syndromes, with the crosstalk between native and artificial organs making this effort even more complicated[17]. The interactions between native organs leading to MOF, the effect of artificial organs on native organs, and the compatibility and harmony between two or more artificial organs in the same patient are procedures requiring special training, a multidisciplinary team approach, and advanced patient monitoring systems. We must always take into consideration that no matter how complex techniques and equipment are used, these therapies remain complementary to the etiological confrontation of the primary insult, which must be our primary and basic approach (e.g., antibiotics and source control for sepsis). These techniques also remain expensive and not widely available; so, we must find ways to increase their cost-effectiveness through studies using biomarkers and genetic sub-groups that will benefit the most from their use. Nevertheless, future research is expected to provide evidence in favor of outcome benefit from ECOS when applied as adjunctive therapies[44]. We must also point out that these revolutionary systems are constantly evolving and their use is increasing over time, leading to more precise and personalized treatment options for critically ill patients. Thus, the future of therapies with ECOS systems is eagerly awaited with great expectations.
This paper is dedicated to the memory of Tilemachos Zafeiridis (1974–2021), an exceptional friend and doctor who will never be forgotten.
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
| 1. | Ronco C, Bagshaw SM, Bellomo R, Clark WR, Husain-Syed F, Kellum JA, Ricci Z, Rimmelé T, Reis T, Ostermann M. Extracorporeal Blood Purification and Organ Support in the Critically Ill Patient during COVID-19 Pandemic: Expert Review and Recommendation. Blood Purif. 2021;50:17-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 2. | Berlot G, Tomasini A, Zanchi S, Moro E. The Techniques of Blood Purification in the Treatment of Sepsis and Other Hyperinflammatory Conditions. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 3. | Ronco C, Ricci Z, Husain-Syed F. From Multiple Organ Support Therapy to Extracorporeal Organ Support in Critically Ill Patients. Blood Purif. 2019;48:99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Ranieri VM, Brodie D, Vincent JL. Extracorporeal Organ Support: From Technological Tool to Clinical Strategy Supporting Severe Organ Failure. JAMA. 2017;318:1105-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Ronco C. Continuous Renal Replacement Therapy: Forty-year Anniversary. Int J Artif Organs. 2017;40:257-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Clark E, Molnar AO, Joannes-Boyau O, Honoré PM, Sikora L, Bagshaw SM. High-volume hemofiltration for septic acute kidney injury: a systematic review and meta-analysis. Crit Care. 2014;18:R7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 7. | Gondouin B, Hutchison CA. High cut-off dialysis membranes: current uses and future potential. Adv Chronic Kidney Dis. 2011;18:180-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Rasch S, Sancak S, Erber J, Wießner J, Schulz D, Huberle C, Algül H, Schmid RM, Lahmer T. Influence of extracorporeal cytokine adsorption on hemodynamics in severe acute pancreatitis: Results of the matched cohort pancreatitis cytosorbents inflammatory cytokine removal (PACIFIC) study. Artif Organs. 2022;46:1019-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Hayanga JWA, Song T, Durham L, Garrison L, Smith D, Molnar Z, Scheier J, Deliargyris EN, Moazami N. Extracorporeal hemoadsorption in critically ill COVID-19 patients on VV ECMO: the CytoSorb therapy in COVID-19 (CTC) registry. Crit Care. 2023;27:243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 10. | Träger K, Skrabal C, Fischer G, Datzmann T, Schroeder J, Fritzler D, Hartmann J, Liebold A, Reinelt H. Hemoadsorption treatment of patients with acute infective endocarditis during surgery with cardiopulmonary bypass - a case series. Int J Artif Organs. 2017;40:240-249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 11. | Becker S, Lang H, Vollmer Barbosa C, Tian Z, Melk A, Schmidt BMW. Efficacy of CytoSorb®: a systematic review and meta-analysis. Crit Care. 2023;27:215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 76] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 12. | Heymann M, Schorer R, Putzu A. Mortality and adverse events of hemoadsorption with CytoSorb® in critically ill patients: A systematic review and meta-analysis of randomized controlled trials. Acta Anaesthesiol Scand. 2022;66:1037-1050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 13. | Cruz DN, Antonelli M, Fumagalli R, Foltran F, Brienza N, Donati A, Malcangi V, Petrini F, Volta G, Bobbio Pallavicini FM, Rottoli F, Giunta F, Ronco C. Early use of polymyxin B hemoperfusion in abdominal septic shock: the EUPHAS randomized controlled trial. JAMA. 2009;301:2445-2452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 538] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 14. | Klein DJ, Foster D, Walker PM, Bagshaw SM, Mekonnen H, Antonelli M. Polymyxin B hemoperfusion in endotoxemic septic shock patients without extreme endotoxemia: a post hoc analysis of the EUPHRATES trial. Intensive Care Med. 2018;44:2205-2212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 132] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 15. | Garbero E, Livigni S, Ferrari F, Finazzi S, Langer M, Malacarne P, Meca MCC, Mosca S, Olivieri C, Pozzato M, Rossi C, Tavola M, Terzitta M, Viaggi B, Bertolini G; GiViTI. High dose coupled plasma filtration and adsorption in septic shock patients. Results of the COMPACT-2: a multicentre, adaptive, randomised clinical trial. Intensive Care Med. 2021;47:1303-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 16. | Carpentier B, Gautier A, Legallais C. Artificial and bioartificial liver devices: present and future. Gut. 2009;58:1690-1702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 124] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 17. | Husain-Syed F, Ricci Z, Brodie D, Vincent JL, Ranieri VM, Slutsky AS, Taccone FS, Gattinoni L, Ronco C. Extracorporeal organ support (ECOS) in critical illness and acute kidney injury: from native to artificial organ crosstalk. Intensive Care Med. 2018;44:1447-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 18. | Huber W, Ruiz de Garibay AP. Options in extracorporeal support of multiple organ failure. Med Klin Intensivmed Notfmed. 2020;115:28-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Tsipotis E, Shuja A, Jaber BL. Albumin Dialysis for Liver Failure: A Systematic Review. Adv Chronic Kidney Dis. 2015;22:382-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 20. | Muciño-Bermejo MJ. Extracorporeal organ support and the kidney. Front Nephrol. 2022;2:924363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 21. | Evenepoel P, Laleman W, Wilmer A, Claes K, Maes B, Kuypers D, Bammens B, Nevens F, Vanrenterghem Y. Detoxifying capacity and kinetics of prometheus--a new extracorporeal system for the treatment of liver failure. Blood Purif. 2005;23:349-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | European Association for the Study of the Liver; Clinical practice guidelines panel, Wendon, J; Panel members, Cordoba J, Dhawan A, Larsen FS, Manns M, Samuel D, Simpson KJ, Yaron I; EASL Governing Board representative, Bernardi M. EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J Hepatol. 2017;66:1047-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 618] [Article Influence: 77.3] [Reference Citation Analysis (1)] |
| 23. | Bauer PR, Ostermann M, Russell L, Robba C, David S, Ferreyro BL, Cid J, Castro P, Juffermans NP, Montini L, Pirani T, Van De Louw A, Nielsen N, Wendon J, Brignier AC, Schetz M, Kielstein JT, Winters JL, Azoulay E; Nine-I Investigators. Plasma exchange in the intensive care unit: a narrative review. Intensive Care Med. 2022;48:1382-1396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 24. | Larsen FS, Schmidt LE, Bernsmeier C, Rasmussen A, Isoniemi H, Patel VC, Triantafyllou E, Bernal W, Auzinger G, Shawcross D, Eefsen M, Bjerring PN, Clemmesen JO, Hockerstedt K, Frederiksen HJ, Hansen BA, Antoniades CG, Wendon J. High-volume plasma exchange in patients with acute liver failure: An open randomised controlled trial. J Hepatol. 2016;64:69-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 440] [Article Influence: 48.9] [Reference Citation Analysis (4)] |
| 25. | Papamichalis P, Oikonomou KG, Valsamaki A, Xanthoudaki M, Katsiafylloudis P, Papapostolou E, Skoura AL, Papamichalis M, Karvouniaris M, Koutras A, Vaitsi E, Sarchosi S, Papadogoulas A, Papadopoulos D. Liver replacement therapy with extracorporeal blood purification techniques current knowledge and future directions. World J Clin Cases. 2023;11:3932-3948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (2)] |
| 26. | Zapol WM, Kitz RJ. Buying time with artificial lungs. N Engl J Med. 1972;286:657-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 16] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Tonna JE, Abrams D, Brodie D, Greenwood JC, Rubio Mateo-Sidron JA, Usman A, Fan E. Management of Adult Patients Supported with Venovenous Extracorporeal Membrane Oxygenation (VV ECMO): Guideline from the Extracorporeal Life Support Organization (ELSO). ASAIO J. 2021;67:601-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 354] [Article Influence: 88.5] [Reference Citation Analysis (0)] |
| 28. | Banfi C, Pozzi M, Siegenthaler N, Brunner ME, Tassaux D, Obadia JF, Bendjelid K, Giraud R. Veno-venous extracorporeal membrane oxygenation: cannulation techniques. J Thorac Dis. 2016;8:3762-3773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 29. | Tonetti T, Zanella A, Pérez-Torres D, Grasselli G, Ranieri VM. Current knowledge gaps in extracorporeal respiratory support. Intensive Care Med Exp. 2023;11:77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 30. | MacLaren G, Combes A, Bartlett RH. Contemporary extracorporeal membrane oxygenation for adult respiratory failure: life support in the new era. Intensive Care Med. 2012;38:210-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 272] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 31. | Nunez JI, Gosling AF, O'Gara B, Kennedy KF, Rycus P, Abrams D, Brodie D, Shaefi S, Garan AR, Grandin EW. Bleeding and thrombotic events in adults supported with venovenous extracorporeal membrane oxygenation: an ELSO registry analysis. Intensive Care Med. 2022;48:213-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 127] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 32. | Giraud R, Banfi C, Assouline B, De Charrière A, Cecconi M, Bendjelid K. The use of extracorporeal CO(2) removal in acute respiratory failure. Ann Intensive Care. 2021;11:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 33. | Schmidt M, Jaber S, Zogheib E, Godet T, Capellier G, Combes A. Feasibility and safety of low-flow extracorporeal CO(2) removal managed with a renal replacement platform to enhance lung-protective ventilation of patients with mild-to-moderate ARDS. Crit Care. 2018;22:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 34. | Combes A, Auzinger G, Capellier G, du Cheyron D, Clement I, Consales G, Dabrowski W, De Bels D, de Molina Ortiz FJG, Gottschalk A, Hilty MP, Pestaña D, Sousa E, Tully R, Goldstein J, Harenski K. ECCO(2)R therapy in the ICU: consensus of a European round table meeting. Crit Care. 2020;24:490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 35. | Syed A, Kerdi S, Qamar A. Bioengineering Progress in Lung Assist Devices. Bioengineering (Basel). 2021;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 36. | Tsangaris A, Alexy T, Kalra R, Kosmopoulos M, Elliott A, Bartos JA, Yannopoulos D. Overview of Veno-Arterial Extracorporeal Membrane Oxygenation (VA-ECMO) Support for the Management of Cardiogenic Shock. Front Cardiovasc Med. 2021;8:686558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (1)] |
| 37. | Nalesso F, Stefanelli F, Cattarin L, Billo M, Gnappi M, Partesano G, Cacciapuoti M, Babuin L, Calò LA. Slow Continuous Ultrafiltration in Regional Citrate Anticoagulation Performed with a Standard Fluid Infusion Central Venous Catheter in Intensive Care Unit for Fluid Overload in Acute on Chronic Heart Failure: A Case Report. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 38. | Lorusso R, Shekar K, MacLaren G, Schmidt M, Pellegrino V, Meyns B, Haft J, Vercaemst L, Pappalardo F, Bermudez C, Belohlavek J, Hou X, Boeken U, Castillo R, Donker DW, Abrams D, Ranucci M, Hryniewicz K, Chavez I, Chen YS, Salazar L, Whitman G. ELSO Interim Guidelines for Venoarterial Extracorporeal Membrane Oxygenation in Adult Cardiac Patients. ASAIO J. 2021;67:827-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 215] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 39. | Chen YS, Ko WJ, Lin FY, Huang SC, Chou TF, Chou NK, Hsu RB, Wang SS, Chu SH. Preliminary result of an algorithm to select proper ventricular assist devices for high-risk patients with extracorporeal membrane oxygenation support. J Heart Lung Transplant. 2001;20:850-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 40. | Yoon JH, Pinsky MR, Clermont G. Artificial Intelligence in Critical Care Medicine. Crit Care. 2022;26:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 41. | Peine A, Hallawa A, Bickenbach J, Dartmann G, Fazlic LB, Schmeink A, Ascheid G, Thiemermann C, Schuppert A, Kindle R, Celi L, Marx G, Martin L. Development and validation of a reinforcement learning algorithm to dynamically optimize mechanical ventilation in critical care. NPJ Digit Med. 2021;4:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 42. | Ronco C, Chawla L, Husain-Syed F, Kellum JA. Rationale for sequential extracorporeal therapy (SET) in sepsis. Crit Care. 2023;27:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 33] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 43. | Ronco C, Reis T. Continuous renal replacement therapy and extended indications. Semin Dial. 2021;34:550-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 44. | Clark WR, Neri M, Garzotto F, Ricci Z, Goldstein SL, Ding X, Xu J, Ronco C. The future of critical care: renal support in 2027. Crit Care. 2017;21:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |