Peer-review started: July 14, 2015
First decision: September 22, 2015
Revised: October 24, 2015
Accepted: November 23, 2015
Article in press: November 25, 2015
Published online: March 27, 2016
Processing time: 263 Days and 9.8 Hours
Effective adaptive immune responses rely upon appropriate activation of T cells by antigenic peptide-major histocompatibility complex on the surface of antigen presenting cells (APCs). Activation relies on additional signals including co-stimulatory molecules on the surface of the APCs that promote T cell expansion. The immune response is further sculpted by the cytokine environment. However, T cells also respond to other environmental signals including hormones, neurotransmitters, and vitamins. In this review, we summarize the mechanisms through which vitamins A and D impact immune responses, particularly in the context of T cell responses.
Core tip: Vitamins are organic compounds that are required in small quantities as nutrients. When used as dietary supplements, vitamins can be used to treat diseases and maintain physiological processes including bone and skin health. Vitamins also play important roles in immune system including lymphocyte activation, and T helper cell differentiation. Among all the vitamins, vitamin A and D have garnered more interest in clinical setting. In this review we have focused on how vitamin A and D regulate adaptive immunity especially how both the vitamins modulate T cell responses. We have highlighted the mechanisms how these vitamins affect T helper cell differentiation. How these vitamins affect inflammatory disorders including allergy and autoimmune diseases have also been described in this review. The purpose of this review is to suggest the potential of dietary supplements such as vitamin A and D can be used to treat inflammatory disorders.
- Citation: Goswami R, Kaplan MH. Essential vitamins for an effective T cell response. World J Immunol 2016; 6(1): 39-59
- URL: https://www.wjgnet.com/2219-2824/full/v6/i1/39.htm
- DOI: https://dx.doi.org/10.5411/wji.v6.i1.39
Vitamins are organic compounds required in small amounts in the human body. As humans cannot synthesize vitamins in sufficient quantity, essential vitamins are provided by diet or dietary supplements. In recent years vitamins have generated considerable clinical interest as they promote pivotal activities in the immune system. Vitamins modulate a wide range of immune responses including lymphocyte activation, homing, and T helper cell differentiation. An association of vitamin B6-deficiency and poor immune parameters has been observed, and a separate study has observed that vitamin B6 is important for normal T-cell function in vivo[1,2]. Systemic administration of vitamin B2 is used to treat septic shock[3]. Food supplementation of vitamin E is used for hepatitis B treatment[4]. The maintenance of healthy bones in inflammatory bowel disease might be enhanced by vitamin K[5]. Vitamins A and D modulate the immune system in very specific ways influencing both the adaptive and innate immune system. The bioactive metabolites of these vitamins have hormone-like properties, making them distinct from other vitamins. Vitamin D deficiency is recognized as a worldwide problem with health consequences including increased incidence of food allergy, while vitamin A deficiency has been acknowledged as a public health problem[6,7]. This review is aimed at highlighting the mechanisms of vitamin A- and D-dependent regulation of an optimal T cell response.
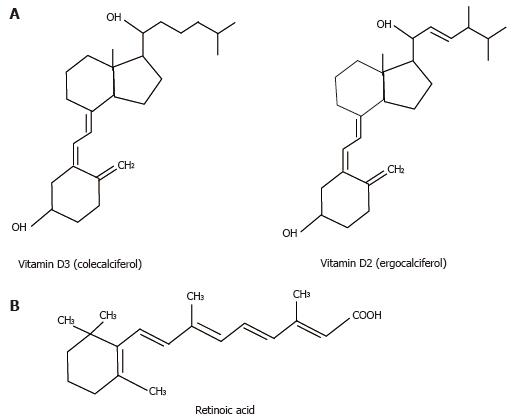
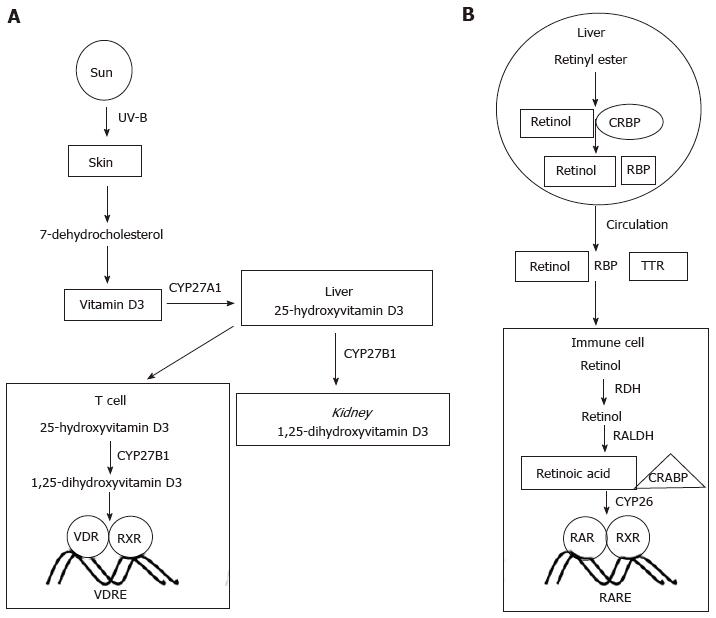
The term vitamin D refers to both vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol) (Figure 1A)[8]. Vitamin D is acquired either from diet or from conversion of 7-dehydrocholesterol in the sebaceous gland of animal skin upon exposure to sunlight, though levels generally decrease with aging[8,9]. To become biologically active, vitamin D undergoes several modification steps (Figure 2A). The enzymes CYP27A1, CYP2R1 among others are required for the formation of 1, 25-hydroxyvitamin D3 (1, 25D3), also known as calcitriol, the biologically active form of vitamin D[8]. CYP27B1 (one of many cytochrome P450 vitamin D hydroxylases) converts 25-hydroxyvitamin D (25-D) to 1, 25-dihydroxy vitamin D3 by 1α-hydroxylation[8,10]. CYP27B1 is found in placenta, monocytes and macrophages[11]. 1, 25D3 is released into circulation from kidney, an important organ of 1α-hydroxylation reaction[8,12]. Transport of vitamin D in blood is aided by vitamin D binding protein[11]. This protein does not affect the amount of 1, 25D3 entering cells or the synthesis of vitamin D target proteins[13]. Cultured alveolar macrophages can secrete 1, 25D3[14]. The level of circulating 25-hydroxyvitamin D is the defining parameter of vitamin D sufficiency or insufficiency. There is considerable debate though as to what should be considered as the cut-off level of required vitamin D in the healthy body. Even though some studies recommend concentrations of 20-30 ng/mL of 25-hydroxyvitamin D as an indicator of vitamin insufficiency, one study has argued that 20-50 ng/mL of 25-hydroxyvitamin D is normal[15,16]. Toxicity induced by high concentrations (> 150 ng/mL) of vitamin D is rare[17,18].
Metabolites of vitamin A include 11-cis-retinaldehyde and all-trans retinoic acid (atRA); also known as retinoic acid (RA) (Figure 1B)[19].The abundance of RA is regulated by retinol dehydrogenases and retinal dehydrogenases (RALDH), while degradation is controlled by CYP26[20]. Though Rdh5-deficient mice are viable, Rdh10 mutants exhibit characteristics similar to RA deficiency[21]. Carotenoids and retinoids are converted to RA in the intestine, liver and finally in target cells[22]. Proteins including cellular retinol-binding proteins (CRBP), cellular retinoic acid binding proteins (CRABP) and retinol-binding proteins play important role in the biochemical conversion of retinoic acid[23]. The key retinoid in circulation binds to retinol-binding protein 4 (RBP4)[24]. STRA6, a retinoic acid inducible gene, facilitates the uptake of RBP4 in tissues[25]. Retinol can be obtained from animal retinyl esters by hydrolysis. Unesterified retinol gets converted to retinyl ester by lecithin-retinol acyltransferase enzyme[22]. Free retinol binds to retinol-binding protein while in plasma it binds to transthyretin[26]. Inside the target cell retinol binds to CRBP to give rise to retinal which subsequently gets converted to RA by RALDH[27]. When RA binds to CRABP, RA translocates to nucleus where it can bind to different nuclear receptors. The synthesis of vitamin A is shown in Figure 2B.
1, 25D3, responsible for most of the biological actions of vitamin D binds to the vitamin D receptor (VDR), a member of the superfamily of the nuclear receptors[28]. While expressed at low to moderate levels in most human tissues and cell-types, the highest expression of VDR is found in kidney, bone and intestine[29]. The presence of a structurally conserved α-helical ligand-binding domain (LBD) and a DNA-binding domain are the hallmarks of the nuclear receptor superfamily[30,31]. A molecule having roughly the size of cholesterol and at nanomolar concentrations can activate VDR[32]. The LBD of ligand-binding receptors have a 400-1400 Å3 ligand binding pocket[33]. VDR forms a heterodimer with retinoid X receptor (RXR) in the presence of vitamin D[8]. This heterodimer is required for the recognition of a vitamin D response element (VDRE) that lies in the regulatory region of vitamin D target genes. The VDRE is found both in proximal promoter regions and in regions located far away from the promoter[34,35]. VDRE is composed of direct repeats (DRs) of PuG(G/T)TCA motifs that are separated by a 3 bp (DR3) or 6 bp or 8 bp separation of everted repeats (ER6 or ER8)[36-38]. The 3 bp spacing of DR3 allows a proper platform for VDR/RXR heterodimer formation that has been confirmed by genome-wide analyses for VDR binding sites[39]. However, VDR binding to DR3 covers approximately one third of all genomic binding sites suggesting additional mechanisms for VDR to associate with the genomic loci. These mechanisms include binding with unrecognized proteins and tying up to other transcription factors[39]. Therefore VDR would possibly recruit co-activators and co-repressors to gain access to its target genes. In addition to transactivation, VDR/RXR heterodimers can cause transrepression at their target genes[40,41].
Chromatin immunoprecipitation (ChIP) has been used extensively to analyze genome-wide binding of VDR[42]. The regulatory regions of VDR target genes including CYP24A1, CYP27B1 and CCNC were analyzed for VDR binding by ChIP[43-45]. More than 2500 genomic VDR-binding sites were observed when human lymphoblastoid cells were treated with vitamin D[46]. Similarly when human monocytes were stimulated with vitamin D, approximately 1200 VDR peaks were observed[47]. After 3 h stimulation with vitamin D, human colorectal cells show 1674 VDR-binding sites that co-localize with RXR[48]. ChIP-seq studies also confirm VDR-binding sites on vitamin D target gene including VDR and MYC[49,50]. When different cell lines and differences in ligand stimulation time were taken into account it was observed that roughly 20% of the genomic sites are identical in assessing VDR-binding sites[39]. It has been suggested that VDR may not bind regulatory genomic regions to open the gene loci as a “pioneer factor”, but rather VDR would act as an “accessory factor”, binding already accessible regions of a gene[39].
VDR and other nuclear receptor interactions with chromatin have been well described[51,52]. Recruitment of RNA pol II and chromatin modifying enzymes has been observed with VDR on human genes including CYP24A1 and MYC[43,50]. VDR-dependent changes in the transcriptome have been assessed by both mRNA and miRNA microarrays as well as by ChIP-seq[39,53]. ChIP-seq data suggested that VDR either has a very promiscuous DNA binding specificity, or that VDR interacts with other DNA-binding transcription factors that alter the DNA binding site[39].
The biologically active form of vitamin A, atRA binds to RXR and retinoic acid receptor (RAR). Both RAR and RXR have three subtypes: α, β, and γ; each encoded by separate gene[54]. Each subunit of the receptor has multiple isoforms[55]. RAR and RXR both belong to the retinoid receptor family. RARα, RXRα and RXRβ are constitutively expressed in mouse embryos[19]. Functional redundancy is observed between RAR and RXR isotypes[20]. The functional domains of retinoid receptor include the N-terminal DNA binding domain (DBD) and a C-terminal LBD separated by a central hinge region[55]. The DBD, a highly conserved domain, contains two zinc finger modules while the activation function-2 (AF-2) domain is part of the LBD[22]. RAR can be activated by atRA, while RXR is activated by 9-cis RA[22]. Ligand binding to LBD causes a conformational change in the receptor complex[22]. AF-2 also plays an important role in the conformational change[22]. RAR-RXR heterodimer binds to the DNA region known as retinoic acid response elements (RAREs) located in the enhancer regions of RA target genes[19]. Even though RA functions by binding to RAR, RA can also signal by binding to PPAR-β/γ, an orphan nuclear receptor[56].
The availability of retinoic acid is governed in a spatiotemporal and tissue-specific fashion[57]. Transcriptional regulation mediated by RA involves many co-activator and co-repressor proteins[19]. In the absence of ligand, the RAR/RXR heterodimer binds to a co-repressor complex containing nuclear receptor co-repressor (NCoR) 1 and 2 (known as SMRT), polycomb repressive complex 2, and proteins having histone deacetylase activity[19,58]. NCoR/SMRT contains two conserved consensus sequences[54]. Once ligand binding occurs (such as RA), the RAR/RXR heterodimer interaction with the co-regulatory protein is altered with increased binding towards co-activator proteins and reduced interactions with the co-repressor proteins. Co-activator proteins have multiple LXXLL motifs that interact with ligand-activated RAR/RXR heterodimers. Proteins including SRC complexes and histone acetyltransferase such as p300 can interact with RAR/RXR heterodimers[54,59]. In the presence of appropriate ligand, co-activators preferentially bind to RAR but not RXR. Functional RARE sites consist of hexameric direct repeats-(A/G)G(T/G)TCA with spacing of either 2 bp (DR2) or 5 bp (DR5)[19]. AGTTCA is the most efficient hexamer for RAR binding[60]. In vitro studies have suggested a role of DR1 as a RARE; however, the in vivo significance is not completely understood[61]. Protein poly (ADP-ribose) polymerase 1 (PARP-1), present on RAR-inducible promoters in cells, can bind directly to RAR and other mediators[62]. The events that determine whether a specific RARE will have activation or repression function are also not well understood.
VDR is located at chromosome 12q13.1 in human and its locus covers more than 100 kb[63]. The locus contains eight introns and nine exons[63]. In the VDR gene four restriction enzyme polymorphisms have been identified: ApaI (rs7975232), BsmI (rs1544410), FokI (rs10735810), and TaqI (rs731236)[63]. FokI is located in exon 2, ApaI and BsmI are located in intron between exon 8 and 9 and TaqI is located in exon 9[63]. There is no structural change in the VDR protein because of the ApaI, BsmI and TaqI single nucleotide polymorphisms[64]. The FokI polymorphism, however, results in a second protein variant[65]. Additionally, the FokI polymorphism has been associated with serum 1, 25D3 concentration in twins with multiple sclerosis[66]. In systemic lupus erythematosus (SLE) patients, an association between serum 1, 25D3 level and FokI polymorphism has been observed[67]. In contrast to vitamin D serum concentrations, there is no association between the FokI polymorphism and the clinical spectrum in SLE and multiple sclerosis (MS) patients[67,68]. However, the BsmI and TaqI polymorphisms are associated with risk of autoimmune diseases including MS, SLE[63]. VDR polymorphisms have also been associated with increased susceptibility to infections including human immunodeficiency virus-1, tuberculosis and hepatitis B virus[69]. Single nucleotide polymorphism (SNPs) in vitamin A and D receptor genes in healthy Caucasian children and adults correlate with the efficacy of vaccine-mediated immunity[70].
Vitamin A receptor allelic variations and haplotypes affect immune responses to measles vaccine[69]. A specific RARB haplotype (resulting from SNPs rs6550978 and rs6777544) was associated with variations in measles antibody titers and measles-specific IL-2 and IFN-γ secretion, while the RARB AAC haplotype was concurrently associated with variations in measles antibody titers, and IL-10, IFN-α, and TNF-α secretion in Caucasians[69]. Using ChIP-seq new RAR-binding loci has been identified along with diverse spacing and topology of DNA[71,72].
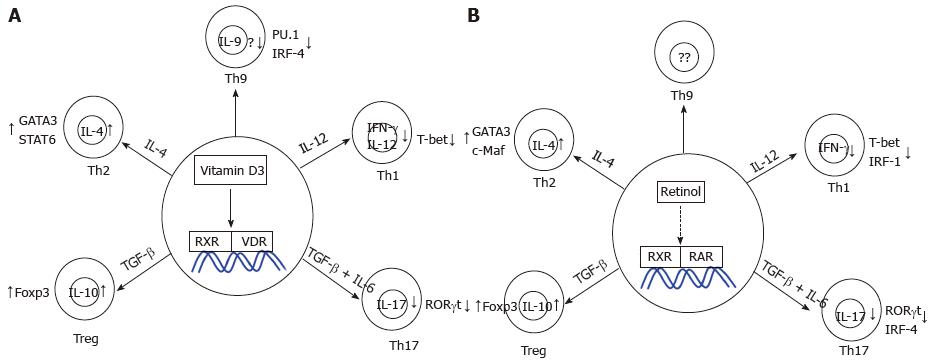
Nutrient status and metabolism affect CD4+ T cell development[73]. Both vitamin A and D control the expression of key players of innate and adaptive immune responses[74,75]. T cells are directly regulated by vitamin D via various mechanisms[76]. VDR expressed in T cells has high affinity for 1, 25D3, and the concentration of free 25-hydroxyvitamin D to antigen presenting cells regulates the balance between inflammatory and regulatory T cell responses[77,78]. Inhibitory responses has been observed in vitro on adaptive immune cells in the presence of vitamin D[79]. Activated T cells have increased VDR expression compared to naïve T cells[80]. Both increased and decreased VDR-dependent Foxp3 expression in murine model has been reported[81]. Murine Th1, Th2, and Th17 cells express significant Vdr transcripts, and the defined functions of VDR in these Th subsets is summarized below[81]. In the following sections we highlight the specific of vitamin D activity on each Th subset. The function of vitamin D in peripheral CD4+ T cells is summarized in Figure 3A.
Th1 cells are characterized by the production of IFN-γ following differentiation stimulated by IL-12. IFN-γ activates and enhances the microbicidal activity of macrophages, and as such Th1 cells provide immunity against intracellular bacteria, fungi and protozoa[82,83]. T-bet is a master regulator of Th1 cell development[84]. In addition, both STAT4 and STAT1, the signaling and transcription factors downstream of IL-12 and IFN-γ, respectively, promote Th1 cell differentiation[85,86]. IL-12 signaling also induces the transcription factor IRF-1, required for Th1 cell differentiation[87].
Several reports have demonstrated the effect of vitamin D in peripheral T cell responses. 1, 25D3 leads to an inhibitory effect on adaptive immune cells[79]. Cell proliferation is inhibited and the expression of IL-2, a T cell growth factor is attenuated in the presence of 1, 25D3[88,89]. Memory T cells that have abundant expression of VDR show profound inhibitory effect of 1, 25D3[90]. Vitamin D suppresses the development of Th1 cells while promoting Th2 cell differentiation[91]. 1, 25D3 directly inhibits IFN-γ production in vitro[92,93]. In vivo Th1 cell development is impaired in the presence of vitamin D that correlates with protection from inflammatory bowel disease[94]. Even though VDR-deficient animals have normal CD4+, and CD8+ cells; IFN-γ production by CD4+ T cells is significantly increased[95]. Vitamin D attenuates the production of IL-12 and IFN-γ from human leukocytes infected with Mycobacteria[96]. Mice lacking VDR augment IFN-γ responses and increase clearance rates of L. major in vivo[97]. The effects of the vitamin D response on host response to Candida infections is not completely understood though vitamin D deficiency results in enhanced replication of M. bovis, while addition of vitamin D suppresses tuberculosis replication[96,98,99]. In summary, vitamin D decreases IFN-γ production and IL-12 by DCs thereby attenuating Th1 responses.
Naïve T cells activated by antigen in the presence of IL-4 differentiate into Th2 cells that secrete IL-4, IL-5 and IL-13. Th2 cells provide immunity against extracellular parasites[100]. IL-4 acts as a positive feedback loop for Th2 differentiation, while IL-5 is eosinophil recruitment and development cytokine[101,102]. IL-13 plays a key role against parasite infections[103]. GATA3 acts as the master regulator of Th2 cells, while c-Maf expressed in Th2 cells is a potent transactivator of IL-4[104,105]. IL-4-induced STAT6 is also required for the development of Th2 cells[106,107].
In Th2 cells 1, 25D3 enhances the production of IL-4[93]. When injected subcutaneously in mice in an allergic asthma model, vitamin D increases allergen-induced T-cell proliferation, IL-4 and IL-13 and IgE production[108]. Surprisingly airway eosinophilia is attenuated following vitamin D administration[108]. 1, 25D3 polarizes human T cells and murine T cells to a Th2 phenotype[109]. With increased concentrations of vitamin D, STAT6 expression is up regulated in human PBMCs, and STAT6 is required for the therapeutic effects of 1, 25D3 in EAE[109]. When pregnant rats are supplemented with high dose of vitamin D, the offspring develop allergic responses due to altered Th1/Th2 ratios[110]. Patients with allergic bronchopulmonary aspergillosis have attenuated Aspergillosis-induced Th2 responses in the presence of vitamin D[111]. However, when vitamin D-deficient healthy females when undergo vitamin D supplementation there is significant difference in the expression of IL-4, IFN-γ, GATA-3, T-bet, STAT6 and STAT4[112]. Overall, vitamin D augments Th2 differentiation by increasing the secretion of IL-4, IL-5, IL-10 and Th2-specific transcription factors.
In the presence of TGF-β, IL-6 and IL-21, naïve CD4+ T cells differentiate into Th17 cells that produce IL-17, IL-21 and IL-22[113-115]. Th17 cells provide immunity to several extracellular pathogens including defense against infections from Candida, Citrobacter and Klebsiella[116,117]. The transcription factor RORγt is required for Th17 differentiation[118]. IRF4, an inducer of GATA3 in Th2 cells is also required for Th17 development[119].
Vitamin D suppresses Th17 cell responses partly by inhibiting IL-6 and IL-23 production, cytokines required for Th17 cell differentiation and commitment[120-123]. One report suggested that vitamin D can prevent experimental autoimmune uveitis owing in part to direct suppression of Th17 cells[124]. In vitro 1, 25D3 inhibits Th17 cytokine production via VDR but does not suppress Th17 gene transcription[125]. Mechanistically, 1, 25D3 induces the expression of C/EBP homologous protein that suppresses the production of Th17 cytokines[125]. The inhibitory effect of 1, 25D3 on IL-17A transcription has been attributed to competition with NFAT at the level of DNA binding, recruitment of histone deacetylase to the IL17A promoter and sequestration of Runx1 by 1, 25D3[126]. The same study has also demonstrated that 1, 25D3 represses mIl17a transcription by attenuating recruitment of the transcription factor Runx1 to the Il17a promoter[126]. 1, 25D3 also inhibits Th17 differentiation in both young asthmatic patients and healthy controls through a dendritic cell-dependent mechanism, inhibiting the expression of IL-17, IL-23R, RORC, and CCR6[127]. Overall, studies suggest Th17 cell polarization is attenuated by vitamin D.
Regulatory T cells (Tregs) control pro-inflammatory responses of effector Th cells and promote self-tolerance[128]. Naïve CD4+ T cells exposed to TGF-β develop into inducible Tregs in the periphery, and these cells have similar properties to natural Tregs, derived from the thymus including having suppressive functions[129,130]. The transcription factor Foxp3 has been identified as a reliable marker for Treg cells in both human and mice[131,132]. IL-10 signaling in Tregs maintains the expression of Foxp3 for immune regulation[133].
1, 25D3 induces the differentiation and expansion of Foxp3+ Treg cells through several mechanisms[122,134,135]. Immunoglobulin-like transcript 3, (a surface molecule expressed on DCs and macrophages) up-regulation by 1, 25D3 in DCs could induce the production of Treg cells, though this is likely dispensable for Treg induction[134]. When applied topically, vitamin D augments the suppressive capacity of CD4+CD25+ cells and CD4+ T cells from skin draining lymph nodes have reduced ability to proliferate in vitro[135]. With the help of IL-2, 1, 25D3 increases the frequency of activation-induced human Foxp3+ T cells[136]. There is increased number of CD4+CD25+Foxp3+ Treg cells and increased CTLA4 expression when CD4+CD25-T cells are cultured in the presence of vitamin D in vitro[136,137]. Vitamin D supplementation is associated with increased percentage of circulating CD4+ Treg cells in healthy individuals[138]. When administered orally, calcitriol induces Tregs and immature DCs via intestinal exposure while decreasing atherosclerosis in mice[139]. There is an in vivo correlation between vitamin D status and Treg suppressive function in relapsing-remitting multiple sclerosis patients[140]. However, there is a negative correlation between the Th1/Th2 ratio and 25-D level[140]. A VDRE is present in the human FOXP3 conserved non-coding sequence (CNS) that is homologous to the enhancer 1 region of the mouse Foxp3 gene and there is enhanced FOXP3 promoter activity in the presence of 1, 25D3[141]. Vitamin D also induces the development of IL-10-producing Treg cells; although, vitamin D does not affect IL-10R expression[142-144]. Monocyte-derived DCs pretreated with 1, 25D3 up-regulate the expression of PD-L1 and when co-cultured these DCs convert CD4+ T cells to IL-10-secreting Treg cells[145]. 1, 25D3 in the presence of dexamethasone induces the population of IL-10-secreting Treg cells in both murine and human CD4+ T cells[146]. Overall, Treg cell development is augmented in the presence of vitamin D.
Naïve CD4+ T cells in the presence of TGF-β and IL-4 differentiate into Th9 cells producing IL-9[147,148]. The transcription factors PU.1 and IRF4 are required for the development of Th9 cells[149,150]. The effect of vitamin D has also been ascertained in IL-9-secreting Th9 cells. 1, 25D3 inhibits the development of Th9 cells in mice concomitant with increased IL-10[151]. Vitamin D attenuates the production of IL-9 in purified memory T cells from healthy controls and patients with asthma[152]. However, vitamin D-mediated inhibition of IL-9 production by Th9 cells is not possible with anti-IL-10 treatment[152].
Vitamin D might regulate Th cell development and function through additional mechanisms that include direct regulation of gene expression by vitamin receptors, regulation of miRNAs, and by altering epigenetic regulatory mechanisms. miRNAs control effector T cell differentiation and function[153]. Vitamin D-mediated regulation of T helper cells via modulating miRNA has not been studied in great detail. Vitamin D induces the expression of miR-22 in colon cancer cells that can potentially be therapeutic[154]. In addition, vitamin D-mediated regulation of miRNA has been established in bone[155]. T helper cell differentiation is also regulated by epigenetic modifications[156]. Vitamin D-mediated signaling via VDR entails both histone acetyltransferase and histone deacetylase (HDAC)[157]. Histone and DNA methylation are also regulated by vitamin D[158]. Vitamin D prevents the migration of T helper cells into CNS thereby attenuating experimental autoimmune encephalomyelitis (EAE)[159].
Retinoic acid affects specific adaptive immune responses and elicits effector CD4+ T cell responses[79,160,161]. Retinoic acid augments T-cell proliferation by inducing IL-2 secretion[162]. RA induces gut-homing receptors on T cells[163]. Depleting vitamin A significantly attenuates the number of IgA-secreting cells in rat ileum[164]. Vitamin A-deficient mice demonstrate defective Th-cell activity[165]. Retinoic acid augments the expression of gut-homing molecules α4β7 and CCR9 on CD4+ T cells maintaining intestinal homeostasis[166]. In the following sections we highlight the specific of retinoic acid on each Th subset. The function of retinoic acid on T helper cells is summarized in Figure 3B.
Th1 cells: Vitamin A deficiency leads to enhanced production of IFN-γ-secreting Th1 cells[167]. Retinoic acid inhibits Th1 cell development by attenuating IFN-γ production directly via RAR but not RXR[168]. In RA-treated immunized mice there is reduced expression of Th1-specific transcription factors T-bet and IRF-1[169]. RA also attenuates the expression of STAT4 and T-bet in a mouse model of autoimmune insulitis[170]. Whether atRA can regulate Th1/Th2 balance in a strong Th1 environment has been assessed[171]. In Gata3-deficient CD4+ T cells, atRA induces the expression of IL-4 while reducing the secretion of IFN-γ[171]. atRA abrogates the induction of Th1 cells in spleen and lymph nodes in vivo[172]. In RARα-transfected Jurkat cells, atRA significantly down-regulates Ifng promoter activity[173]. However, a report published this year argues RA is important for Th1 cell stability while preventing a switch to Th17 cells[174]. RA is required for STAT4-dependent T-bet expression in Th1 cells[174]. At Th1 lineage associated loci, RA-RARα regulates enhancer activity[174]. Overall, retinoic acid inhibits the production of Th1 cytokines both in vitro and in vivo.
Th2 cells: Vitamin A-deficient mice demonstrate poor antibody responses owing to significant decreases in Th2-cell frequency[165]. In the absence of exogenous cytokines, atRA significantly attenuates IFN-γ production but does not change IL-4 production[175]. When Th2-polarizing cytokines are present, atRA significantly enhances IL-4 secretion in the presence of antigen-presenting cells[175]. atRA enhances the transcription of Th2-associated genes including Gata-3, c-Maf and IL-4Rα in murine CD4+ T cells[168]. atRA has also been shown to have a similar Th2-promoting effect on human MBP-specific T cell lines in vitro[176]. A recent report suggested that in vitamin A-deficient mice, mesenteric lymph node DCs induce a Th subset producing high IL-13 and TNF-α[177]. Induction of this specific subset was mediated by B220-CD8α-CD103-CD11b+ DCs and was dependent on IL-6 and OX40L but inhibited by RA[177]. Both atRA and 9-cis RA are equally potent in promoting human Th2 cell development[178]. The role of specific retinoic acid receptors in either Th1 or Th2-mediated disease pathology has remained unclear. RARα induces human Th2 cytokine production that correlates with the expression of CD38 and CD69[179]. Similarly, RAR agonists, Am80 and Tp80 mimic the effect of RA[168]. However, one study reported that Am80 did not have any effect on IFN-γ production by Th1 clones[180]. atRA enhances the percentage of IL-5+ Th2 cells while RARα antagonist Ro415253 reduces the percentage of IL-5+ Th2 cells from house dust mite specific short-term cell lines[181]. Therefore, the studies indicate that retinoic acid promotes Th2 differentiation and maintains Th1-Th2 balance.
Th17 cells: atRA and RARα suppress the formation of Th17 cells and induce the production of Foxp3+ Treg cells[182,183]. The atRA-mediated inhibition of Th17 cells is independent of IL-2, STAT3 and STAT5 to overcome the effect of IL-6; however some initial IL-2 signaling may be required for the regulatory function of atRA[182,183]. In Th17 cells, RA attenuates the expression of TGF-β-induced IL-6Rα and the transcription factor IRF4[184]. RA also suppresses Th17 cell development from Th1 precursors[174]. In the absence of RA signaling there is increased expression of Th17-specific genes including Rorc, Il23r, Il22, Il21[174]. Overall, retinoic acid blocks the development of Th17 cells by down-regulating RORγt expression.
Treg cells: atRA plays an important role in immune tolerance via induction of Treg cells. In the presence of RA there is an induction of Foxp3+ Treg cells in murine CD4+ T cells[184]. 1, 25D3 augments dexamethasone-induced expression of IL-10 by Tregs[185]. There is increased expression and phosphorylation of the TGF-β-induced molecule Smad3 but not Smad2 in the presence of RA[184]. RA can override the negative effect of co-stimulatory molecules and specific secreted cytokines in enhancing the conversion of naïve T cells to Treg cells[186]. Even though there is RA-mediated induction of Smad3, enhanced Treg conversion is Smad3-independent[186]. The same study demonstrated that RARα, but not RARα1 deficiency blocks Treg cell conversion[186]. TGF-β also induces ERK1/2-signaling pathway and Smad3 and ERK1/2 have complex interaction that is cell type-specific[187]. In human Tregs generated from cord blood ERK1/2 signaling is impaired, while in relapsing-remitting multiple sclerosis patients the activity of ERK1/2 is altered[188,189]. In contrast, ERK1/2 signaling is activated by atRA leading to enhanced Foxp3 expression[190]. atRA induces CTLA-4, part of the T-cell co-stimulatory signal that differentially regulates ERK signaling pathway and is required by TGF-β to generate CD4+CD25+ Tregs[191-193]. For the development of human inducible Tregs (iTregs), the addition of atRA is required, even though atRA does not affect proliferation or survival of human CD4+ T cells[194,195]. After atRA treatment, human natural Tregs (nTregs) become resistant to conversion to Th1 and Th17 cells with sustained expression of Foxp3[191]. atRA induces the expression of Foxp3+Tregs from naïve CD4+ T cells but not from memory CD4+ T cells[195]. Maintenance of Foxp3 expression during nTreg expansion is mediated by atRA[196]. Even in the presence of inflammatory cytokines, atRA-pretreated Tregs maintain their suppressive capabilities[191]. Mechanistically atRA increases histone acetylation and methylation of Foxp3 promoter as well as conserved non-coding sites at the gene promoter[190]. In nTregs, atRA can inhibit methylation of Foxp3 in the presence of inflammatory cytokines[191]. In contrast to Th17 cells, retinoic acid induces the development of Foxp3+ Tregs indicating the reciprocal regulation between Th17 and Treg cells. Overall, the potential role of retinoic acid in peripheral T cells is described in Figure 3.
Retinoic acid has been shown to mediate epigenetic changes in embryonic stem cells[197]. Retinoic acid induces the expression of specific microRNAs and their precursors in human AML and in neuroblastoma cells[198,199]. miR-10a, which can act as tumor suppressor in breast cancer is also induced by retinoic acid and TGF-β altering the plasticity of T helper cells in process[200,201].
Therefore, both vitamin D and retinoic acid could potentially regulate T helper cell differentiation via epigenetic modification and miRNA.
Both vitamin D and retinoic acid have been implicated in allergy and autoimmune diseases[81,202]. This section highlights the role of both vitamins in allergy and autoimmune diseases such as EAE and type 1 diabetes (T1D).
As both the prevalence of asthma and vitamin D deficiency has been increased it has been hypothesized that both of these parameters might be linked[203,204]. A cross-sectional study from the Third National Health and Nutrition Examination Survey suggests that serum vitamin D level is positively associated with FEV-1, the maximum amount of air to be expelled during the first second of forced expiration[205]. In Chinese adults with asthma, vitamin D deficiency has been associated with reduced lung function[206]. Low vitamin D levels have also been associated with markers of allergy and asthma severity[207]. Corticosteroids that are used to treat asthma patients function better in the presence of vitamin D, suggesting that low vitamin D concentrations might also impair therapy[208]. Serum 25-D level of less than 30 ng/mL has been associated with impaired lung function, airway hyper responsiveness and increased asthma exacerbations[209]. Vitamin D deficiency in children with asthma being treated with inhaled corticosteroids is associated with reduced lung function[209]. However, one study did not find any correlation between the level of vitamin D and airway inflammation in a group of asthmatic children[210]. However, vitamin D supplementation has not been suggested as a therapeutic in patients with symptomatic asthma[211]. A case control study performed in asthmatic children observed a possible association of vitamin D sufficiency status and VDR gene polymorphism[212]. Impaired maternal 1, 25D3 level during pregnancy impairs the production of Treg cells[213]. Even though most cohort studies have suggested that there is inverse correlation between maternal vitamin D level and early childhood risk of allergic sensitization, this is not consistent in all studies[214-216]. A recent study argued that neither serum 25-D level nor dietary intake of calcitriol is associated with allergy[217].
Retinoic acid-treated rabbit tracheal epithelial cells lead to enhanced mucin gene expression suggesting the potential role of retinoic acid in asthma[218]. Normal bone marrow cultures treated with atRA suppress eosinophil and basophil differentiation[219]. Furthermore, human bronchial epithelial cell line BEAS-2B treated with RA, abrogates IL-4-induced production of eotaxin, an eosinophil chemoattractant[220]. The transrepression of eotaxin is not due to altered STAT6 binding or AP-1 activity[220]. atRA reduces total cell counts and inflammatory cells in BAL fluid in asthmatic rats which could be due to reduced expression of NF-κB and ICAM1[221]. RA also inhibits human bronchial smooth muscle cell migration by modulating PI3K/Akt pathway and is associated with inhibition of platelet- derived growth factor-induced actin migration[222]. In order to prevent atRA catabolism and clearance, liposome encapsulated atRA has been developed. When liposome-encapsulated atRA is administered intraperitoneally, OVA-induced IgE production and accumulation of IL-5 is increased[223]. However, when administered intranasally those parameters were not affected[223]. When inhaled atRA is given to a patient suffering from severe emphysema, inflammation and lung destruction are reduced[224]. In mouse models of allergic inflammation, atRA attenuates the asthmatic phenotype by inhibiting both Th2 and Th17 responses[225,226]. In contrast, the expression of Foxp3 is not altered after atRA treatment in the spleen of the asthmatic mice[226]. In an allergic asthma model when mice are treated with RAR inhibitor to block RA signaling the number of Foxp3+ cells were reduced in vivo[227]. A study has shown that atRA upregulates the expression of ORMDL3, a childhood asthma onset candidate gene via PKC/CREB pathway[227].
Autoimmune diseases: The function of vitamin D and retinoic acid have been studied in EAE (mouse model of multiple sclerosis) and T1D[228].
EAE: When naïve T cells with a TCR specific for myelin basic protein are transferred to recipient mice that are immunized later with MBP, recipients do not have EAE symptoms when they are treated with 1, 25D3[229]. This effect is mediated via VDR in T cells[230,231]. Peripheral CD4+ T cell priming and myelin-specific T cell trafficking remain unchanged after vitamin D treatment owing to an anergic T cell phenotype in vitamin D-treated groups[229,230]. A reduced number of Th1 cells in the periphery could also contribute to the therapeutic effect of vitamin D in treating EAE[232]. Vitamin D administered to rodents correlated with fewer CD4+ T cells in CNS, attenuated CNS pathology and reduced IFN-γ-production[81]. In healthy volunteers, vitamin D decreases the percentage of Th1 cells in circulation[233]. In contrast, it has been reported that vitamin D deficiency actually delays the onset of EAE[234]. Oral vitamin D treatment in healthy volunteers and in MS patients does not have a significant effect on the level of circulating IFN-γ[235,236].
Apart from Th1 cells, development of Th17 cells is affected by vitamin D in EAE. In EAE, there are reduced numbers of Th17 cells in the CNS after vitamin D treatment[237]. There are fewer Th17 cells in spleen in vitamin D-treated mice compared to placebo controls[125,238]. Vitamin D can either inhibit Th17 cytokine production or can suppress Il17 gene transcription in EAE[125,126]. When human CD4+ T cells are treated with vitamin D, the frequency of Th17 cells are attenuated, whereas increased doses of vitamin D reduce the proportion of Th17 cells in peripheral blood[137,233]. However, vitamin D supplementation may not affect Th17 cells in MS patients[239]. Th2 cells might also play a role in EAE[240]. Disruption of IL-4 slightly reduces the protective function of vitamin D in EAE, and there are increased IL-4 transcripts in the lymph nodes when vitamin D is administered before EAE induction[241,242].
In autoimmune diseases including MS there are defects in CD4+Foxp3+ Treg cells in peripheral blood cells and thymus[243-245]. There is correlation between percentage of Tregs and 25-D level as well as correlation of suppressive capacity of CD4+CD25+ Treg cells with serum 25-D level[239,246]. Seasonal increases in 25-D and 1, 25D3 in healthy volunteers correlate with enhanced Foxp3+ Treg expression[247].
Retinoic acid suppresses EAE in rodents[176,248]. Production of IL-4 is correlated with better disease outcome when retinoic acid is used to treat EAE, while the level of IFN-γ, TNF-α, and IL-2 mRNA is attenuated[249]. Intraperitoneal injection of high dose of atRA protects animals from developing EAE and is associated with reduced IFN-γ and IL-17 production[184]. When synthetic retinoid AM80 is administered orally, early development of EAE is ameliorated concomitant with reduced Th17 cell differentiation[250]. The amelioration of EAE is mediated by down-regulation of MHC II molecules, CD80 and CD86 on the surface of DCs that results in altered polarization of both Th1 and Th17 cells[172]. As an additional potential mechanism, one report has suggested AM80 induces MDSCs thereby increasing the severity of EAE[251]. When atRA is combined with atorvastatin, a synergistic effect is observed in ameliorating EAE[252]. Tributylin, which activates RXR delays the onset of EAE[253]. Ligands for PPARγ and RXR generate additive anti-inflammatory effects on EAE[254]. The decrease of pro-inflammatory cytokine production following helminth infection in multiple sclerosis patients is dependent on RA[255].
T1D: T1D is an autoimmune disorder where the immune system attacks the insulin-producing beta cells in the pancreas. The relationship between T1D and vitamin D is still not completely understood as most of the studies have been observational. Studies have observed that vitamin D supplementation may improve insulin sensitivity whereas, vitamin D deficiency has been suggested to affect the risk of T1D[256]. People diagnosed with T1D have a reduced level of vitamin D[257]. Approximately 15%-90% prevalence of vitamin D deficiency in patients with T1D has been reported[258-260]. A steady dose of vitamin D could reduce the risk of T1D[261,262]. Lower serum vitamin D level and insufficient supplementation during infancy may enhance the risk of T1D[263]. In addition, genetic association of VDR and age of onset of T1D has been assessed[264-266]. One progressive study observed no association between diabetes progression and vitamin D level[267]. Vitamin D deficiency during pregnancy may increase the incidence of T1D[268]. However, whether vitamin D intake during pregnancy would prevent children from developing T1D is not clear due to contradictory results[269]. A reduced level of vitamin D has been observed in children with T1D and multiple islet autoantibodies[270]. Both 1, 25D3 and its analog TX527 suppress effector cytokine production and induce the production of IL-10-secreting CD4+CD25hiCD127lo T cells with regulatory function[271]. In NOD mice 1, 25D3 establishes tolerogenic DCs which are responsible for Treg expansion and IL-10 production[272]. In the presence of vitamin D there is increased frequency of Tregs in the pancreatic lymph node in NOD mice[273]. Overall these results suggest that vitamin D deficiency correlates with increased risk of T1D. Thus vitamin D could help in managing some spectrum of the disease.
RA has been shown to attenuate inflammation in diabetic rat kidney and reduced proteinuria in diabetic rats[274]. In NOD mice with insulitis, atRA treatment suppresses diabetes associated with reduced IFN-γ-producing T cells[170]. However, the percentages of IL-17- or IL-4-secreting cells are not affected. When CD8+ T cells from 8.3-NOD splenocytes are cultured in the presence of atRA and TGF-β, an induction of Foxp3+ CD8+ T cells is observed that suppress diabetogenic T cells both in vitro and in vivo[275]. atRA and exendin-4 (which increases beta-cell mass and function) treatment before the onset of T1D prevents and reverses the disease; however, after the onset of the disease reversal of hyperglycemia or improved survival is not observed[276]. The suppression of T1D by retinoids is dependent on the presence of Tregs[277].
As vitamin D deficiency is endemic and associated with various diseases, it is no surprise that vitamin D has its uses in the clinical practice. As previously mentioned, vitamin D aids in the suppression of allergic inflammation and autoimmune diseases including T1D and multiple sclerosis. A meta-analysis of 18 randomized controlled trials have suggested that even at a low dose vitamin D significantly reduces mortality[278]. Subsequent studies have indicated that the statin class of drug could attenuate mortality rate by enhancing the level of 25-D[279]. A 30% increase in the baseline dose of 25-D reduces internal cancer by 60%, while the level of serum 25-D is an independent predictor of cancer risk[280]. Vitamin D attenuates the incidence of respiratory infections in children[281]. Vitamin D supplementation in food has been suggested to decrease the risk of autism[282]. Deficiency of vitamin D has been associated with increased risk of multiple chronic disorders[283,284]. Vitamin D is recommended to treat osteoporosis[285]. Seasonal deficiency of antimicrobial peptide (AMP) is secondary to vitamin D seasonal deficiency and could be linked, to flu epidemics[281]. Interestingly though vitamin D-sensitive AMPs inhibit HIV virus but vitamin D has been suggested play a role in the pathogenesis of HIV[286]. Vitamin D could provide respite against invasive bacterial diseases including meningococci and pneumococci. A therapeutic effect of vitamin D is also seen in non-small-cell lung cancer patients[287]. Consistent with its anti-inflammatory property, vitamin D enhances the life span in mouse model of SLE and inhibits contact hypersensitivity[288,289]. Vitamin D has also been successfully used to treat psoriasis[290]. It has also been suggested that vitamin D can potentially be used during transplantation. In rat model of renal transplantation vitamin D and its analogue prevents chronic allograft rejection while vitamin D delayed chronic allograft rejection in mouse model of aortic transplantation[291,292]. Intriguingly, VDR polymorphisms are associated with increased GVHD in bone-marrow transplantation patients[293]. It has been noted that the vitamin D analogue may be safer alternative to vitamin D-mediated immune modulation owing to its adverse effect in bone resorption and hypercalcemia[91].
Retinoic acid also has multiple clinical applications. A correlation between vitamin A supplementation and reduced diarrhea and mortality in HIV-infected children has been reported[294,295]. Retinoic acid has been suggested to induce terminal cellular differentiation in tumor cells[296]. Vitamin A has been demonstrated to maintain natural killer cell numbers that play important role in anti-tumor immunity[297]. atRA treatment doubles the survival rate of acute promyelocytic leukemia (APL) patients[298]. In the presence of HDAC inhibitor sodium phenylbutyrate, atRA induces remission in APL patient[299]. In addition, atRA along with trichostatin A, another HDAC inhibitor demonstrated significant inhibition of tumor growth associated with increased level of RARβ2 level[300]. RARα proteins play crucial role in the attenuating APL[54]. RA also initiates growth arrest of leukemia-initiating cells in APL mouse model[301]. Retinoids have been suggested to induce irreversible differentiation of cancer stem cells[54]. Apart from cancer treatment, retinoic acid has also been suggested to treat autoimmune inflammation including EAE, adjuvant arthritis and experimental nephritis[180,249,302]. Retinoic acid has also been used for treating atopic dermatitis and psoriasis[303,304].
There is a paucity of data on the role of vitamin C, E, and K on adaptive immune system including the function in Th cell development. We have highlighted the role of vitamin D and retinoic acid on CD4+ T cells and their role in inflammatory disorders, but other vitamins likely also impact many of the responses we have described. Vitamin D-mediated paracrine signaling to CD4+ T cells affects its immunoregulatory mechanisms. The sources of vitamin D and the signals required to induce and inhibit vitamin D in vivo is crucial to the understanding of its role in health issues. Because of the use of common receptor RXR, vitamin D and retinoic acid could potentially oppose each other’s contribution in the development of T helper cells and modulation of inflammatory responses.
A better understanding of the role of vitamin D in controlling effector CD4+ T cells could be achieved using animals that have T-cell-specific deletion of VDR. A study has demonstrated that vitamin D in combination with IL-2 induce the production of CTLA-4-expressing Treg cells[136]. CTLA-4 regulates the spectrum of T cell responses and CTLA-4 deficiency increases follicular T helper cells (Tfh cell) numbers[305]. Vitamin D also up-regulates the expression of Helios and CD200, other markers of Tfh cells[81,306]. Whether vitamin D plays a role in the differentiation of Tfh cells has not been ascertained. Consumption of foods enriched with specific vitamins could alter the microbiota in our body leading to better immune responses.
There is ample indication that both vitamin D and retinoic acid could be used as future therapeutics to treat inflammatory diseases. Vitamin D and oral mycophenolate mofetil lead to tolerance and acceptance of fully mismatched mouse islet isograft associated with increased frequency of CD4+CD25+CD152+ Tregs in a transplant model[307]. Mucosal vaccination might be aided by retinoic acid while vitamin D could be applied in transplant patients[308]. The role of vitamin D in cardiovascular disease remains unclear. The function of retinoic acid is also not explored in other inflammatory disease pathologies. However, the challenge remains in translating promising animal model results into humans. Once established, purchasing vitamins over the counter could be a safe and efficient approach to modifying immune responses.
Dietary supplementation of vitamin D and retinoic acid can be used to treat certain diseases; Skin, bone, dental health, cognitive function, and other physiological processes are maintained by vitamin D and retinoic acid; both vitamins A and D regulate immune system by affecting CD4+ T helper cell differentiation and lymphocyte activation; allergy and autoimmune diseases such as type I diabetes and EAE can be modulated indirectly by vitamin D and retinoic acid; Both vitamin D and retinoic acid have clinical implications for treating various inflammatory disorders.
P- Reviewer: Martinez-Lostao L, Webb TJ S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Baum MK, Mantero-Atienza E, Shor-Posner G, Fletcher MA, Morgan R, Eisdorfer C, Sauberlich HE, Cornwell PE, Beach RS. Association of vitamin B6 status with parameters of immune function in early HIV-1 infection. J Acquir Immune Defic Syndr. 1991;4:1122-1132. [PubMed] |
| 2. | Ha C, Miller LT, Kerkvliet NI. The effect of vitamin B6 deficiency on cytotoxic immune responses of T cells, antibodies, and natural killer cells, and phagocytosis by macrophages. Cell Immunol. 1984;85:318-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Toyosawa T, Suzuki M, Kodama K, Araki S. Effects of intravenous infusion of highly purified vitamin B2 on lipopolysaccharide-induced shock and bacterial infection in mice. Eur J Pharmacol. 2004;492:273-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Andreone P, Fiorino S, Cursaro C, Gramenzi A, Margotti M, Di Giammarino L, Biselli M, Miniero R, Gasbarrini G, Bernardi M. Vitamin E as treatment for chronic hepatitis B: results of a randomized controlled pilot trial. Antiviral Res. 2001;49:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 56] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Iijima H, Shinzaki S, Takehara T. The importance of vitamins D and K for the bone health and immune function in inflammatory bowel disease. Curr Opin Clin Nutr Metab Care. 2012;15:635-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S-1086S. [PubMed] |
| 7. | Suaini NH, Zhang Y, Vuillermin PJ, Allen KJ, Harrison LC. Immune Modulation by Vitamin D and Its Relevance to Food Allergy. Nutrients. 2015;7:6088-6108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 8. | White JH. Vitamin D metabolism and signaling in the immune system. Rev Endocr Metab Disord. 2012;13:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 174] [Article Influence: 13.4] [Reference Citation Analysis (1)] |
| 9. | Kumar J, Muntner P, Kaskel FJ, Hailpern SM, Melamed ML. Prevalence and associations of 25-hydroxyvitamin D deficiency in US children: NHANES 2001-2004. Pediatrics. 2009;124:e362-e370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 434] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 10. | Prosser DE, Jones G. Enzymes involved in the activation and inactivation of vitamin D. Trends Biochem Sci. 2004;29:664-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 456] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 11. | Christakos S, Ajibade DV, Dhawan P, Fechner AJ, Mady LJ. Vitamin D: metabolism. Endocrinol Metab Clin North Am. 2010;39:243-253, table of contents. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 310] [Cited by in RCA: 268] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 12. | Jones G, Strugnell SA, DeLuca HF. Current understanding of the molecular actions of vitamin D. Physiol Rev. 1998;78:1193-1231. [PubMed] |
| 13. | Zella LA, Shevde NK, Hollis BW, Cooke NE, Pike JW. Vitamin D-binding protein influences total circulating levels of 1,25-dihydroxyvitamin D3 but does not directly modulate the bioactive levels of the hormone in vivo. Endocrinology. 2008;149:3656-3667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 14. | Adams JS, Gacad MA. Characterization of 1 alpha-hydroxylation of vitamin D3 sterols by cultured alveolar macrophages from patients with sarcoidosis. J Exp Med. 1985;161:755-765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 201] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2655] [Cited by in RCA: 2830] [Article Influence: 202.1] [Reference Citation Analysis (0)] |
| 16. | Thomas MK, Lloyd-Jones DM, Thadhani RI, Shaw AC, Deraska DJ, Kitch BT, Vamvakas EC, Dick IM, Prince RL, Finkelstein JS. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338:777-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1002] [Cited by in RCA: 897] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 17. | Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9399] [Cited by in RCA: 9400] [Article Influence: 522.2] [Reference Citation Analysis (1)] |
| 18. | Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr. 1999;69:842-856. [PubMed] |
| 19. | Cunningham TJ, Duester G. Mechanisms of retinoic acid signalling and its roles in organ and limb development. Nat Rev Mol Cell Biol. 2015;16:110-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 431] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 20. | Niederreither K, Dollé P. Retinoic acid in development: towards an integrated view. Nat Rev Genet. 2008;9:541-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 543] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 21. | Rhinn M, Schuhbaur B, Niederreither K, Dollé P. Involvement of retinol dehydrogenase 10 in embryonic patterning and rescue of its loss of function by maternal retinaldehyde treatment. Proc Natl Acad Sci USA. 2011;108:16687-16692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 22. | Das BC, Thapa P, Karki R, Das S, Mahapatra S, Liu TC, Torregroza I, Wallace DP, Kambhampati S, Van Veldhuizen P. Retinoic acid signaling pathways in development and diseases. Bioorg Med Chem. 2014;22:673-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 193] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 23. | Bushue N, Wan YJ. Retinoid pathway and cancer therapeutics. Adv Drug Deliv Rev. 2010;62:1285-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 255] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 24. | Rhinn M, Dollé P. Retinoic acid signalling during development. Development. 2012;139:843-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 624] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 25. | Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge J, Ping P, Wiita P, Bok D, Sun H. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science. 2007;315:820-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 562] [Cited by in RCA: 599] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 26. | Noy N, Slosberg E, Scarlata S. Interactions of retinol with binding proteins: studies with retinol-binding protein and with transthyretin. Biochemistry. 1992;31:11118-11124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Duester G. Families of retinoid dehydrogenases regulating vitamin A function: production of visual pigment and retinoic acid. Eur J Biochem. 2000;267:4315-4324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 426] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 28. | Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: opening the X-files. Science. 2001;294:1866-1870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1535] [Cited by in RCA: 1498] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 29. | Verstuyf A, Carmeliet G, Bouillon R, Mathieu C. Vitamin D: a pleiotropic hormone. Kidney Int. 2010;78:140-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 241] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 30. | Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5272] [Cited by in RCA: 5176] [Article Influence: 172.5] [Reference Citation Analysis (0)] |
| 31. | Rochel N, Wurtz JM, Mitschler A, Klaholz B, Moras D. The crystal structure of the nuclear receptor for vitamin D bound to its natural ligand. Mol Cell. 2000;5:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 657] [Cited by in RCA: 593] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 32. | Molnár F, Peräkylä M, Carlberg C. Vitamin D receptor agonists specifically modulate the volume of the ligand-binding pocket. J Biol Chem. 2006;281:10516-10526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Nagy L, Schwabe JW. Mechanism of the nuclear receptor molecular switch. Trends Biochem Sci. 2004;29:317-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 285] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 34. | Wang TT, Tavera-Mendoza LE, Laperriere D, Libby E, MacLeod NB, Nagai Y, Bourdeau V, Konstorum A, Lallemant B, Zhang R. Large-scale in silico and microarray-based identification of direct 1,25-dihydroxyvitamin D3 target genes. Mol Endocrinol. 2005;19:2685-2695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 421] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 35. | Kim S, Yamazaki M, Zella LA, Shevde NK, Pike JW. Activation of receptor activator of NF-kappaB ligand gene expression by 1,25-dihydroxyvitamin D3 is mediated through multiple long-range enhancers. Mol Cell Biol. 2006;26:6469-6486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 157] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 36. | Lin R, White JH. The pleiotropic actions of vitamin D. Bioessays. 2004;26:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 188] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 37. | Thompson PD, Jurutka PW, Whitfield GK, Myskowski SM, Eichhorst KR, Dominguez CE, Haussler CA, Haussler MR. Liganded VDR induces CYP3A4 in small intestinal and colon cancer cells via DR3 and ER6 vitamin D responsive elements. Biochem Biophys Res Commun. 2002;299:730-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 88] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | Tavera-Mendoza L, Wang TT, Lallemant B, Zhang R, Nagai Y, Bourdeau V, Ramirez-Calderon M, Desbarats J, Mader S, White JH. Convergence of vitamin D and retinoic acid signalling at a common hormone response element. EMBO Rep. 2006;7:180-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 39. | Carlberg C, Campbell MJ. Vitamin D receptor signaling mechanisms: integrated actions of a well-defined transcription factor. Steroids. 2013;78:127-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 206] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 40. | Takeuchi A, Reddy GS, Kobayashi T, Okano T, Park J, Sharma S. Nuclear factor of activated T cells (NFAT) as a molecular target for 1alpha,25-dihydroxyvitamin D3-mediated effects. J Immunol. 1998;160:209-218. [PubMed] |
| 41. | An BS, Tavera-Mendoza LE, Dimitrov V, Wang X, Calderon MR, Wang HJ, White JH. Stimulation of Sirt1-regulated FoxO protein function by the ligand-bound vitamin D receptor. Mol Cell Biol. 2010;30:4890-4900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 42. | Orlando V. Mapping chromosomal proteins in vivo by formaldehyde-crosslinked-chromatin immunoprecipitation. Trends Biochem Sci. 2000;25:99-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 345] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 43. | Väisänen S, Dunlop TW, Sinkkonen L, Frank C, Carlberg C. Spatio-temporal activation of chromatin on the human CYP24 gene promoter in the presence of 1alpha,25-Dihydroxyvitamin D3. J Mol Biol. 2005;350:65-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 109] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 44. | Turunen MM, Dunlop TW, Carlberg C, Väisänen S. Selective use of multiple vitamin D response elements underlies the 1 alpha,25-dihydroxyvitamin D3-mediated negative regulation of the human CYP27B1 gene. Nucleic Acids Res. 2007;35:2734-2747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 45. | Sinkkonen L, Malinen M, Saavalainen K, Väisänen S, Carlberg C. Regulation of the human cyclin C gene via multiple vitamin D3-responsive regions in its promoter. Nucleic Acids Res. 2005;33:2440-2451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 46. | Ramagopalan SV, Heger A, Berlanga AJ, Maugeri NJ, Lincoln MR, Burrell A, Handunnetthi L, Handel AE, Disanto G, Orton SM. A ChIP-seq defined genome-wide map of vitamin D receptor binding: associations with disease and evolution. Genome Res. 2010;20:1352-1360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 684] [Cited by in RCA: 625] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 47. | Heikkinen S, Väisänen S, Pehkonen P, Seuter S, Benes V, Carlberg C. Nuclear hormone 1α,25-dihydroxyvitamin D3 elicits a genome-wide shift in the locations of VDR chromatin occupancy. Nucleic Acids Res. 2011;39:9181-9193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 178] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 48. | Meyer MB, Goetsch PD, Pike JW. VDR/RXR and TCF4/β-catenin cistromes in colonic cells of colorectal tumor origin: impact on c-FOS and c-MYC gene expression. Mol Endocrinol. 2012;26:37-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 165] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 49. | Zella LA, Meyer MB, Nerenz RD, Lee SM, Martowicz ML, Pike JW. Multifunctional enhancers regulate mouse and human vitamin D receptor gene transcription. Mol Endocrinol. 2010;24:128-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 50. | Toropainen S, Väisänen S, Heikkinen S, Carlberg C. The down-regulation of the human MYC gene by the nuclear hormone 1alpha,25-dihydroxyvitamin D3 is associated with cycling of corepressors and histone deacetylases. J Mol Biol. 2010;400:284-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 51. | Trotter KW, Archer TK. Nuclear receptors and chromatin remodeling machinery. Mol Cell Endocrinol. 2007;265-266:162-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 52. | Thorne JL, Maguire O, Doig CL, Battaglia S, Fehr L, Sucheston LE, Heinaniemi M, O’Neill LP, McCabe CJ, Turner BM. Epigenetic control of a VDR-governed feed-forward loop that regulates p21(waf1/cip1) expression and function in non-malignant prostate cells. Nucleic Acids Res. 2011;39:2045-2056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 53. | Wang WL, Chatterjee N, Chittur SV, Welsh J, Tenniswood MP. Effects of 1α,25 dihydroxyvitamin D3 and testosterone on miRNA and mRNA expression in LNCaP cells. Mol Cancer. 2011;10:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 54. | Tang XH, Gudas LJ. Retinoids, retinoic acid receptors, and cancer. Annu Rev Pathol. 2011;6:345-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 458] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 55. | Rastinejad F. Retinoid X receptor and its partners in the nuclear receptor family. Curr Opin Struct Biol. 2001;11:33-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 118] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 56. | Schug TT, Berry DC, Shaw NS, Travis SN, Noy N. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell. 2007;129:723-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 563] [Cited by in RCA: 531] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 57. | Duester G. Retinoic acid synthesis and signaling during early organogenesis. Cell. 2008;134:921-931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 874] [Cited by in RCA: 793] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 58. | Perissi V, Jepsen K, Glass CK, Rosenfeld MG. Deconstructing repression: evolving models of co-repressor action. Nat Rev Genet. 2010;11:109-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 417] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 59. | O’Malley BW, Kumar R. Nuclear receptor coregulators in cancer biology. Cancer Res. 2009;69:8217-8222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 60. | Phan TQ, Jow MM, Privalsky ML. DNA recognition by thyroid hormone and retinoic acid receptors: 3,4,5 rule modified. Mol Cell Endocrinol. 2010;319:88-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 61. | Balmer JE, Blomhoff R. A robust characterization of retinoic acid response elements based on a comparison of sites in three species. J Steroid Biochem Mol Biol. 2005;96:347-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 62. | Pavri R, Lewis B, Kim TK, Dilworth FJ, Erdjument-Bromage H, Tempst P, de Murcia G, Evans R, Chambon P, Reinberg D. PARP-1 determines specificity in a retinoid signaling pathway via direct modulation of mediator. Mol Cell. 2005;18:83-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 193] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 63. | Yang CY, Leung PS, Adamopoulos IE, Gershwin ME. The implication of vitamin D and autoimmunity: a comprehensive review. Clin Rev Allergy Immunol. 2013;45:217-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 203] [Article Influence: 18.5] [Reference Citation Analysis (1)] |
| 64. | Smolders J, Peelen E, Thewissen M, Menheere P, Tervaert JW, Hupperts R, Damoiseaux J. The relevance of vitamin D receptor gene polymorphisms for vitamin D research in multiple sclerosis. Autoimmun Rev. 2009;8:621-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 65. | Uitterlinden AG, Fang Y, Van Meurs JB, Pols HA, Van Leeuwen JP. Genetics and biology of vitamin D receptor polymorphisms. Gene. 2004;338:143-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 965] [Cited by in RCA: 1067] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 66. | Orton SM, Morris AP, Herrera BM, Ramagopalan SV, Lincoln MR, Chao MJ, Vieth R, Sadovnick AD, Ebers GC. Evidence for genetic regulation of vitamin D status in twins with multiple sclerosis. Am J Clin Nutr. 2008;88:441-447. [PubMed] |
| 67. | Smolders J, Damoiseaux J, Menheere P, Tervaert JW, Hupperts R. Fok-I vitamin D receptor gene polymorphism (rs10735810) and vitamin D metabolism in multiple sclerosis. J Neuroimmunol. 2009;207:117-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 68. | Simon KC, Munger KL, Xing Yang A. Polymorphisms in vitamin D metabolism related genes and risk of multiple sclerosis. Mult Scler. 2010;16:133-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 69. | Ovsyannikova IG, Haralambieva IH, Vierkant RA, O’Byrne MM, Jacobson RM, Poland GA. Effects of vitamin A and D receptor gene polymorphisms/haplotypes on immune responses to measles vaccine. Pharmacogenet Genomics. 2012;22:20-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 70. | Ovsyannikova IG, Haralambieva IH, Dhiman N, O’Byrne MM, Pankratz VS, Jacobson RM, Poland GA. Polymorphisms in the vitamin A receptor and innate immunity genes influence the antibody response to rubella vaccination. J Infect Dis. 2010;201:207-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 71. | Mahony S, Mazzoni EO, McCuine S, Young RA, Wichterle H, Gifford DK. Ligand-dependent dynamics of retinoic acid receptor binding during early neurogenesis. Genome Biol. 2011;12:R2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 72. | Moutier E, Ye T, Choukrallah MA, Urban S, Osz J, Chatagnon A, Delacroix L, Langer D, Rochel N, Moras D. Retinoic acid receptors recognize the mouse genome through binding elements with diverse spacing and topology. J Biol Chem. 2012;287:26328-26341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 73. | Fox CJ, Hammerman PS, Thompson CB. Fuel feeds function: energy metabolism and the T-cell response. Nat Rev Immunol. 2005;5:844-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 604] [Cited by in RCA: 671] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 74. | Miller J, Gallo RL. Vitamin D and innate immunity. Dermatol Ther. 2010;23:13-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 75. | Wojtal KA, Wolfram L, Frey-Wagner I, Lang S, Scharl M, Vavricka SR, Rogler G. The effects of vitamin A on cells of innate immunity in vitro. Toxicol In Vitro. 2013;27:1525-1532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 76. | Cantorna MT, Snyder L, Lin YD, Yang L. Vitamin D and 1,25(OH)2D regulation of T cells. Nutrients. 2015;7:3011-3021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 284] [Cited by in RCA: 376] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 77. | Jeffery LE, Wood AM, Qureshi OS, Hou TZ, Gardner D, Briggs Z, Kaur S, Raza K, Sansom DM. Availability of 25-hydroxyvitamin D(3) to APCs controls the balance between regulatory and inflammatory T cell responses. J Immunol. 2012;189:5155-5164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 155] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 78. | Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221:1181-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 749] [Cited by in RCA: 745] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 79. | Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol. 2008;8:685-698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1184] [Cited by in RCA: 1065] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 80. | Brennan A, Katz DR, Nunn JD, Barker S, Hewison M, Fraher LJ, O’Riordan JL. Dendritic cells from human tissues express receptors for the immunoregulatory vitamin D3 metabolite, dihydroxycholecalciferol. Immunology. 1987;61:457-461. [PubMed] |
| 81. | Hayes CE, Hubler SL, Moore JR, Barta LE, Praska CE, Nashold FE. Vitamin D Actions on CD4(+) T Cells in Autoimmune Disease. Front Immunol. 2015;6:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 82. | Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O’Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2363] [Cited by in RCA: 2394] [Article Influence: 74.8] [Reference Citation Analysis (0)] |
| 83. | Suzuki Y, Orellana MA, Schreiber RD, Remington JS. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 872] [Cited by in RCA: 886] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 84. | Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2577] [Cited by in RCA: 2714] [Article Influence: 108.6] [Reference Citation Analysis (0)] |
| 85. | Kaplan MH, Sun YL, Hoey T, Grusby MJ. Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature. 1996;382:174-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 937] [Cited by in RCA: 936] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 86. | Afkarian M, Sedy JR, Yang J, Jacobson NG, Cereb N, Yang SY, Murphy TL, Murphy KM. T-bet is a STAT1-induced regulator of IL-12R expression in naïve CD4+ T cells. Nat Immunol. 2002;3:549-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 766] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 87. | Galon J, Sudarshan C, Ito S, Finbloom D, O’Shea JJ. IL-12 induces IFN regulating factor-1 (IRF-1) gene expression in human NK and T cells. J Immunol. 1999;162:7256-7262. [PubMed] |
| 88. | Rigby WF, Stacy T, Fanger MW. Inhibition of T lymphocyte mitogenesis by 1,25-dihydroxyvitamin D3 (calcitriol). J Clin Invest. 1984;74:1451-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 346] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 89. | Lemire JM, Adams JS, Kermani-Arab V, Bakke AC, Sakai R, Jordan SC. 1,25-Dihydroxyvitamin D3 suppresses human T helper/inducer lymphocyte activity in vitro. J Immunol. 1985;134:3032-3035. [PubMed] |
| 90. | Müller K, Bendtzen K. Inhibition of human T lymphocyte proliferation and cytokine production by 1,25-dihydroxyvitamin D3. Differential effects on CD45RA+ and CD45R0+ cells. Autoimmunity. 1992;14:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 91. | van Etten E, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. J Steroid Biochem Mol Biol. 2005;97:93-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 597] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 92. | Staeva-Vieira TP, Freedman LP. 1,25-dihydroxyvitamin D3 inhibits IFN-gamma and IL-4 levels during in vitro polarization of primary murine CD4+ T cells. J Immunol. 2002;168:1181-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 199] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 93. | Mahon BD, Wittke A, Weaver V, Cantorna MT. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J Cell Biochem. 2003;89:922-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 324] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 94. | Cantorna MT, Munsick C, Bemiss C, Mahon BD. 1,25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J Nutr. 2000;130:2648-2652. [PubMed] |
| 95. | Froicu M, Weaver V, Wynn TA, McDowell MA, Welsh JE, Cantorna MT. A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Mol Endocrinol. 2003;17:2386-2392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 315] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 96. | Cantorna MT, Yu S, Bruce D. The paradoxical effects of vitamin D on type 1 mediated immunity. Mol Aspects Med. 2008;29:369-375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 97. | Ehrchen J, Helming L, Varga G, Pasche B, Loser K, Gunzer M, Sunderkötter C, Sorg C, Roth J, Lengeling A. Vitamin D receptor signaling contributes to susceptibility to infection with Leishmania major. FASEB J. 2007;21:3208-3218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 98. | Waters WR, Palmer MV, Nonnecke BJ, Whipple DL, Horst RL. Mycobacterium bovis infection of vitamin D-deficient NOS2-/- mice. Microb Pathog. 2004;36:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 99. | Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770-1773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2664] [Cited by in RCA: 2696] [Article Influence: 141.9] [Reference Citation Analysis (0)] |
| 100. | Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112:1557-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1196] [Article Influence: 70.4] [Reference Citation Analysis (0)] |
| 101. | Le Gros G, Ben-Sasson SZ, Seder R, Finkelman FD, Paul WE. Generation of interleukin 4 (IL-4)-producing cells in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4-producing cells. J Exp Med. 1990;172:921-929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 622] [Cited by in RCA: 737] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 102. | Coffman RL, Seymour BW, Hudak S, Jackson J, Rennick D. Antibody to interleukin-5 inhibits helminth-induced eosinophilia in mice. Science. 1989;245:308-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 455] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 103. | Wynn TA. IL-13 effector functions. Annu Rev Immunol. 2003;21:425-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 709] [Cited by in RCA: 727] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 104. | Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1765] [Cited by in RCA: 1799] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 105. | Ho IC, Lo D, Glimcher LH. c-maf promotes T helper cell type 2 (Th2) and attenuates Th1 differentiation by both interleukin 4-dependent and -independent mechanisms. J Exp Med. 1998;188:1859-1866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 235] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 106. | Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1231] [Cited by in RCA: 1248] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 107. | Shimoda K, van Deursen J, Sangster MY, Sarawar SR, Carson RT, Tripp RA, Chu C, Quelle FW, Nosaka T, Vignali DA. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996;380:630-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 995] [Cited by in RCA: 996] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 108. | Matheu V, Bäck O, Mondoc E, Issazadeh-Navikas S. Dual effects of vitamin D-induced alteration of TH1/TH2 cytokine expression: enhancing IgE production and decreasing airway eosinophilia in murine allergic airway disease. J Allergy Clin Immunol. 2003;112:585-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 176] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 109. | Sloka S, Silva C, Wang J, Yong VW. Predominance of Th2 polarization by vitamin D through a STAT6-dependent mechanism. J Neuroinflammation. 2011;8:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 110. | Li W, Duan H, Chen F, Wang Z, Huang X, Deng X, Liu Y. Identification of quantitative trait loci controlling high Calcium response in Arabidopsis thaliana. PLoS One. 2014;9:e112511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 111. | Kreindler JL, Steele C, Nguyen N, Chan YR, Pilewski JM, Alcorn JF, Vyas YM, Aujla SJ, Finelli P, Blanchard M. Vitamin D3 attenuates Th2 responses to Aspergillus fumigatus mounted by CD4+ T cells from cystic fibrosis patients with allergic bronchopulmonary aspergillosis. J Clin Invest. 2010;120:3242-3254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 118] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 112. | Das M, Tomar N, Sreenivas V, Gupta N, Goswami R. Effect of vitamin D supplementation on cathelicidin, IFN-γ, IL-4 and Th1/Th2 transcription factors in young healthy females. Eur J Clin Nutr. 2014;68:338-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 113. | Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5130] [Cited by in RCA: 5465] [Article Influence: 287.6] [Reference Citation Analysis (0)] |
| 114. | Korn T, Mitsdoerffer M, Croxford AL, Awasthi A, Dardalhon VA, Galileos G, Vollmar P, Stritesky GL, Kaplan MH, Waisman A. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc Natl Acad Sci USA. 2008;105:18460-18465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 439] [Cited by in RCA: 419] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 115. | Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2372] [Cited by in RCA: 2486] [Article Influence: 130.8] [Reference Citation Analysis (0)] |
| 116. | Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 2004;190:624-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 664] [Cited by in RCA: 691] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 117. | Happel KI, Dubin PJ, Zheng M, Ghilardi N, Lockhart C, Quinton LJ, Odden AR, Shellito JE, Bagby GJ, Nelson S. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med. 2005;202:761-769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 463] [Cited by in RCA: 472] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 118. | Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3769] [Cited by in RCA: 4117] [Article Influence: 216.7] [Reference Citation Analysis (0)] |
| 119. | Brüstle A, Heink S, Huber M, Rosenplänter C, Stadelmann C, Yu P, Arpaia E, Mak TW, Kamradt T, Lohoff M. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nat Immunol. 2007;8:958-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 555] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 120. | Stritesky GL, Yeh N, Kaplan MH. IL-23 promotes maintenance but not commitment to the Th17 lineage. J Immunol. 2008;181:5948-5955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 321] [Cited by in RCA: 315] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 121. | Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40:1830-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 978] [Cited by in RCA: 1234] [Article Influence: 82.3] [Reference Citation Analysis (0)] |
| 122. | Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J Pharmacol Exp Ther. 2008;324:23-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 360] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 123. | Penna G, Amuchastegui S, Cossetti C, Aquilano F, Mariani R, Sanvito F, Doglioni C, Adorini L. Treatment of experimental autoimmune prostatitis in nonobese diabetic mice by the vitamin D receptor agonist elocalcitol. J Immunol. 2006;177:8504-8511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 124. | Tang J, Zhou R, Luger D, Zhu W, Silver PB, Grajewski RS, Su SB, Chan CC, Adorini L, Caspi RR. Calcitriol suppresses antiretinal autoimmunity through inhibitory effects on the Th17 effector response. J Immunol. 2009;182:4624-4632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 279] [Cited by in RCA: 261] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 125. | Chang SH, Chung Y, Dong C. Vitamin D suppresses Th17 cytokine production by inducing C/EBP homologous protein (CHOP) expression. J Biol Chem. 2010;285:38751-38755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 145] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 126. | Joshi S, Pantalena LC, Liu XK, Gaffen SL, Liu H, Rohowsky-Kochan C, Ichiyama K, Yoshimura A, Steinman L, Christakos S. 1,25-dihydroxyvitamin D(3) ameliorates Th17 autoimmunity via transcriptional modulation of interleukin-17A. Mol Cell Biol. 2011;31:3653-3669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 383] [Cited by in RCA: 352] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 127. | Hamzaoui A, Berraïes A, Hamdi B, Kaabachi W, Ammar J, Hamzaoui K. Vitamin D reduces the differentiation and expansion of Th17 cells in young asthmatic children. Immunobiology. 2014;219:873-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 128. | Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151-1164. [PubMed] |
| 129. | DiPaolo RJ, Brinster C, Davidson TS, Andersson J, Glass D, Shevach EM. Autoantigen-specific TGFbeta-induced Foxp3+ regulatory T cells prevent autoimmunity by inhibiting dendritic cells from activating autoreactive T cells. J Immunol. 2007;179:4685-4693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 168] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 130. | Baecher-Allan C, Viglietta V, Hafler DA. Inhibition of human CD4(+)CD25(+high) regulatory T cell function. J Immunol. 2002;169:6210-6217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 203] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 131. | Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6077] [Cited by in RCA: 6378] [Article Influence: 289.9] [Reference Citation Analysis (0)] |
| 132. | Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5576] [Cited by in RCA: 5845] [Article Influence: 265.7] [Reference Citation Analysis (0)] |
| 133. | Murai M, Turovskaya O, Kim G, Madan R, Karp CL, Cheroutre H, Kronenberg M. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol. 2009;10:1178-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 613] [Cited by in RCA: 685] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 134. | Penna G, Roncari A, Amuchastegui S, Daniel KC, Berti E, Colonna M, Adorini L. Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4+Foxp3+ regulatory T cells by 1,25-dihydroxyvitamin D3. Blood. 2005;106:3490-3497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 310] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 135. | Gorman S, Kuritzky LA, Judge MA, Dixon KM, McGlade JP, Mason RS, Finlay-Jones JJ, Hart PH. Topically applied 1,25-dihydroxyvitamin D3 enhances the suppressive activity of CD4+CD25+ cells in the draining lymph nodes. J Immunol. 2007;179:6273-6283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 203] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 136. | Jeffery LE, Burke F, Mura M, Zheng Y, Qureshi OS, Hewison M, Walker LS, Lammas DA, Raza K, Sansom DM. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J Immunol. 2009;183:5458-5467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 571] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 137. | Correale J, Ysrraelit MC, Gaitán MI. Immunomodulatory effects of Vitamin D in multiple sclerosis. Brain. 2009;132:1146-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 359] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 138. | Prietl B, Pilz S, Wolf M, Tomaschitz A, Obermayer-Pietsch B, Graninger W, Pieber TR. Vitamin D supplementation and regulatory T cells in apparently healthy subjects: vitamin D treatment for autoimmune diseases? Isr Med Assoc J. 2010;12:136-139. [PubMed] |
| 139. | Takeda M, Yamashita T, Sasaki N, Nakajima K, Kita T, Shinohara M, Ishida T, Hirata K. Oral administration of an active form of vitamin D3 (calcitriol) decreases atherosclerosis in mice by inducing regulatory T cells and immature dendritic cells with tolerogenic functions. Arterioscler Thromb Vasc Biol. 2010;30:2495-2503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 140. | Smolders J, Thewissen M, Peelen E, Menheere P, Tervaert JW, Damoiseaux J, Hupperts R. Vitamin D status is positively correlated with regulatory T cell function in patients with multiple sclerosis. PLoS One. 2009;4:e6635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 206] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 141. | Kang SW, Kim SH, Lee N, Lee WW, Hwang KA, Shin MS, Lee SH, Kim WU, Kang I. 1,25-Dihyroxyvitamin D3 promotes FOXP3 expression via binding to vitamin D response elements in its conserved noncoding sequence region. J Immunol. 2012;188:5276-5282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 148] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 142. | Stohlman SA, Pei L, Cua DJ, Li Z, Hinton DR. Activation of regulatory cells suppresses experimental allergic encephalomyelitis via secretion of IL-10. J Immunol. 1999;163:6338-6344. [PubMed] |
| 143. | Spach KM, Nashold FE, Dittel BN, Hayes CE. IL-10 signaling is essential for 1,25-dihydroxyvitamin D3-mediated inhibition of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:6030-6037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 171] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 144. | Urry Z, Xystrakis E, Richards DF, McDonald J, Sattar Z, Cousins DJ, Corrigan CJ, Hickman E, Brown Z, Hawrylowicz CM. Ligation of TLR9 induced on human IL-10-secreting Tregs by 1alpha,25-dihydroxyvitamin D3 abrogates regulatory function. J Clin Invest. 2009;119:387-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 145. | Unger WW, Laban S, Kleijwegt FS, van der Slik AR, Roep BO. Induction of Treg by monocyte-derived DC modulated by vitamin D3 or dexamethasone: differential role for PD-L1. Eur J Immunol. 2009;39:3147-3159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 320] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 146. | Barrat FJ, Cua DJ, Boonstra A, Richards DF, Crain C, Savelkoul HF, de Waal-Malefyt R, Coffman RL, Hawrylowicz CM, O’Garra A. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195:603-616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 882] [Cited by in RCA: 857] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 147. | Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(-) effector T cells. Nat Immunol. 2008;9:1347-1355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 914] [Cited by in RCA: 875] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 148. | Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, Martin B, Wilhelm C, Stockinger B. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008;9:1341-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 874] [Cited by in RCA: 913] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 149. | Chang HC, Sehra S, Goswami R, Yao W, Yu Q, Stritesky GL, Jabeen R, McKinley C, Ahyi AN, Han L. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nat Immunol. 2010;11:527-534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 479] [Cited by in RCA: 466] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 150. | Staudt V, Bothur E, Klein M, Lingnau K, Reuter S, Grebe N, Gerlitzki B, Hoffmann M, Ulges A, Taube C. Interferon-regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity. 2010;33:192-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 435] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 151. | Palmer MT, Lee YK, Maynard CL, Oliver JR, Bikle DD, Jetten AM, Weaver CT. Lineage-specific effects of 1,25-dihydroxyvitamin D(3) on the development of effector CD4 T cells. J Biol Chem. 2011;286:997-1004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 180] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 152. | Keating P, Munim A, Hartmann JX. Effect of vitamin D on T-helper type 9 polarized human memory cells in chronic persistent asthma. Ann Allergy Asthma Immunol. 2014;112:154-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 153. | Baumjohann D, Ansel KM. MicroRNA-mediated regulation of T helper cell differentiation and plasticity. Nat Rev Immunol. 2013;13:666-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 292] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 154. | Alvarez-Díaz S, Valle N, Ferrer-Mayorga G, Lombardía L, Herrera M, Domínguez O, Segura MF, Bonilla F, Hernando E, Muñoz A. MicroRNA-22 is induced by vitamin D and contributes to its antiproliferative, antimigratory and gene regulatory effects in colon cancer cells. Hum Mol Genet. 2012;21:2157-2165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 124] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 155. | Lisse TS, Adams JS, Hewison M. Vitamin D and microRNAs in bone. Crit Rev Eukaryot Gene Expr. 2013;23:195-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 156. | Kanno Y, Vahedi G, Hirahara K, Singleton K, O’Shea JJ. Transcriptional and epigenetic control of T helper cell specification: molecular mechanisms underlying commitment and plasticity. Annu Rev Immunol. 2012;30:707-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 270] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 157. | Karlic H, Varga F. Impact of vitamin D metabolism on clinical epigenetics. Clin Epigenetics. 2011;2:55-61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 158. | Fetahu IS, Höbaus J, Kállay E. Vitamin D and the epigenome. Front Physiol. 2014;5:164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 191] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 159. | Grishkan IV, Fairchild AN, Calabresi PA, Gocke AR. 1,25-Dihydroxyvitamin D3 selectively and reversibly impairs T helper-cell CNS localization. Proc Natl Acad Sci USA. 2013;110:21101-21106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 160. | Hall JA, Grainger JR, Spencer SP, Belkaid Y. The role of retinoic acid in tolerance and immunity. Immunity. 2011;35:13-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 422] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 161. | Brown CC, Noelle RJ. Seeing through the dark: New insights into the immune regulatory functions of vitamin A. Eur J Immunol. 2015;45:1287-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 162. | Ertesvag A, Engedal N, Naderi S, Blomhoff HK. Retinoic acid stimulates the cell cycle machinery in normal T cells: involvement of retinoic acid receptor-mediated IL-2 secretion. J Immunol. 2002;169:5555-5563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 163. | Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1148] [Cited by in RCA: 1217] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 164. | Bjersing JL, Telemo E, Dahlgren U, Hanson LA. Loss of ileal IgA+ plasma cells and of CD4+ lymphocytes in ileal Peyer’s patches of vitamin A deficient rats. Clin Exp Immunol. 2002;130:404-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 165. | Carman JA, Smith SM, Hayes CE. Characterization of a helper T lymphocyte defect in vitamin A-deficient mice. J Immunol. 1989;142:388-393. [PubMed] |
| 166. | Mora JR. Homing imprinting and immunomodulation in the gut: role of dendritic cells and retinoids. Inflamm Bowel Dis. 2008;14:275-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 167. | Cantorna MT, Nashold FE, Hayes CE. Vitamin A deficiency results in a priming environment conducive for Th1 cell development. Eur J Immunol. 1995;25:1673-1679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 98] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 168. | Iwata M, Eshima Y, Kagechika H. Retinoic acids exert direct effects on T cells to suppress Th1 development and enhance Th2 development via retinoic acid receptors. Int Immunol. 2003;15:1017-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 220] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 169. | Ma Y, Chen Q, Ross AC. Retinoic acid and polyriboinosinic: polyribocytidylic acid stimulate robust anti-tetanus antibody production while differentially regulating type 1/type 2 cytokines and lymphocyte populations. J Immunol. 2005;174:7961-7969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 170. | Van YH, Lee WH, Ortiz S, Lee MH, Qin HJ, Liu CP. All-trans retinoic acid inhibits type 1 diabetes by T regulatory (Treg)-dependent suppression of interferon-gamma-producing T-cells without affecting Th17 cells. Diabetes. 2009;58:146-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 171. | Zhu YF, Hu JZ, Zhao PN, Liu LX, Li Y. All-transretinoic acid regulates Th1/Th2 balance in CD4+ T cells when GATA-3 is deficient. Biomed Environ Sci. 2013;26:774-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 172. | Zhan XX, Liu Y, Yang JF, Wang GY, Mu L, Zhang TS, Xie XL, Wang JH, Liu YM, Kong QF. All-trans-retinoic acid ameliorates experimental allergic encephalomyelitis by affecting dendritic cell and monocyte development. Immunology. 2013;138:333-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 173. | Cippitelli M, Ye J, Viggiano V, Sica A, Ghosh P, Gulino A, Santoni A, Young HA. Retinoic acid-induced transcriptional modulation of the human interferon-gamma promoter. J Biol Chem. 1996;271:26783-26793. [PubMed] |
| 174. | Brown CC, Esterhazy D, Sarde A, London M, Pullabhatla V, Osma-Garcia I, Al-Bader R, Ortiz C, Elgueta R, Arno M. Retinoic acid is essential for Th1 cell lineage stability and prevents transition to a Th17 cell program. Immunity. 2015;42:499-511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 175. | Hoag KA, Nashold FE, Goverman J, Hayes CE. Retinoic acid enhances the T helper 2 cell development that is essential for robust antibody responses through its action on antigen-presenting cells. J Nutr. 2002;132:3736-3739. [PubMed] |
| 176. | Lovett-Racke AE, Racke MK. Retinoic acid promotes the development of Th2-like human myelin basic protein-reactive T cells. Cell Immunol. 2002;215:54-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 177. | Yokota-Nakatsuma A, Takeuchi H, Ohoka Y, Kato C, Song SY, Hoshino T, Yagita H, Ohteki T, Iwata M. Retinoic acid prevents mesenteric lymph node dendritic cells from inducing IL-13-producing inflammatory Th2 cells. Mucosal Immunol. 2014;7:786-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 178. | Dawson HD, Collins G, Pyle R, Key M, Weeraratna A, Deep-Dixit V, Nadal CN, Taub DD. Direct and indirect effects of retinoic acid on human Th2 cytokine and chemokine expression by human T lymphocytes. BMC Immunol. 2006;7:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 91] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 179. | Dawson HD, Collins G, Pyle R, Key M, Taub DD. The Retinoic Acid Receptor-alpha mediates human T-cell activation and Th2 cytokine and chemokine production. BMC Immunol. 2008;9:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 180. | Nagai H, Matsuura S, Bouda K, Takaoka Y, Wang T, Niwa S, Shudo K. Effect of Am-80, a synthetic derivative of retinoid, on experimental arthritis in mice. Pharmacology. 1999;58:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 181. | Wansley DL, Yin Y, Prussin C. The retinoic acid receptor-α modulators ATRA and Ro415253 reciprocally regulate human IL-5+ Th2 cell proliferation and cytokine expression. Clin Mol Allergy. 2013;11:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 182. | Elias KM, Laurence A, Davidson TS, Stephens G, Kanno Y, Shevach EM, O’Shea JJ. Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood. 2008;111:1013-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 351] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 183. | Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1505] [Cited by in RCA: 1588] [Article Influence: 88.2] [Reference Citation Analysis (0)] |
| 184. | Xiao S, Jin H, Korn T, Liu SM, Oukka M, Lim B, Kuchroo VK. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J Immunol. 2008;181:2277-2284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 411] [Cited by in RCA: 416] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 185. | Xystrakis E, Kusumakar S, Boswell S, Peek E, Urry Z, Richards DF, Adikibi T, Pridgeon C, Dallman M, Loke TK. Reversing the defective induction of IL-10-secreting regulatory T cells in glucocorticoid-resistant asthma patients. J Clin Invest. 2006;116:146-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 430] [Cited by in RCA: 407] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 186. | Nolting J, Daniel C, Reuter S, Stuelten C, Li P, Sucov H, Kim BG, Letterio JJ, Kretschmer K, Kim HJ. Retinoic acid can enhance conversion of naive into regulatory T cells independently of secreted cytokines. J Exp Med. 2009;206:2131-2139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 187. | Chen X, Xiao W, Wang W, Luo L, Ye S, Liu Y. The complex interplay between ERK1/2, TGFβ/Smad, and Jagged/Notch signaling pathways in the regulation of epithelial-mesenchymal transition in retinal pigment epithelium cells. PLoS One. 2014;9:e96365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 188. | Carbone F, De Rosa V, Carrieri PB, Montella S, Bruzzese D, Porcellini A, Procaccini C, La Cava A, Matarese G. Regulatory T cell proliferative potential is impaired in human autoimmune disease. Nat Med. 2014;20:69-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 177] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 189. | Li L, Godfrey WR, Porter SB, Ge Y, June CH, Blazar BR, Boussiotis VA. CD4+CD25+ regulatory T-cell lines from human cord blood have functional and molecular properties of T-cell anergy. Blood. 2005;106:3068-3073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 110] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 190. | Lu L, Ma J, Li Z, Lan Q, Chen M, Liu Y, Xia Z, Wang J, Han Y, Shi W. All-trans retinoic acid promotes TGF-β-induced Tregs via histone modification but not DNA demethylation on Foxp3 gene locus. PLoS One. 2011;6:e24590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 191. | Lu L, Lan Q, Li Z, Zhou X, Gu J, Li Q, Wang J, Chen M, Liu Y, Shen Y. Critical role of all-trans retinoic acid in stabilizing human natural regulatory T cells under inflammatory conditions. Proc Natl Acad Sci USA. 2014;111:E3432-E3440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 188] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 192. | Zheng SG, Wang JH, Stohl W, Kim KS, Gray JD, Horwitz DA. TGF-beta requires CTLA-4 early after T cell activation to induce FoxP3 and generate adaptive CD4+CD25+ regulatory cells. J Immunol. 2006;176:3321-3329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 226] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 193. | Schneider H, Mandelbrot DA, Greenwald RJ, Ng F, Lechler R, Sharpe AH, Rudd CE. Cutting edge: CTLA-4 (CD152) differentially regulates mitogen-activated protein kinases (extracellular signal-regulated kinase and c-Jun N-terminal kinase) in CD4+ T cells from receptor/ligand-deficient mice. J Immunol. 2002;169:3475-3479. [PubMed] |
| 194. | Lu L, Zhou X, Wang J, Zheng SG, Horwitz DA. Characterization of protective human CD4CD25 FOXP3 regulatory T cells generated with IL-2, TGF-β and retinoic acid. PLoS One. 2010;5:e15150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 195. | Wang J, Huizinga TW, Toes RE. De novo generation and enhanced suppression of human CD4+CD25+ regulatory T cells by retinoic acid. J Immunol. 2009;183:4119-4126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 196. | Zhou X, Kong N, Wang J, Fan H, Zou H, Horwitz D, Brand D, Liu Z, Zheng SG. Cutting edge: all-trans retinoic acid sustains the stability and function of natural regulatory T cells in an inflammatory milieu. J Immunol. 2010;185:2675-2679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 193] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 197. | Urvalek AM, Gudas LJ. Retinoic acid and histone deacetylases regulate epigenetic changes in embryonic stem cells. J Biol Chem. 2014;289:19519-19530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 198. | Garzon R, Pichiorri F, Palumbo T, Visentini M, Aqeilan R, Cimmino A, Wang H, Sun H, Volinia S, Alder H. MicroRNA gene expression during retinoic acid-induced differentiation of human acute promyelocytic leukemia. Oncogene. 2007;26:4148-4157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 277] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 199. | Meseguer S, Mudduluru G, Escamilla JM, Allgayer H, Barettino D. MicroRNAs-10a and -10b contribute to retinoic acid-induced differentiation of neuroblastoma cells and target the alternative splicing regulatory factor SFRS1 (SF2/ASF). J Biol Chem. 2011;286:4150-4164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 200. | Takahashi H, Kanno T, Nakayamada S, Hirahara K, Sciumè G, Muljo SA, Kuchen S, Casellas R, Wei L, Kanno Y. TGF-β and retinoic acid induce the microRNA miR-10a, which targets Bcl-6 and constrains the plasticity of helper T cells. Nat Immunol. 2012;13:587-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 230] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 201. | Khan S, Wall D, Curran C, Newell J, Kerin MJ, Dwyer RM. MicroRNA-10a is reduced in breast cancer and regulated in part through retinoic acid. BMC Cancer. 2015;15:345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 202. | Pino-Lagos K, Benson MJ, Noelle RJ. Retinoic acid in the immune system. Ann N Y Acad Sci. 2008;1143:170-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 203. | Litonjua AA, Weiss ST. Is vitamin D deficiency to blame for the asthma epidemic? J Allergy Clin Immunol. 2007;120:1031-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 323] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 204. | Li LB, Leung DY, Martin RJ, Goleva E. Inhibition of histone deacetylase 2 expression by elevated glucocorticoid receptor beta in steroid-resistant asthma. Am J Respir Crit Care Med. 2010;182:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 205. | Black PN, Scragg R. Relationship between serum 25-hydroxyvitamin d and pulmonary function in the third national health and nutrition examination survey. Chest. 2005;128:3792-3798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 408] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 206. | Li F, Peng M, Jiang L, Sun Q, Zhang K, Lian F, Litonjua AA, Gao J, Gao X. Vitamin D deficiency is associated with decreased lung function in Chinese adults with asthma. Respiration. 2011;81:469-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 207. | Brehm JM, Celedón JC, Soto-Quiros ME, Avila L, Hunninghake GM, Forno E, Laskey D, Sylvia JS, Hollis BW, Weiss ST. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am J Respir Crit Care Med. 2009;179:765-771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 470] [Cited by in RCA: 437] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 208. | Searing DA, Zhang Y, Murphy JR, Hauk PJ, Goleva E, Leung DY. Decreased serum vitamin D levels in children with asthma are associated with increased corticosteroid use. J Allergy Clin Immunol. 2010;125:995-1000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 293] [Cited by in RCA: 274] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 209. | Wu AC, Tantisira K, Li L, Fuhlbrigge AL, Weiss ST, Litonjua A. Effect of vitamin D and inhaled corticosteroid treatment on lung function in children. Am J Respir Crit Care Med. 2012;186:508-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 210. | Dabbah H, Bar Yoseph R, Livnat G, Hakim F, Bentur L. Bronchial Reactivity, Inflammatory and Allergic Parameters, and Vitamin D Levels in Children With Asthma. Respir Care. 2015;60:1157-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 211. | Castro M, King TS, Kunselman SJ, Cabana MD, Denlinger L, Holguin F, Kazani SD, Moore WC, Moy J, Sorkness CA. Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: the VIDA randomized clinical trial. JAMA. 2014;311:2083-2091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 205] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 212. | Einisman H, Reyes ML, Angulo J, Cerda J, López-Lastra M, Castro-Rodriguez JA. Vitamin D levels and vitamin D receptor gene polymorphisms in asthmatic children: a case-control study. Pediatr Allergy Immunol. 2015;26:545-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 213. | Vijayendra Chary A, Hemalatha R, Seshacharyulu M, Vasudeva Murali M, Jayaprakash D, Dinesh Kumar B. Vitamin D deficiency in pregnant women impairs regulatory T cell function. J Steroid Biochem Mol Biol. 2015;147:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 214. | Morales E, Romieu I, Guerra S, Ballester F, Rebagliato M, Vioque J, Tardón A, Rodriguez Delhi C, Arranz L, Torrent M. Maternal vitamin D status in pregnancy and risk of lower respiratory tract infections, wheezing, and asthma in offspring. Epidemiology. 2012;23:64-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 215. | Wills AK, Shaheen SO, Granell R, Henderson AJ, Fraser WD, Lawlor DA. Maternal 25-hydroxyvitamin D and its association with childhood atopic outcomes and lung function. Clin Exp Allergy. 2013;43:1180-1188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 216. | Gale CR, Robinson SM, Harvey NC, Javaid MK, Jiang B, Martyn CN, Godfrey KM, Cooper C. Maternal vitamin D status during pregnancy and child outcomes. Eur J Clin Nutr. 2008;62:68-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 514] [Cited by in RCA: 469] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 217. | Barman M, Jonsson K, Hesselmar B, Sandin A, Sandberg AS, Wold AE. No association between allergy and current 25-hydroxy vitamin D in serum or vitamin D intake. Acta Paediatr. 2015;104:405-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 218. | Manna B, Ashbaugh P, Bhattacharyya SN. Retinoic acid-regulated cellular differentiation and mucin gene expression in isolated rabbit tracheal-epithelial cells in culture. Inflammation. 1995;19:489-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 219. | Denburg JA, Sehmi R, Upham J. Regulation of IL-5 receptor on eosinophil progenitors in allergic inflammation: role of retinoic acid. Int Arch Allergy Immunol. 2001;124:246-248. [PubMed] |
| 220. | Takamura K, Nasuhara Y, Kobayashi M, Betsuyaku T, Tanino Y, Kinoshita I, Yamaguchi E, Matsukura S, Schleimer RP, Nishimura M. Retinoic acid inhibits interleukin-4-induced eotaxin production in a human bronchial epithelial cell line. Am J Physiol Lung Cell Mol Physiol. 2004;286:L777-L785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 221. | Fang H, Jin H, Wang H. Effect of all-trans retinoic acid on airway inflammation in asthmatic rats and its mechanism. J Huazhong Univ Sci Technolog Med Sci. 2004;24:229-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 222. | Day RM, Lee YH, Park AM, Suzuki YJ. Retinoic acid inhibits airway smooth muscle cell migration. Am J Respir Cell Mol Biol. 2006;34:695-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 223. | Maret M, Ruffie C, Periquet B, Campo AM, Menevret M, Phelep A, Dziewiszek K, Druilhe A, Pretolani M. Liposomal retinoic acids modulate asthma manifestations in mice. J Nutr. 2007;137:2730-2736. [PubMed] |
| 224. | Frankenberger M, Heimbeck I, Möller W, Mamidi S, Kassner G, Pukelsheim K, Wjst M, Neiswirth M, Kroneberg P, Lomas D. Inhaled all-trans retinoic acid in an individual with severe emphysema. Eur Respir J. 2009;34:1487-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 225. | Goswami S, Angkasekwinai P, Shan M, Greenlee KJ, Barranco WT, Polikepahad S, Seryshev A, Song LZ, Redding D, Singh B. Divergent functions for airway epithelial matrix metalloproteinase 7 and retinoic acid in experimental asthma. Nat Immunol. 2009;10:496-503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 226. | Wu J, Zhang Y, Liu Q, Zhong W, Xia Z. All-trans retinoic acid attenuates airway inflammation by inhibiting Th2 and Th17 response in experimental allergic asthma. BMC Immunol. 2013;14:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 227. | Soroosh P, Doherty TA, Duan W, Mehta AK, Choi H, Adams YF, Mikulski Z, Khorram N, Rosenthal P, Broide DH. Lung-resident tissue macrophages generate Foxp3+ regulatory T cells and promote airway tolerance. J Exp Med. 2013;210:775-788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 273] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 228. | Ben-Nun A, Kaushansky N, Kawakami N, Krishnamoorthy G, Berer K, Liblau R, Hohlfeld R, Wekerle H. From classic to spontaneous and humanized models of multiple sclerosis: impact on understanding pathogenesis and drug development. J Autoimmun. 2014;54:33-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 229. | Nashold FE, Hoag KA, Goverman J, Hayes CE. Rag-1-dependent cells are necessary for 1,25-dihydroxyvitamin D(3) prevention of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2001;119:16-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 81] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 230. | Mayne CG, Spanier JA, Relland LM, Williams CB, Hayes CE. 1,25-Dihydroxyvitamin D3 acts directly on the T lymphocyte vitamin D receptor to inhibit experimental autoimmune encephalomyelitis. Eur J Immunol. 2011;41:822-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 231. | Cantorna MT, Hayes CE, DeLuca HF. 1,25-Dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proc Natl Acad Sci USA. 1996;93:7861-7864. [PubMed] |
| 232. | Mattner F, Smiroldo S, Galbiati F, Muller M, Di Lucia P, Poliani PL, Martino G, Panina-Bordignon P, Adorini L. Inhibition of Th1 development and treatment of chronic-relapsing experimental allergic encephalomyelitis by a non-hypercalcemic analogue of 1,25-dihydroxyvitamin D(3). Eur J Immunol. 2000;30:498-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 233. | Drozdenko G, Heine G, Worm M. Oral vitamin D increases the frequencies of CD38+ human B cells and ameliorates IL-17-producing T cells. Exp Dermatol. 2014;23:107-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 234. | DeLuca HF, Plum LA. Vitamin D deficiency diminishes the severity and delays onset of experimental autoimmune encephalomyelitis. Arch Biochem Biophys. 2011;513:140-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 235. | Müller K, Gram J, Bollerslev J, Diamant M, Barington T, Hansen MB, Bendtzen K. Down-regulation of monocyte functions by treatment of healthy adults with 1 alpha,25 dihydroxyvitamin D3. Int J Immunopharmacol. 1991;13:525-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 236. | Mahon BD, Gordon SA, Cruz J, Cosman F, Cantorna MT. Cytokine profile in patients with multiple sclerosis following vitamin D supplementation. J Neuroimmunol. 2003;134:128-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 197] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 237. | Spanier JA, Nashold FE, Olson JK, Hayes CE. The Ifng gene is essential for Vdr gene expression and vitamin D₃-mediated reduction of the pathogenic T cell burden in the central nervous system in experimental autoimmune encephalomyelitis, a multiple sclerosis model. J Immunol. 2012;189:3188-3197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 238. | Chang JH, Cha HR, Lee DS, Seo KY, Kweon MN. 1,25-Dihydroxyvitamin D3 inhibits the differentiation and migration of T(H)17 cells to protect against experimental autoimmune encephalomyelitis. PLoS One. 2010;5:e12925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 125] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 239. | Smolders J, Peelen E, Thewissen M, Cohen Tervaert JW, Menheere P, Hupperts R, Damoiseaux J. Safety and T cell modulating effects of high dose vitamin D3 supplementation in multiple sclerosis. PLoS One. 2010;5:e15235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 126] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 240. | Lafaille JJ, Keere FV, Hsu AL, Baron JL, Haas W, Raine CS, Tonegawa S. Myelin basic protein-specific T helper 2 (Th2) cells cause experimental autoimmune encephalomyelitis in immunodeficient hosts rather than protect them from the disease. J Exp Med. 1997;186:307-312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 358] [Cited by in RCA: 332] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 241. | Cantorna MT, Woodward WD, Hayes CE, DeLuca HF. 1,25-dihydroxyvitamin D3 is a positive regulator for the two anti-encephalitogenic cytokines TGF-beta 1 and IL-4. J Immunol. 1998;160:5314-5319. [PubMed] |
| 242. | Cantorna MT, Humpal-Winter J, DeLuca HF. In vivo upregulation of interleukin-4 is one mechanism underlying the immunoregulatory effects of 1,25-dihydroxyvitamin D(3). Arch Biochem Biophys. 2000;377:135-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 53] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 243. | Buckner JH. Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nat Rev Immunol. 2010;10:849-859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 613] [Cited by in RCA: 604] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 244. | Haas J, Hug A, Viehöver A, Fritzsching B, Falk CS, Filser A, Vetter T, Milkova L, Korporal M, Fritz B. Reduced suppressive effect of CD4+CD25high regulatory T cells on the T cell immune response against myelin oligodendrocyte glycoprotein in patients with multiple sclerosis. Eur J Immunol. 2005;35:3343-3352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 322] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 245. | Venken K, Hellings N, Broekmans T, Hensen K, Rummens JL, Stinissen P. Natural naive CD4+CD25+CD127low regulatory T cell (Treg) development and function are disturbed in multiple sclerosis patients: recovery of memory Treg homeostasis during disease progression. J Immunol. 2008;180:6411-6420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 194] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 246. | Royal W, Mia Y, Li H, Naunton K. Peripheral blood regulatory T cell measurements correlate with serum vitamin D levels in patients with multiple sclerosis. J Neuroimmunol. 2009;213:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 247. | Khoo AL, Koenen HJ, Chai LY, Sweep FC, Netea MG, van der Ven AJ, Joosten I. Seasonal variation in vitamin D₃ levels is paralleled by changes in the peripheral blood human T cell compartment. PLoS One. 2012;7:e29250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 248. | Massacesi L, Abbamondi AL, Giorgi C, Sarlo F, Lolli F, Amaducci L. Suppression of experimental allergic encephalomyelitis by retinoic acid. J Neurol Sci. 1987;80:55-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 249. | Racke MK, Burnett D, Pak SH, Albert PS, Cannella B, Raine CS, McFarlin DE, Scott DE. Retinoid treatment of experimental allergic encephalomyelitis. IL-4 production correlates with improved disease course. J Immunol. 1995;154:450-458. [PubMed] |
| 250. | Klemann C, Raveney BJ, Klemann AK, Ozawa T, von Hörsten S, Shudo K, Oki S, Yamamura T. Synthetic retinoid AM80 inhibits Th17 cells and ameliorates experimental autoimmune encephalomyelitis. Am J Pathol. 2009;174:2234-2245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 251. | Moliné-Velázquez V, Ortega MC, Vila del Sol V, Melero-Jerez C, de Castro F, Clemente D. The synthetic retinoid Am80 delays recovery in a model of multiple sclerosis by modulating myeloid-derived suppressor cell fate and viability. Neurobiol Dis. 2014;67:149-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 252. | Abtahi Froushani SM, Delirezh N, Hobbenaghi R, Mosayebi G. Synergistic effects of atorvastatin and all-trans retinoic acid in ameliorating animal model of multiple sclerosis. Immunol Invest. 2014;43:54-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 253. | Takeuchi H, Yokota-Nakatsuma A, Ohoka Y, Kagechika H, Kato C, Song SY, Iwata M. Retinoid X receptor agonists modulate Foxp3⁺ regulatory T cell and Th17 cell differentiation with differential dependence on retinoic acid receptor activation. J Immunol. 2013;191:3725-3733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 254. | Diab A, Hussain RZ, Lovett-Racke AE, Chavis JA, Drew PD, Racke MK. Ligands for the peroxisome proliferator-activated receptor-gamma and the retinoid X receptor exert additive anti-inflammatory effects on experimental autoimmune encephalomyelitis. J Neuroimmunol. 2004;148:116-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 95] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 255. | Correale J, Farez MF. Parasite infections in multiple sclerosis modulate immune responses through a retinoic acid-dependent pathway. J Immunol. 2013;191:3827-3837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 256. | Chakhtoura M, Azar ST. The role of vitamin d deficiency in the incidence, progression, and complications of type 1 diabetes mellitus. Int J Endocrinol. 2013;2013:148673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 257. | Pozzilli P, Manfrini S, Crinò A, Picardi A, Leomanni C, Cherubini V, Valente L, Khazrai M, Visalli N. Low levels of 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 in patients with newly diagnosed type 1 diabetes. Horm Metab Res. 2005;37:680-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 141] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 258. | Svoren BM, Volkening LK, Wood JR, Laffel LM. Significant vitamin D deficiency in youth with type 1 diabetes mellitus. J Pediatr. 2009;154:132-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 259. | Bener A, Alsaied A, Al-Ali M, Al-Kubaisi A, Basha B, Abraham A, Guiter G, Mian M. High prevalence of vitamin D deficiency in type 1 diabetes mellitus and healthy children. Acta Diabetol. 2009;46:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 260. | Feng R, Li Y, Li G, Li Z, Zhang Y, Li Q, Sun C. Lower serum 25 (OH) D concentrations in type 1 diabetes: A meta-analysis. Diabetes Res Clin Pract. 2015;108:e71-e75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 261. | Luong Kv, Nguyen LT, Nguyen DN. The role of vitamin D in protecting type 1 diabetes mellitus. Diabetes Metab Res Rev. 2005;21:338-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 262. | Mathieu C, Waer M, Laureys J, Rutgeerts O, Bouillon R. Prevention of autoimmune diabetes in NOD mice by 1,25 dihydroxyvitamin D3. Diabetologia. 1994;37:552-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 295] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 263. | Hyppönen E, Läärä E, Reunanen A, Järvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358:1500-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1277] [Cited by in RCA: 1154] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 264. | Fassbender WJ, Goertz B, Weismüller K, Steinhauer B, Stracke H, Auch D, Linn T, Bretzel RG. VDR gene polymorphisms are overrepresented in german patients with type 1 diabetes compared to healthy controls without effect on biochemical parameters of bone metabolism. Horm Metab Res. 2002;34:330-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 265. | Frederiksen B, Liu E, Romanos J, Steck AK, Yin X, Kroehl M, Fingerlin TE, Erlich H, Eisenbarth GS, Rewers M. Investigation of the vitamin D receptor gene (VDR) and its interaction with protein tyrosine phosphatase, non-receptor type 2 gene (PTPN2) on risk of islet autoimmunity and type 1 diabetes: the Diabetes Autoimmunity Study in the Young (DAISY). J Steroid Biochem Mol Biol. 2013;133:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 266. | De Azevêdo Silva J, Guimarães RL, Brandão LA, Araujo J, Segat L, Crovella S, Sandrin-Garcia P. Vitamin D receptor (VDR) gene polymorphisms and age onset in type 1 diabetes mellitus. Autoimmunity. 2013;46:382-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 267. | Simpson M, Brady H, Yin X, Seifert J, Barriga K, Hoffman M, Bugawan T, Barón AE, Sokol RJ, Eisenbarth G. No association of vitamin D intake or 25-hydroxyvitamin D levels in childhood with risk of islet autoimmunity and type 1 diabetes: the Diabetes Autoimmunity Study in the Young (DAISY). Diabetologia. 2011;54:2779-2788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 268. | Mathieu C, Badenhoop K. Vitamin D and type 1 diabetes mellitus: state of the art. Trends Endocrinol Metab. 2005;16:261-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 144] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 269. | Sørensen IM, Joner G, Jenum PA, Eskild A, Torjesen PA, Stene LC. Maternal serum levels of 25-hydroxy-vitamin D during pregnancy and risk of type 1 diabetes in the offspring. Diabetes. 2012;61:175-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 270. | Raab J, Giannopoulou EZ, Schneider S, Warncke K, Krasmann M, Winkler C, Ziegler AG. Prevalence of vitamin D deficiency in pre-type 1 diabetes and its association with disease progression. Diabetologia. 2014;57:902-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 271. | Van Belle TL, Vanherwegen AS, Feyaerts D, De Clercq P, Verstuyf A, Korf H, Gysemans C, Mathieu C. 1,25-Dihydroxyvitamin D3 and its analog TX527 promote a stable regulatory T cell phenotype in T cells from type 1 diabetes patients. PLoS One. 2014;9:e109194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 272. | Ferreira GB, Gysemans CA, Demengeot J, da Cunha JP, Vanherwegen AS, Overbergh L, Van Belle TL, Pauwels F, Verstuyf A, Korf H. 1,25-Dihydroxyvitamin D3 promotes tolerogenic dendritic cells with functional migratory properties in NOD mice. J Immunol. 2014;192:4210-4220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 273. | Gregori S, Giarratana N, Smiroldo S, Uskokovic M, Adorini L. A 1alpha,25-dihydroxyvitamin D(3) analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes. 2002;51:1367-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 348] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 274. | Han SY, So GA, Jee YH, Han KH, Kang YS, Kim HK, Kang SW, Han DS, Han JY, Cha DR. Effect of retinoic acid in experimental diabetic nephropathy. Immunol Cell Biol. 2004;82:568-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 275. | Kishi M, Yasuda H, Abe Y, Sasaki H, Shimizu M, Arai T, Okumachi Y, Moriyama H, Hara K, Yokono K. Regulatory CD8+ T cells induced by exposure to all-trans retinoic acid and TGF-beta suppress autoimmune diabetes. Biochem Biophys Res Commun. 2010;394:228-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 276. | Juang JH, Van YH, Kuo CH, Lin MY, Liu YH, Chang HY. Prevention and Reversal of Diabetes by All-Trans Retinoid Acid and Exendin-4 in NOD Mice. Int J Endocrinol. 2014;2014:435481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 277. | Iakhiaev MA, Iakhiaev AV. Graph-theoretical comparison of protein surfaces reveals potential determinants of cross-reactivity and the molecular mimicry. Mol Immunol. 2010;47:719-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 278. | Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167:1730-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 807] [Cited by in RCA: 741] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 279. | Aloia JF, Li-Ng M, Pollack S. Statins and vitamin D. Am J Cardiol. 2007;100:1329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 280. | Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85:1586-1591. [PubMed] |
| 281. | Cannell JJ, Vieth R, Umhau JC, Holick MF, Grant WB, Madronich S, Garland CF, Giovannucci E. Epidemic influenza and vitamin D. Epidemiol Infect. 2006;134:1129-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 707] [Cited by in RCA: 689] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 282. | Cannell JJ. Autism and vitamin D. Med Hypotheses. 2008;70:750-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 187] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 283. | Peterlik M, Cross HS. Vitamin D and calcium deficits predispose for multiple chronic diseases. Eur J Clin Invest. 2005;35:290-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 234] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 284. | Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1272] [Cited by in RCA: 1229] [Article Influence: 64.7] [Reference Citation Analysis (0)] |
| 285. | Vieth R. The role of vitamin D in the prevention of osteoporosis. Ann Med. 2005;37:278-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 286. | Villamor E. A potential role for vitamin D on HIV infection? Nutr Rev. 2006;64:226-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 287. | Zhou W, Suk R, Liu G, Park S, Neuberg DS, Wain JC, Lynch TJ, Giovannucci E, Christiani DC. Vitamin D is associated with improved survival in early-stage non-small cell lung cancer patients. Cancer Epidemiol Biomarkers Prev. 2005;14:2303-2309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 95] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 288. | Yamanaka K, Dimitroff CJ, Fuhlbrigge RC, Kakeda M, Kurokawa I, Mizutani H, Kupper TS. Vitamins A and D are potent inhibitors of cutaneous lymphocyte-associated antigen expression. J Allergy Clin Immunol. 2008;121:148-157.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 289. | Lemire JM, Ince A, Takashima M. 1,25-Dihydroxyvitamin D3 attenuates the expression of experimental murine lupus of MRL/l mice. Autoimmunity. 1992;12:143-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 170] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 290. | Durakovic C, Ray S, Holick MF. Topical paricalcitol (19-nor-1 alpha,25-dihydroxyvitamin D2) is a novel, safe and effective treatment for plaque psoriasis: a pilot study. Br J Dermatol. 2004;151:190-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 291. | Amuchastegui S, Daniel KC, Adorini L. Inhibition of acute and chronic allograft rejection in mouse models by BXL-628, a nonhypercalcemic vitamin D receptor agonist. Transplantation. 2005;80:81-87. [PubMed] |
| 292. | Hullett DA, Laeseke PF, Malin G, Nessel R, Sollinger HW, Becker BN. Prevention of chronic allograft nephropathy with vitamin D. Transpl Int. 2005;18:1175-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 293. | Middleton PG, Cullup H, Dickinson AM, Norden J, Jackson GH, Taylor PR, Cavet J. Vitamin D receptor gene polymorphism associates with graft-versus-host disease and survival in HLA-matched sibling allogeneic bone marrow transplantation. Bone Marrow Transplant. 2002;30:223-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 294. | Sommer A, Tarwotjo I, Djunaedi E, West KP, Loeden AA, Tilden R, Mele L. Impact of vitamin A supplementation on childhood mortality. A randomised controlled community trial. Lancet. 1986;1:1169-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 376] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 295. | Semba RD, Ndugwa C, Perry RT, Clark TD, Jackson JB, Melikian G, Tielsch J, Mmiro F. Effect of periodic vitamin A supplementation on mortality and morbidity of human immunodeficiency virus-infected children in Uganda: A controlled clinical trial. Nutrition. 2005;21:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 296. | Camacho LH. Clinical applications of retinoids in cancer medicine. J Biol Regul Homeost Agents. 2003;17:98-114. [PubMed] |
| 297. | Zhao Z, Murasko DM, Ross AC. The role of vitamin A in natural killer cell cytotoxicity, number and activation in the rat. Nat Immun. 1994;13:29-41. [PubMed] |
| 298. | Warrell RP, Frankel SR, Miller WH, Scheinberg DA, Itri LM, Hittelman WN, Vyas R, Andreeff M, Tafuri A, Jakubowski A. Differentiation therapy of acute promyelocytic leukemia with tretinoin (all-trans-retinoic acid). N Engl J Med. 1991;324:1385-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 916] [Cited by in RCA: 886] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 299. | Warrell RP, He LZ, Richon V, Calleja E, Pandolfi PP. Therapeutic targeting of transcription in acute promyelocytic leukemia by use of an inhibitor of histone deacetylase. J Natl Cancer Inst. 1998;90:1621-1625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 371] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 300. | Touma SE, Goldberg JS, Moench P, Guo X, Tickoo SK, Gudas LJ, Nanus DM. Retinoic acid and the histone deacetylase inhibitor trichostatin a inhibit the proliferation of human renal cell carcinoma in a xenograft tumor model. Clin Cancer Res. 2005;11:3558-3566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 301. | Nasr R, Guillemin MC, Ferhi O, Soilihi H, Peres L, Berthier C, Rousselot P, Robledo-Sarmiento M, Lallemand-Breitenbach V, Gourmel B. Eradication of acute promyelocytic leukemia-initiating cells through PML-RARA degradation. Nat Med. 2008;14:1333-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 279] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 302. | Escribese MM, Conde E, Martín A, Sáenz-Morales D, Sancho D, Pérez de Lema G, Lucio-Cazaña J, Sánchez-Madrid F, García-Bermejo ML, Mampaso FM. Therapeutic effect of all-trans-retinoic acid (at-RA) on an autoimmune nephritis experimental model: role of the VLA-4 integrin. BMC Nephrol. 2007;8:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 303. | Niwa S, Ochi T, Hirano Y, Wang T, Inagaki N, Shudo K, Nagai H. Effect of Am-80, a retinoid derivative, on 2, 4-dinitrofluorobenzene-induced contact dermatitis in mice. Pharmacology. 2000;60:208-214. [PubMed] |
| 304. | van de Kerkhof PC. Update on retinoid therapy of psoriasis in: an update on the use of retinoids in dermatology. Dermatol Ther. 2006;19:252-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 305. | Wang CJ, Heuts F, Ovcinnikovs V, Wardzinski L, Bowers C, Schmidt EM, Kogimtzis A, Kenefeck R, Sansom DM, Walker LS. CTLA-4 controls follicular helper T-cell differentiation by regulating the strength of CD28 engagement. Proc Natl Acad Sci USA. 2015;112:524-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 149] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 306. | Choi YS, Yang JA, Yusuf I, Johnston RJ, Greenbaum J, Peters B, Crotty S. Bcl6 expressing follicular helper CD4 T cells are fate committed early and have the capacity to form memory. J Immunol. 2013;190:4014-4026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 193] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 307. | Gregori S, Casorati M, Amuchastegui S, Smiroldo S, Davalli AM, Adorini L. Regulatory T cells induced by 1 alpha,25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J Immunol. 2001;167:1945-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 485] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 308. | Moore C, Fuentes C, Sauma D, Morales J, Bono MR, Rosemblatt M, Fierro JA. Retinoic acid generates regulatory T cells in experimental transplantation. Transplant Proc. 2011;43:2334-2337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |