Peer-review started: December 11, 2022
First decision: January 3, 2023
Revised: January 14, 2023
Accepted: February 2, 2023
Article in press: February 2, 2023
Published online: May 31, 2023
Processing time: 169 Days and 14.7 Hours
Diagnosis of coronavirus disease 2019 (COVID-19)-related neurological events in the pediatric population is challenging. Overlapping clinical picture of children with altered neurological state and inborn errors of metabolism, in addition to the frequency of asymptomatic COVID-19 cases, pose the main challenges for diagnosis. Diagnostic approaches to the onset post-COVID 19 subacute encephalopathy are still troublesome as seronegative autoimmune encephalitis (AIE) is reported.
A 27-mo-old boy was admitted for stormy refractory seizure of polymorphic semiology and altered mental status followed by various neuropsychiatric features that were suggestive of AIE. Brain magnetic resonance imaging and cerebrospinal fluid analysis were normal. Neither the immunological assessment, including viral serologies, antinuclear antibodies, autoimmune antibodies (NMDA, AMPA, CASPR2, LG11, GABARB, Hu, Yo, Ri, CV2, PNMA2, SOX1, Titin, amphiphysin, Recoverin), nor the metabolic assessment for lactate and pyruvate showed significant anomaly. Both positive history of COVID-19 infection and the findings of characteristic repetitive extreme delta brush played a key role in the diagnosis of COVID-19-related AIE. A remarkable improvement in the state of the child was noted after two pulse doses of intravenous Veino-globulin and high dose of intravenous Corticosteroid.
Diagnostic biomarkers for AIE might aid effective treatment.
Core Tip: Overlapping epileptic syndromes and etiologies in infancy constitute a real challenge to the diagnostic and therapeutic approach to autoimmune epilepsy related to post-coronavirus disease 2019 (COVID-19) autoimmune encephalitis (AIE). Indeed, epileptic seizures could entail the onset of seronegative form of post-COVID-19 AIE. Adequate seizure management and control might be hampered by potential misdiagnosis. Defining clinical and paraclinical biomarkers of seronegative form of post-COVID-19 AIE would enable early diagnosis and management, and better prognosis.
- Citation: Zouari Mallouli S, Jallouli O, Bouchaala W, Ben Nsir S, Kamoun Feki F, Charfi Triki C. Challenges to associate early onset epilepsy with COVID-19 autoimmune encephalitis: A case report. World J Immunol 2023; 13(1): 1-10
- URL: https://www.wjgnet.com/2219-2824/full/v13/i1/1.htm
- DOI: https://dx.doi.org/10.5411/wji.v13.i1.1
Since the outbreak of novel coronavirus 2 (SARS-CoV-2) in China in late December 2019, several acute and post-acute neurological events have been reported with the increasing number of infections[1]. These disorders were believed to arise either out of direct viral cytopathogenic effect in the acute phase or an indirect immune-mediated and vascular process in the post-acute phase. Recent literature on coronavirus disease 2019 (COVID-19)-related well-defined immune-mediated neurological conditions included central nervous system (CNS) disorders such as post-infectious immune encephalitis, CNS vasculitis, and myelitis as well as peripheral nervous system injury, mainly Guillain–Barre syndrome[2]. Although seizures occurred in conjunction with SARS-CoV-2 as acute symptomatic seizures or post-acute period due to brain immune response, little interest was given to seizure features and their underlying pathogenic mechanisms[3].
We here report an infant initially admitted for refractory seizures. Diagnosis and subsequent management of COVID-19-related AIE were delayed. Due to insufficient clinical and paraclinical biomarkers, it took a long time to establish the diagnostic criteria for a sero-negative form of autoimmune encephalitis (AIE). Data of similar cases were collected in order to establish clinical and para-clinical biomarkers for post-COVID-19 AIE diagnosis. Key words such as “COVID-19 related”, “neurological disorders”, neurological events”, “seizure”, “autoimmune encephalitis” were used to collect the data.
Despite the huge number of reports of different neurological disorders, data about the delayed development of neurological events after the onset of SARS-CoV-2 infection, and pathognomonic features of COVID-19-related features remain scarce. The causal link between COVID-19 and specific neurological manifestations has not been established yet. In addition, various factors should be considered before ascertaining the triggering role of COVID-19. Indeed, neurological symptoms may be due to COVID-19 systemic dysfunction including hypoxia, hypotension, electrolytic homeostasis disturbances, or even iatrogenic origin. Some COVID-19 patients seemed to be more prone to develop neurological events due to unknown genetic, demographic and/or environmental factors. Associating COVID-19 with neurological events is even more challenging in the pediatric population, especially those with prior disabling condition. Future studies on these factors would strengthen our understanding of neurological sequelae of COVID-19.
Epileptic seizures.
A 24-mo-old boy was admitted to our department for stormy onset of multifocal motor seizures.
Multifocal motor seizures occurred 2 wk after the child was infected with mild form of COVID-19 with flu-like syndrome.
Medical and family histories were unremarkable.
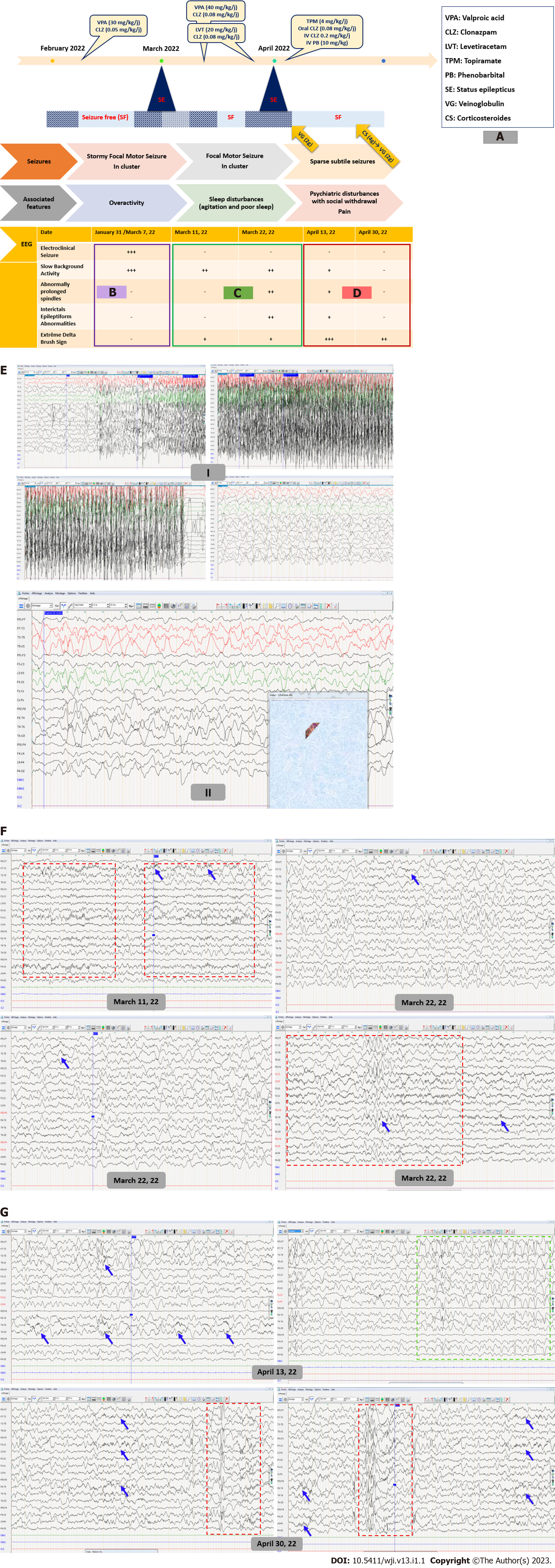
On neurological examination, the child showed some behavioral changes with unsteadiness and over-activity. No further neurological focal signs were noted. There was no fever or headache. On video filmed seizure during hospitalization, the child often yielded focal elementary semiology with machining, slow eye and head deviation associated with autonomic changes such as pallor, labial cyanosis, and hyperventilation. The motor component was made of clonic movements. Electroencephalography (EEG) showed slowed background activity without inter-ictal epileptiform abnormalities (IEA) with a recording of a tonic-clonic focal motor seizure synchronous to frontal discharges. Another EEG performed in the setting of altered mental status and fluctuating awareness showed irregular anterior high amplitude slow waves alternating with short periods of low-voltage activity in favor of non-motor epileptic seizure (Figure 1A and E). Diagnosed with early onset of severe epilepsy, the child was kept on valproate (VPA; 30 mg/kg/d) and clonazepam (CLZ; 0.05 mg/kg/d).
Repetitive peripheral blood biochemistry, C-reactive protein and blood cell count were found normal. Antinuclear antibodies and autoimmune antibodies serologies were negative. Cerebrospinal fluid (CSF) analysis was also normal. COVID-19 serology was positive.
Brain magnetic resonance imaging (MRI) showed no abnormalities.
After a seizure-free period of 2 wk, the child was readmitted for recurrent cluster seizures. Clinical seizure activity continued for several days despite anti-seizure medication (ASM) dose adjustment. Ultimately, the child developed a generalized motor status epilepticus requiring intensive care. Later, seizure control was maintained for one week with a higher dose of VPA (40 mg/kg/d). Then, the child exhibited another seizure storm with motor seizures of a tonic, clonic and myoclonic nature. At this stage, three standard EEGs found a global slowing of the background activity with Delta-brush activity. Abnormally extended and widespread spindles were also noticed (Figure 1A and F). Seizures were often accompanied by a slight increase in body temperature (38.5°C) without perceptible infectious site or biological inflammatory syndrome. Concurrently, the child showed restless sleep. Even on Levetiracetam (LVT; 20 mg/kg/d), the child ended up with a second refractory status epilepticus.
The child was diagnosed with autoimmune epilepsy due to post-COVID-19 AIE.
Disease-modifying treatment with intravenous veinoglobulin (2 g/kg) was started in addition to switching to Topiramate with a good seizure control outcome. Over the monitoring period, social withdrawal and sleep moaning were noted. EEG recording at that time showed better awake and sleep background activity organization but higher load of IEA and the classic aspect of “extreme delta brush” (EDB) (Figure 1A and G). Thus, three weeks later, the child received a second veinoglobulin pulse (0.4/kg/d for 5 d) after administration of high-dose intravenous methylprednisolone (30 mg/kg/d for 4 d) relayed by oral corticosteroids. Second-line treatment such as Rituximab and cyclophosphamide has been avoided because it has no AMM in children. Several new therapies including proteasome inhibitors (bortezomib), (IL)-6 inhibitors (tocilizumab), IL-2 at low dose and anti-CD20 antibodies (Ocrelizumab) are still under test.
During hospitalization, the child still had some subtle and brief seizures with ataxic gait. At outpatient follow-up visit 6 mo later, gait was back to normal. According to his mother, sleep disturbances and behavioral problems had disappeared. However, the child uttered no more than single words. Follow-up EEG recorded electroclinical seizures made of tonic spasms.
Here we report the case of a child with refractory seizure who was later diagnosed with seronegative post-COVID 19 AIE. Clinical and para-clinical red flags that led to this diagnosis were highlighted.
Since the COVID-19 outbreak, few researches have been carried out on pediatric population [4-7]. Thus, there is insufficient data to establish specific recommendations in this regard[4]. By mid-November 2021, the spread of the SARS-CoV-2 Omicron variant was associated with a notable increase in COVID-19 pediatric cases[8,9]. Until late 2021, Alpha and Delta variants, which infected mainly adults, had been the most investigated COVID-19 cases[10]. Therefore, the clinical picture of adult COVID-19 patients with neurological manifestation was by far superior to reports on pediatric population. By 2022, the emergence of Omicron variant was a pivotal event. It prompted an insight on the neurological clinical picture of children with increasing pediatric COVID-19 cases. A wide spectrum of neurological symptoms has been reported. Among these, infant seizures were deemed as an inaugural sign of onset COVID-19 infection and later in the para-infectious period[1,11]. It is particularly challenging to establish a direct and unique cause-effect link between COVID-19 disease and seizure in this age range due to the already higher frequency of such neurological feature. The spectrum of SARS-CoV-2-related neurological features includes headache, intracranial hypertension, meningismus, altered mental status, stroke, seizure, brainstem and cerebellar signs, as well peripheral nervous system involvement. Neurological symptoms develop at some points of the disease course and may even constitute a part of the onset initial presentation[1,12,13]. A paper on Omicron variant in South Africa reported convulsions as a major reason for the hospitalization of pediatric COVID-19 patients[8]. However, seizure types and EEG features were not reported. The present report addresses diagnostic pitfalls of COVID-19 autoimmune encephalitis with inaugural seizures and three-stage electro-clinical disease course (Figure 1A-D). In the present case, delta brush sign on EEG and the response to immunomodulatory treatment were key elements for a definitive diagnosis of AIE.
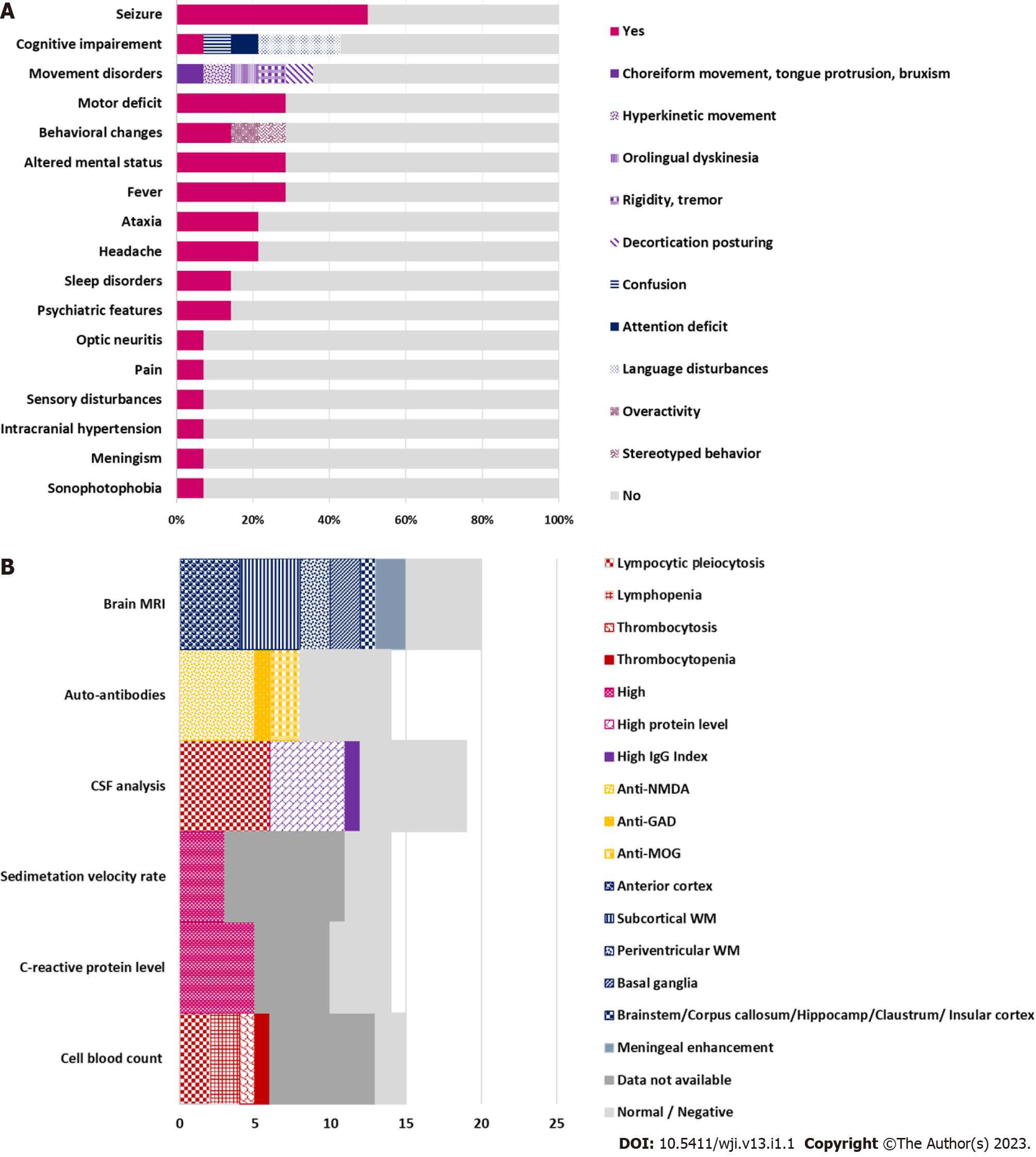
Despite the growing association between COVID-19 and various neurological features, the causal link between the novel virus and onset neurological presentation has not been established with sufficient certainty. So far, there are no clear criteria, risk scores and definite biomarkers that sustain the plausible link. This seems an imminent need notably faced with onset seizure at early childhood, a life-period known for its higher frequency of certain epileptic syndromes and encephalopathies. A recent paper linked seizures at onset COVID-19 infection with fever or hypoxia due to upper respiratory distress falling into the umbrella of febrile or provoked seizures. However, the authors stressed a probable predisposition of male sex and some ethnic groups to onset COVID-19-related febrile seizures which had the peculiarity to occur outside the typical age range for febrile seizures[11]. Altogether seizures were not attributed to the direct effect of the virus but rather to the preexistent potential in some individuals to develop seizure, a condition mandatory for epilepsy definition[14]. As for seizures occurring after a delay from onset COVID-19 infection, AIE was the major underlying cause. According data from six papers reporting 14 pediatric SARS-CoV-2-related AIE cases, seven patients (4 girls and 3 boys) presented seizures. Two of these children had a history of chronic neurological condition including migraine and developmental disorders with autism and intellectual disability. About 42.8% of patients had seizures as their main presenting symptom. The others developed seizures in the course of the disease. Seizure types included cluster generalized tonic-clonic seizures (28.5%), migraine aura triggered seizures (14.2%) and status epilepticus (14.2%). Associated neurological features included mainly cognitive impairment (42.8%) followed by movement disorders (35.7%). Fever, altered mental status, behavioral changes and motor deficit were present in 28.5% children. Other features were in decreasing order of frequency - headache and ataxia (21.4%), psychiatric symptoms and sleep disturbances (14.2%) (Table 1; Figure 2A)[15-20]. Most reports focused on describing clinical picture whilst little interest was given to disease course. The three-stage disease course with alternate period of initial response and resistance to ASM found in this case was previously reported in a child with migraine-aura triggered seizure inaugurating a seronegative AIE[20]. Hence, it is still unclear whether such evolution arises only from underlying pathogenic process or a probable interaction between ASM and autoantibodies’ target[21]. Previous studies on pediatric post-COVID-19 AIE reported a delay of diagnosis in three children. The delay was due to initial long-lasting mono-symptomatic presentation limited to epileptic seizures or isolated psychiatric features with transient improvement under brain-tropic molecules (LVT, Topiramate, atypical neuroleptic) as well as the failure of immunological assessment and neuroimaging to provide additional support elements[17,20]. The key diagnosis proof in the present report of post-COVID-19 seronegative AIE was evidenced by responsiveness to immunomodulatory treatment. The other less pronounced signs which gradually enrich the clinical picture can be attributed to a postictal state or an entry mode in an epileptic encephalopathy in a child with frequent and drug-resistant seizures. Later appearance of EDB sign sustained the diagnosis. While the stormy seizure period was associated with diffuse slow background activity, the occurrence of EDB sign was correlated with lower seizure activity and greater psychiatric problems. This finding is inconsistent with previous reports in which various EEG phenotypes were associated with anti-NMDA AIE. Among these, excessive beta activity and EDB are more likely to occur along with seizure and psychiatric symptoms, anxiety and cognitive impairment[22]. According to the literature, para-clinical biomarkers mostly include anterior cortex and subcortical white matter involvement on brain MRI (28.5%), lymphocytes rates changes (28.5%), lymphocytes pleiocytosis and high protein level on cerebrospinal fluid analysis (35.7%) and positive auto-immune antibodies mainly anti-NMDA (35.7%) (Table 1; Figure 2B). Although such biomarkers are not specific to post-COVID-19 AIE, response to immunomodulatory treatment was a hallmark of disease outcome in all reported cases, with complete recovery in 42.8% of patients, gradual improvement of one patient and incomplete recovery in 50% of patients. Residual symptoms included memory deficits (3/7), asomatognosia (1/7), psychiatric problems, ataxia, dysarthria and visual impairment (1/7)[15-20].
| Patient number | ||
| Clinical features | Epileptic seizures | 7/14 |
| Cognitive impairment | 6/14 | |
| Motor deficit | 4/14 | |
| Movement disorders | 5/14 | |
| Ataxia | 3/14 | |
| Pain/Headache | 4/14 | |
| Behavioral changes | 4/14 | |
| Psychiatric features | 2/14 | |
| Laboratory analysis | Cell blood count changes | 6/14 |
| High C-reactive protein level | 5/14 | |
| High sedimentation velocity rate | 3/14 | |
| CSF analysis: Pleiocytosis | 6/14 | |
| High protein level | 5/14 | |
| High IgG index | 1/14 | |
| Auto-antibodies | 8/14 | |
| Neuroimaging | Cortex involvement | 4/14 |
| Basal ganglia involvement | 2/14 | |
| White matter involvement | 6/14 |
The diagnosis of seronegative AIE in the setting of onset first seizure especially after COVID-19 with long-lasting mono-symptomatic presentation is still challenging. Nevertheless, this case report supports previous studies which suggest the following biomarkers. Clinical biomarkers: Febrile seizure outside the typical age range for febrile convulsions; Stormy, in cluster refractory seizure of various types; Transient response to ASM. EEG biomarkers: Interictal focal or diffuse slowing; Abnormally extended and widespread sleep spindles; Delta brush sign. Biological biomarkers: Lymphocyte count changes; Lymphocytic pleiocytosis, high protein and immunoglobulin G level in CSF analysis. Neuroimaging biomarkers: Predominant involvement of anterior (frontal, temporal and parietal) cortex and basal ganglia.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Neurosciences
Country/Territory of origin: Tunisia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dey J, India; El Sayed S, Egypt S-Editor: Liu JH L-Editor: Ma JY P-Editor: Liu JH
| 1. | Aljomah L, Almedlej S, Baarmah D, Altwaijri W, Alrumayyan A, Alrifai MT, Almuqbil M, Alshalaan M. Pediatrics COVID-19 and neurological manifestations: Single tertiary centre experience. eNeurologicalSci. 2021;24:100355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Beghi E, Giussani G, Westenberg E, Allegri R, Garcia-Azorin D, Guekht A, Frontera J, Kivipelto M, Mangialasche F, Mukaetova-Ladinska EB, Prasad K, Chowdhary N, Winkler AS. Acute and post-acute neurological manifestations of COVID-19: present findings, critical appraisal, and future directions. J Neurol. 2022;269:2265-2274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 3. | Westman G, Zelano J. Epilepsy diagnosis after Covid-19: A population-wide study. Seizure. 2022;101:11-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | García-Azorín D, Seeher KM, Newton CR, Okubadejo NU, Pilotto A, Saylor D, Winkler AS, Charfi Triki C, Leonardi M. Disruptions of neurological services, its causes and mitigation strategies during COVID-19: a global review. J Neurol. 2021;268:3947-3960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Triki CC, Leonardi M, Mallouli SZ, Cacciatore M, Karlshoej KC, Magnani FG, Newton CR, Pilotto A, Saylor D, Westenberg E, Walsh D, Winkler AS, Thakur KT, Okubadejo NU, Garcia-Azorin D. Global survey on disruption and mitigation of neurological services during COVID-19: the perspective of global international neurological patients and scientific associations. J Neurol. 2022;269:26-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | von Oertzen TJ, Macerollo A, Leone MA, Beghi E, Crean M, Oztuk S, Bassetti C, Twardzik A, Bereczki D, Di Liberto G, Helbok R, Oreja-Guevara C, Pisani A, Sauerbier A, Sellner J, Soffietti R, Zedde M, Bianchi E, Bodini B, Cavallieri F, Campiglio L, Maia LF, Priori A, Rakusa M, Taba P, Moro E, Jenkins TM; EANcore COVID-19 task force. EAN consensus statement for management of patients with neurological diseases during the COVID-19 pandemic. Eur J Neurol. 2021;28:7-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 7. | Direction Qualité des Soins et Sécurité des Patients. Guide parcours du patient suspect ou confirme COVID-19 reponse rapide. Septembre 2020. Available from: https://www.ineas.tn/sites/default/files/gps_covid-19.pdf. |
| 8. | Cloete J, Kruger A, Masha M, du Plessis NM, Mawela D, Tshukudu M, Tabea Manyane, Komane L, Venter M, Jassat W, Goga A, Feucht U. Rapid rise in paediatric COVID-19 hospitalisations during the early stages of the Omicron wave, Tshwane District, South Africa. medRxiv. . [DOI] [Full Text] |
| 9. | Cloete J, Kruger A, Masha M, du Plessis NM, Mawela D, Tshukudu M, Manyane T, Komane L, Venter M, Jassat W, Goga A, Feucht U. Paediatric hospitalisations due to COVID-19 during the first SARS-CoV-2 omicron (B.1.1.529) variant wave in South Africa: a multicentre observational study. Lancet Child Adolesc Health. 2022;6:294-302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 140] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 10. | Réseau de Veille et de Riposte « EPICOV-TUN ». Bulletin de veille SARS-CoV-2-Tunisie. Octobre 2021. Available from: https://www.onmne.tn/wp-content/uploads/2021/10/Bulletin-de-veille-SARS-CoV-2-10102021-VFC.pdf. |
| 11. | Ludvigsson JF. Convulsions in children with COVID-19 during the Omicron wave. Acta Paediatr. 2022;111:1023-1026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 54] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 12. | Abdel-Mannan O, Eyre M, Löbel U, Bamford A, Eltze C, Hameed B, Hemingway C, Hacohen Y. Neurologic and Radiographic Findings Associated With COVID-19 Infection in Children. JAMA Neurol. 2020;77:1440-1445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 271] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 13. | Chen TH. Neurological involvement associated with COVID-19 infection in children. J Neurol Sci. 2020;418:117096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 14. | Falco-Walter JJ, Scheffer IE, Fisher RS. The new definition and classification of seizures and epilepsy. Epilepsy Res. 2018;139:73-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 214] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 15. | Payus AO, Jeffree MS, Ohn MH, Tan HJ, Ibrahim A, Chia YK, Raymond AA. Immune-mediated neurological syndrome in SARS-CoV-2 infection: a review of literature on autoimmune encephalitis in COVID-19. Neurol Sci. 2022;43:1533-1547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 16. | Stoian A, Stoian M, Bajko Z, Maier S, Andone S, Cioflinc RA, Motataianu A, Barcutean L, Balasa R. Autoimmune Encephalitis in COVID-19 Infection: Our Experience and Systematic Review of the Literature. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 17. | Nabizadeh F, Balabandian M, Sodeifian F, Rezaei N, Rostami MR, Naser Moghadasi A. Autoimmune encephalitis associated with COVID-19: A systematic review. Mult Scler Relat Disord. 2022;62:103795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 18. | Sarigecili E, Arslan I, Ucar HK, Celik U. Pediatric anti-NMDA receptor encephalitis associated with COVID-19. Childs Nerv Syst. 2021;37:3919-3922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 19. | Sánchez-Morales AE, Urrutia-Osorio M, Camacho-Mendoza E, Rosales-Pedraza G, Dávila-Maldonado L, González-Duarte A, Herrera-Mora P, Ruiz-García M. Neurological manifestations temporally associated with SARS-CoV-2 infection in pediatric patients in Mexico. Childs Nerv Syst. 2021;37:2305-2312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 20. | Hilado M, Banh M, Homans J, Partikian A. Pediatric Autoimmune Encephalitis Following COVID-19 Infection. J Child Neurol. 2022;37:268-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Jang Y, Kim DW, Yang KI, Byun JI, Seo JG, No YJ, Kang KW, Kim D, Kim KT, Cho YW, Lee ST; Drug Committee of Korean Epilepsy Society. Clinical Approach to Autoimmune Epilepsy. J Clin Neurol. 2020;16:519-529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Huang Q, Liao Y, Ma M, Wu Y. Delta brush variant: A novel ictal EEG pattern in anti-NMDAR encephalitis. Epilepsia Open. 2020;5:507-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |