Published online Oct 30, 2021. doi: 10.5411/wji.v11.i2.11
Peer-review started: May 9, 2021
First decision: July 27, 2021
Revised: August 16, 2021
Accepted: October 13, 2021
Article in press: October 13, 2021
Published online: October 30, 2021
Processing time: 171 Days and 6.9 Hours
In liver transplant patients, solid tumors and post-transplant lymphoproliferative disorders have emerged as significant long-term mortality causes. In addition, it is assumed that de novo malignancy after liver transplantation (LT) is the second-leading cause of death after cardiovascular complications. Well-established risk factors for post-transplant lymphoproliferative disorders and solid tumors are calcineurin inhibitors, tacrolimus, and cyclosporine, the cornerstones of all immunosuppressive therapies used after LT. The loss of immunocompetence facilitated by the host immune system due to prolonged immunosuppressive therapy leads to cancer development, including LT patients. Furthermore, various mechanisms such as bacterial dysbiosis, activation through microbe-associated molecular patterns, leaky gut, and bacterial metabolites can drive cancer-promoting liver inflammation, fibrosis, and genotoxicity. Therefore, changes in human microbiota composition may contribute further to de novo carcinogenesis associated with the severe immunosuppression after LT.
Core Tip: Liver transplant recipients have a higher risk of developing de novo malignancy compared to the general population. Immunosuppressive therapy used after liver transplantation is a substantial risk factor for the development of de novo malignancy. Tumorigenesis in liver transplantation patients is linked to the length and intensity of immunosuppression. Data show that the microbiota could significantly affect the survival and acceptance of transplanted allographs. This once again indicates the incredibly complex interaction between the immune system and microbiome in the settings of liver transplantation and raises the possible strategies to induce immunotolerance and reduce complications such as de novo malignancy.
- Citation: Peruhova M, Peshevska-Sekulovska M, Velikova T. Interactions between human microbiome, liver diseases, and immunosuppression after liver transplant. World J Immunol 2021; 11(2): 11-16
- URL: https://www.wjgnet.com/2219-2824/full/v11/i2/11.htm
- DOI: https://dx.doi.org/10.5411/wji.v11.i2.11
Liver transplantation (LT) is the definitive curable treatment method for patients with decompensated liver disease, cirrhosis, acute hepatic dysfunction, and hepatocellular cancer[1]. The mortality rate following LT, on the other hand, remains high. Infections and surgical complications continue to be the leading causes of death in the early post-transplantation period[2]. Furthermore, solid tumors and post-transplant lymphoproliferative disorders have arisen as a major long-term cause of death in liver transplant patients[3], where microbiota could significantly contribute. It is of great importance to underline the subtle difference between the two terms microbiome and microbiota. Microbiome is characterized as a collection of all microorganism with their belonging genetic materials in environment. On the other hand, the term microbiota is defined as an aggregation of microorganisms (bacteria, viruses, fungi) found within a specific environment. For example, gut microbiota differs from bronchial and skin microbiota, thus the origin of the microbiota is essential[4].
Dysbiosis is a condition of alterations in microbiota due to exposure to various environment factors, such as drugs, pathogens, toxins, and diet. It is well established that gut microbiota is one of the most important factors for vital functions, thus the dysbiosis may contribute to many different pathogenic disorders in the human body[5].
In transplantation settings, there is an extremely complex relationship between the immune system and the microbiome. The early post-LT period carries a risk of infectious complications due to surgical or ischemia-reperfusion injury as well as those relate to immunosuppressive (IS) treatment[6]. Furthermore, LT patients are projected to have a greater chance of contracting de novo malignancy than the general public, with standardized incidence rates ranging from 2.3 to 4.3[7,8]. Furthermore, it is thought that de novo malignancy after LT is the second-leading cause of death after cardiovascular complications, especially for smoking-induced cancers (e.g., head and neck, lung, and esophageal) and virus-induced cancers (e.g., cervical and Kaposi’s sarcoma)[9].
The aim of this paper is to provide an outline of the interaction between the immune system and microbiome after LT and the loss of immunocompetence mediated by the host immune system as a result of extended IS therapy. Although gut microbiota is essential for the host, especially in terms of metabolism and immunity, the role of the gut microbiota in disease processes, including cancer, is also increasingly unclear[10].
In terms of the microbiota and the underlying disease (e.g., the indications of LT), the avoidance of reoccurring chronic liver diseases after LT depends primarily on the IS treatment. However, it can occur in combination with microbial dysbiosis. Additional complications that may influence the microbiome include the progression of newly occurring diabetes mellitus and other complications post-transplant by changes in host metabolic homeostasis connected to immunosuppression and related to gut microbiota. This once again indicates the incredibly complex interaction between the immune system and microbiome in transplantation settings and raises the possible strategies to induce immunotolerance and reduce the complications and mortality rate[11].
Recently, we reviewed the antibiotic changes of gut microbiota and their impact on the failure of checkpoint inhibitor treatment of colorectal carcinoma[12].
One can assume that changes in human microbiota composition may contribute further to de novo carcinogenesis associated with the severe immunosuppression after LT[13]. Indeed, the microbiome composition and function can be altered directly or indirectly by IS drugs, including chemotherapeutics, corticosteroids, biologics, etc.[14]. The research area of immunosuppression agents and gut microbiome interactions is a highly evolving topic of interest[15]. For example, dysbiosis associated with tacrolimus (TAC) is characterized by reducing the total diversity of the gut microbiota and a lack of butyrate-producing species. Furthermore, patients on TAC and mycophenolate mofetil have a distinct bacterial metagenome compared with everolimus and mycophenolate mofetil, indicating that functional variations in gut microbiota are based on the IS regimen regardless of the shift in taxonomy[16,17].
Jiang et al[18] conducted a study on liver transplanted mice in order to assess the influence of TAC dosing on the gut microbiota. They established that 30 d after LT, rats that received a dose of 0.5 mg/kg had an overabundance of Faecalibacterium prausnitzii and Bifidobacterium spp and low amount of Bacteroides-Prevotella and Enterobacteriaceae. The authors pointed out that pathogenic endotoxin-producing bacteria could be altered by administration of probiotics in the post-LT period. Therefore, the optimal IS dosages for recipients following LT is of paramount importance for maintaining microbiota balance[18].
We have to mention that this interaction is reciprocal: Microbiota can impact drug absorption and metabolism. This was observed with Lactobacillus and TAC-induced hypertension, for example[19]. Several longitudinal trials of patients after LT showed different levels of gut dysbiosis. The metabolic consequences of dysbiosis were related to the risk of disease recurrence and metabolic-associated immunosuppressants side effects. Thus, microbial therapies may be considered to reduce many of the anticipated complications[20].
An interesting study by Parrilli et al[21] explained the effect of chronic usage of cyclosporin A after LT on gastroduodenal and intestinal permeability and blood endotoxin levels in patients 2 to 3 years after LT. Their study revealed that cyclosporin A is well tolerated, and it did not carry a risk of potential substantial injury in the gut for LT patients. However, this study is limited because only 32 patients were included, thus more studies with similar designs have to be conducted[21].
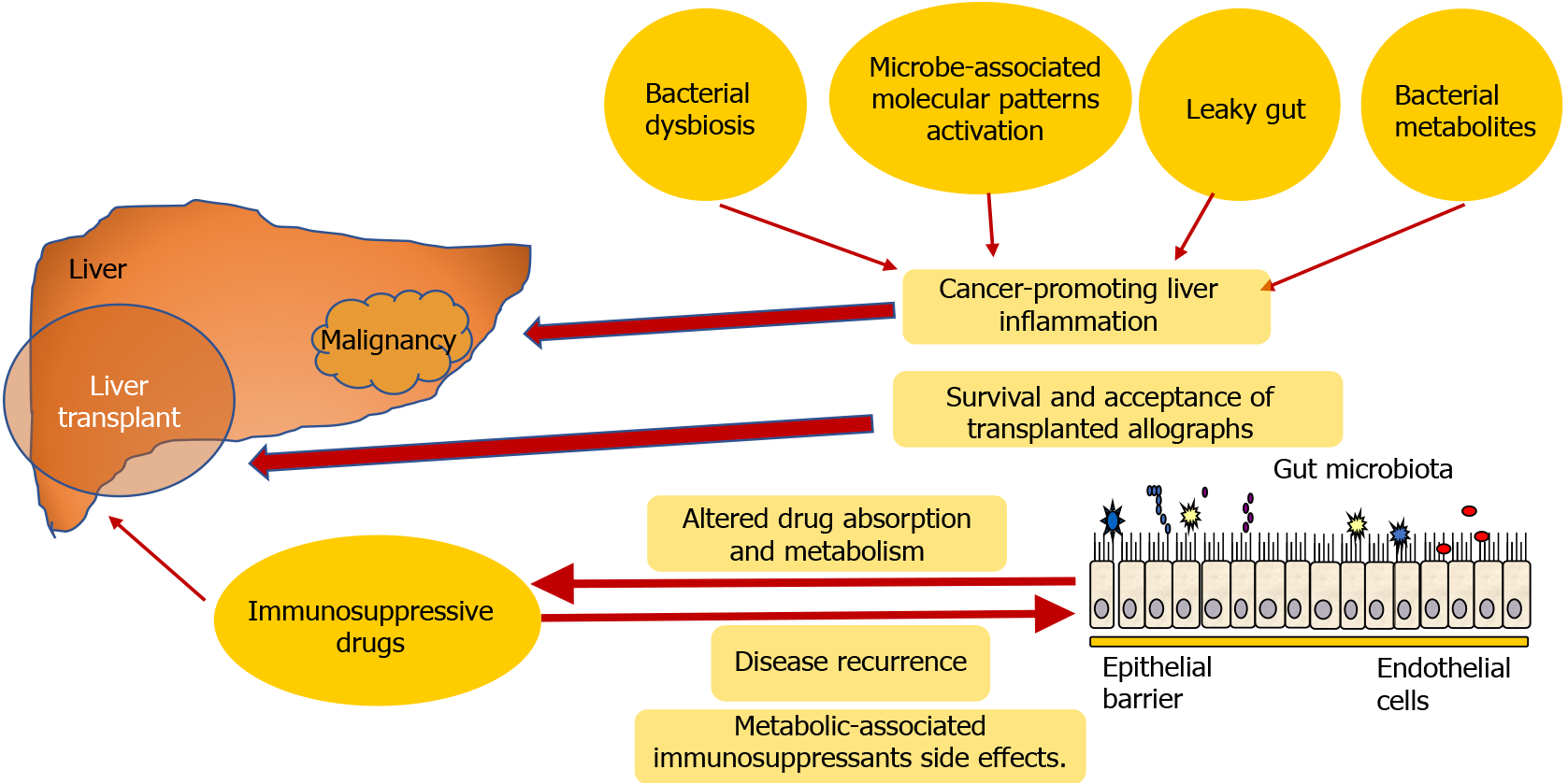
Recent data showed that the microbiota could greatly affect the survival and acceptance of transplanted allographs. This supposed relationship has also become a field of increasing interest, catalyzed by advancements in methodology (e.g., -omics and depth sequencing techniques). Furthermore, microbiota and the immune system reciprocally influence each other. This is especially valid, considering that the allografts themselves harbor immune cells and/or perform immunobiological activities[14]. We present the possible mechanisms in Figure 1. Furthermore, rat models suggested that a microbiota profile or changes might predict acute rejection after transplantation[22]. As ischemia-reperfusion injury is associated with the influence of the gut microbiota on early innate immune activation, it can predict early allograft failure and long-term graft survival and is of particular significance to research[22]. Some animal studies have shown the impact of intestinal microbiota on early rejection after LT[23,24].
A correlation was established between acute rejection and overabundance of Bacteroides and Ruminococcus after LT[23]. Another important data from a study by Ren et al[24] demonstrated that acute cellular rejection in rats after LT is associated with increased levels of Clostridium bolteae and decreased levels of Faecalibacterum prausnitzii and Lactobacillus spp. The possible mechanism of acute rejection and changes in the human microbiome after LT remains unclear. However, a significant increase in intestinal permeability was found in rats after LT with acute rejection. Thus, further human studies need to be conducted in order to evaluate the possible interaction between the microbiome and acute rejection[6].
It was shown that gut microbiota foster the development of various liver diseases, including nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, alcoholic fatty liver disease, cirrhosis, and hepatocellular carcinoma (HCC)[25].
Various mechanisms were linked to HCC carcinogenesis promotion of the gut microbiota as well: Bacterial dysbiosis, activation through microbe-associated molecular patterns, leaky gut, and bacterial metabolites. All these mechanisms can drive cancer-promoting liver inflammation, fibrosis, and genotoxicity[26].
Furthermore, activation of the toll-like receptor 4 signaling pathways by lipopolysaccharide in Kupffer cells has been shown to contribute to compensatory hepatocyte proliferation in tumor necrosis factor- and interleukin-6-dependent cells as well as to oxidative stress and apoptosis reduction. In addition, toll-like receptor 4 activation in HCC cell lines with lipopolysaccharide causes epithelial-mesenchymal transition[27].
Sivan et al[28] published an interesting study about the potential anti-HCC tumor role of Bifidobacterium in patients after LT. In their study, they proved that administration of probiotics containing Bifidobacterium strains reduces tumor progression in a rat HCC model[28]. Taken together, these findings suggest that Bifidobacterium-based therapy can play a role in reducing the risk of recurrent HCC after transplantation.
The gut microbiome plays a critical role in controlling hepatic metabolism and immunity, and our knowledge of its effect on post-LT physiology is rapidly expanding. Therapeutic goals for microbial-based treatments will continue to develop as evidence from longitudinal studies of post-LT patients is collected, in order to enhance allograft function and reduce the risk of post-LT complications. In order to increase clinical results in post-LT patients, we hope to use microbial-based therapies in conjunction with our existing standard of care.
Manuscript source: Unsolicited manuscript
Specialty type: Immunology
Country/Territory of origin: Bulgaria
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bhanji R, Sira AM S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
| 1. | Fox AN, Brown RS Jr. Is the patient a candidate for liver transplantation? Clin Liver Dis. 2012;16:435-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Adam R, Karam V, Delvart V, O'Grady J, Mirza D, Klempnauer J, Castaing D, Neuhaus P, Jamieson N, Salizzoni M, Pollard S, Lerut J, Paul A, Garcia-Valdecasas JC, Rodríguez FS, Burroughs A; All contributing centers (www. eltr.org); European Liver and Intestine Transplant Association (ELITA). Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR). J Hepatol. 2012;57:675-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 643] [Article Influence: 49.5] [Reference Citation Analysis (2)] |
| 3. | Engels EA, Pfeiffer RM, Fraumeni JF Jr, Kasiske BL, Israni AK, Snyder JJ, Wolfe RA, Goodrich NP, Bayakly AR, Clarke CA, Copeland G, Finch JL, Fleissner ML, Goodman MT, Kahn A, Koch L, Lynch CF, Madeleine MM, Pawlish K, Rao C, Williams MA, Castenson D, Curry M, Parsons R, Fant G, Lin M. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306:1891-1901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1114] [Cited by in RCA: 1093] [Article Influence: 78.1] [Reference Citation Analysis (0)] |
| 4. | Ursell LK, Metcalf JL, Parfrey LW, Knight R. Defining the human microbiome. Nutr Rev. 2012;70 Suppl 1:S38-S44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 714] [Article Influence: 54.9] [Reference Citation Analysis (1)] |
| 5. | Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ. Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis. 2015;26:26191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 514] [Cited by in RCA: 801] [Article Influence: 80.1] [Reference Citation Analysis (0)] |
| 6. | Doycheva I, Leise MD, Watt KD. The Intestinal Microbiome and the Liver Transplant Recipient: What We Know and What We Need to Know. Transplantation. 2016;100:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Na R, Grulich AE, Meagher NS, McCaughan GW, Keogh AM, Vajdic CM. Comparison of de novo cancer incidence in Australian liver, heart and lung transplant recipients. Am J Transplant. 2013;13:174-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Krynitz B, Edgren G, Lindelöf B, Baecklund E, Brattström C, Wilczek H, Smedby KE. Risk of skin cancer and other malignancies in kidney, liver, heart and lung transplant recipients 1970 to 2008--a Swedish population-based study. Int J Cancer. 2013;132:1429-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 261] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 9. | Carenco C, Faure S, Ursic-Bedoya J, Herrero A, Pageaux GP. Solid, non-skin, post-liver transplant tumors: Key role of lifestyle and immunosuppression management. World J Gastroenterol. 2016;22:427-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16:341-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1428] [Cited by in RCA: 2246] [Article Influence: 280.8] [Reference Citation Analysis (1)] |
| 11. | Ling Q, Xu X, Wang B, Li L, Zheng S. The Origin of New-Onset Diabetes After Liver Transplantation: Liver, Islets, or Gut? Transplantation. 2016;100:808-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Velikova T, Krastev B, Lozenov S, Gencheva R, Peshevska-Sekulovska M, Nikolaev G, Peruhova M. Antibiotic-Related Changes in Microbiome: The Hidden Villain behind Colorectal Carcinoma Immunotherapy Failure. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Peruhova M, Peshevska-Sekulovska M, Panayotova G, Velikova T. Foremost Concepts in Mechanisms of De Novo Post-Liver Transplantation Malignancy. Gastroenterol Ins. 2021;12:283-292. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Kanangat S. Modulation of alloimmune response by commensal gut microbiota and potential new avenues to influence the outcome of allogeneic transplantation by modification of the 'gut culture'. Int J Immunogenet. 2017;44:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Acharya C, Bajaj JS. Gut Microbiota and Complications of Liver Disease. Gastroenterol Clin North Am. 2017;46:155-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 16. | Toral M, Romero M, Rodríguez-Nogales A, Jiménez R, Robles-Vera I, Algieri F, Chueca-Porcuna N, Sánchez M, de la Visitación N, Olivares M, García F, Pérez-Vizcaíno F, Gálvez J, Duarte J. Lactobacillus fermentum Improves Tacrolimus-Induced Hypertension by Restoring Vascular Redox State and Improving eNOS Coupling. Mol Nutr Food Res. 2018;e1800033. [RCA] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 17. | Zaza G, Dalla Gassa A, Felis G, Granata S, Torriani S, Lupo A. Impact of maintenance immunosuppressive therapy on the fecal microbiome of renal transplant recipients: Comparison between an everolimus- and a standard tacrolimus-based regimen. PLoS One. 2017;12:e0178228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 18. | Jiang JW, Ren ZG, Lu HF, Zhang H, Li A, Cui GY, Jia JJ, Xie HY, Chen XH, He Y, Jiang L, Li LJ. Optimal immunosuppressor induces stable gut microbiota after liver transplantation. World J Gastroenterol. 2018;24:3871-3883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Lee JR, Muthukumar T, Dadhania D, Taur Y, Jenq RR, Toussaint NC, Ling L, Pamer E, Suthanthiran M. Gut microbiota and tacrolimus dosing in kidney transplantation. PLoS One. 2015;10:e0122399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 139] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 20. | Qi X, Yang M, Stenberg J, Dey R, Fogwe L, Alam MS, Kimchi ET, Staveley-O'Carroll KF, Li G. Gut microbiota mediated molecular events and therapy in liver diseases. World J Gastroenterol. 2020;26:7603-7618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Parrilli G, Abazia C, Sarnelli G, Corsaro MM, Coccoli P, Viglione L, Cuomo R, Budillon G. Effect of chronic administration of tacrolimus and cyclosporine on human gastrointestinal permeability. Liver Transpl. 2003;9:484-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Davies YK, Tsay CJ, Caccamo DV, Cox KM, Castillo RO, Cox KL. Successful treatment of recurrent primary sclerosing cholangitis after orthotopic liver transplantation with oral vancomycin. Case Rep Transplant. 2013;2013:314292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Xie Y, Luo Z, Li Z, Deng M, Liu H, Zhu B, Ruan B, Li L. Structural shifts of fecal microbial communities in rats with acute rejection after liver transplantation. Microb Ecol. 2012;64:546-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Ren Z, Jiang J, Lu H, Chen X, He Y, Zhang H, Xie H, Wang W, Zheng S, Zhou L. Intestinal microbial variation may predict early acute rejection after liver transplantation in rats. Transplantation. 2014;98:844-852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 25. | Brandi G, De Lorenzo S, Candela M, Pantaleo MA, Bellentani S, Tovoli F, Saccoccio G, Biasco G. Microbiota, NASH, HCC and the potential role of probiotics. Carcinogenesis. 2017;38:231-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 123] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 26. | Yu LX, Schwabe RF. The gut microbiome and liver cancer: mechanisms and clinical translation. Nat Rev Gastroenterol Hepatol. 2017;14:527-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 413] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 27. | Jing YY, Han ZP, Sun K, Zhang SS, Hou J, Liu Y, Li R, Gao L, Zhao X, Zhao QD, Wu MC, Wei LX. Toll-like receptor 4 signaling promotes epithelial-mesenchymal transition in human hepatocellular carcinoma induced by lipopolysaccharide. BMC Med. 2012;10:98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 28. | Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B, Alegre ML, Chang EB, Gajewski TF. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1979] [Cited by in RCA: 2830] [Article Influence: 283.0] [Reference Citation Analysis (1)] |