Published online Mar 24, 2016. doi: 10.5410/wjcu.v5.i1.53
Peer-review started: August 31, 2015
First decision: October 8, 2015
Revised: October 30, 2015
Accepted: February 14, 2016
Article in press: February 16, 2016
Published online: March 24, 2016
Processing time: 202 Days and 17.6 Hours
AIM: To evaluate the perioperative and long term outcomes of cystectomy in obese patients.
METHODS: This is a retrospective review of 580 patients for whom radical cystectomy (RC) was performed for primary urothelial bladder cancer between November 1996-April 2013 at a single institution. Body mass index (BMI) was available for 424 patients who were categorized as underweight (< 18.5), normal (18.5-24.9), overweight (25.0-29.9), and obese (≥ 30). Baseline demographics, perioperative outcomes, and survival were assessed. Overall survival (OS) and disease specific survival (DSS) was estimated by Kaplan-Meier method. Medians were compared using the Mann-Whitney U Test. Categorical variables were compared using the χ2 test. A P-value of < 0.05 was considered statistically significant. Statistical analyses were performed using the Software Package for the Social Sciences (SPSS), Version 20 (International Business Machines SPSS, Chicago, IL, United States).
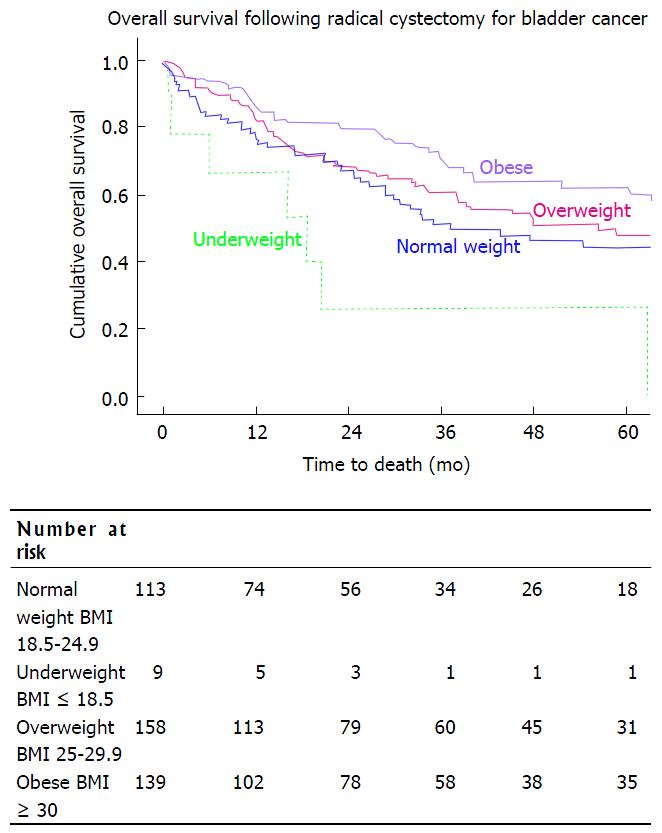
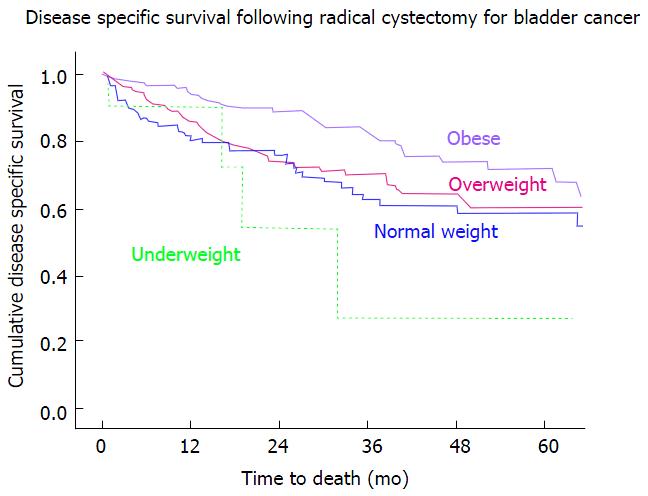
RESULTS: The median age of all patients was 69 years (inter-quartile range 60-75) and median follow-up was 23.4 mo (8.7-55.1). Patients were characterized as underweight [9, (2.1%)], normal [113, (26.7%)], overweight [160, (37.8%)], or obese [142, (33.5%)]. Estimated blood loss during RC was higher in the obese group (800 mL) as compared to the normal weight group (500 mL). However, need for transfusion (47.7% vs 52.1%), number of lymph nodes resected (32 vs 30), length of stay (9 d vs 8 d), and 30-d readmission (29.7% vs 25.2%) between obese and normal BMI patients were similar. Obese patients underwent ileal neobladder diversion in 42% of cases, compared to 24% of normal BMI patients (0.003). Normal BMI and obese patients had comparable urinary incontinence (21.4% vs 25.6%, P = 0.343), and need for intermittent catheterization (14.3% vs 5.2%, P = 0.685) at 2 years follow-up. Overall survival was better in obese compared to normal BMI patients on univariate analysis, with median survival of 67 mo vs 37 mo, respectively (P = 0.031). Disease specific survival in these populations followed the same Kaplan Meier curve, with the obese group having a significantly improved OS, P = 0.016. Underweight patients had a significantly worse prognosis, with a median overall survival of 19 mo (P = 0.018). Disease specific survival was significantly worse in the underweight group compared to the obese group, P = 0.007. On multivariate analysis underweight patients remained at increased risk for death (HR = 3.1, P = 0.006), as were older patients (HR = 1.6, P = 0.006), those with multiple nodal metastases (HR = 3.7, P = 0.007), and those who had received neoadjuvant chemotherapy (HR = 2.0, P = 0.015).
CONCLUSION: Perioperative outcomes and survival following RC in obese patients is comparable with non-obese patients. Underweight patients have the worst OS and DSS.
Core tip: As obesity rates increase, more obese patients will require radical cystectomy for muscle invasive bladder cancer. Anecdotally, obesity increases the technical difficulty of an operation. Literature regarding outcomes of obese patients undergoing radical cystectomy is limited. This study shows obese patients do better than their non-obese counterparts in terms of perioperative outcomes and overall and disease specific survival. However, we found that underweight patients have a significantly decreased overall and disease specific survival compared with obese and patients.
- Citation: Burge BK, Blackwell RH, Wilson A, Flanigan RC, Gupta GN, Quek ML. Perioperative outcomes and survival of radical cystectomy as a function of body mass index. World J Clin Urol 2016; 5(1): 53-59
- URL: https://www.wjgnet.com/2219-2816/full/v5/i1/53.htm
- DOI: https://dx.doi.org/10.5410/wjcu.v5.i1.53
The prevalence of obesity in the United States continues to rise on a yearly basis[1]. Defined as a body mass index (BMI) > 30, it is estimated that 27.2% of the American population in 2013 was obese, increased from 25.5% in 2008. The estimated population that is overweight, defined as a BMI of 25 to < 30, accounted for 35.5%, making greater than half of the population heavier than their ideal body weight[1]. These rates may be underestimated, as the National Center for Health Sciences estimated in 2009-2010 that obesity was present in 35.7% in adults and 16.9% in children[2].
Regardless of the discrepancies, it is clear that an ever growing percentage of our population is overweight or obese. It follows then that an increasing number of patients presenting for surgical management of invasive bladder cancer will be subject to this trend. Anecdotally, increasing body habitus negatively impacts technical ease of surgery. For example, in the general surgery literature, obesity has been correlated with longer operating times during cholecystectomy, mastectomy, and colectomy[3]. Similar findings were demonstrated by Maurer et al[4] in 2009 for radical cystectomy, with increased operative time in obese compared with non-obese patients.
The current literature regarding other surgical outcomes of radical cystectomy in obese patients is limited. Furthermore, to our knowledge, studies evaluating the impact of BMI on surgical outcomes do not differentiate underweight patients from the normal weight cohort. Herein we examine the impact of BMI on perioperative and long term outcomes of radical cystectomy.
A retrospective chart review was performed for 580 patients who underwent radical cystectomy at a single institution for urothelial cell carcinoma of the bladder between November 1996 and April 2013. Baseline patient demographics, comorbidities, and clinical cancer characteristics were recorded (Table 1). Surgical technique (open vs robotic-assisted laparoscopic) was chosen at the discretion of the primary surgeon (557 vs 23, respectively). BMI was calculated from height and weight data recorded prior to cystectomy (kg/m2). BMI information was available for 424 patients, who were used in the final analysis. Patients were categorized into underweight (BMI < 18.5), normal weight (BMI 18.5-24.9), overweight (BMI 25-29.9), and obese (BMI ≥ 30) using World Health Organization BMI criteria[5].
| Normal Weight(BMI 18.5-24.9) | Underweight(BMI < 18.5) | Overweight(BMI 25.0-29.9) | Obese(BMI > 30) | P value | ||
| Age at surgery (median, IQR), yr | 70 (63-76) | 78 (68-79) | 70 (61-76) | 67 (58-72) | 0.126 | |
| Gender | Male | 74 (66%) | 4 (44%) | 130 (81%) | 106 (75%) | 0.005 |
| Female | 39 (35%) | 5 (56%) | 30 (19%) | 36 (25%) | ||
| Clinical tumor stage | cT0 | 1 (1%) | 0 (0%) | 1 (1%) | 1 (1%) | 0.05 |
| cTis | 0 (0%) | 0 (0%) | 8 (5%) | 11 (8%) | ||
| cTa | 7 (7%) | 1 (11%) | 9 (6%) | 10 (8%) | ||
| cT1 | 30 (28%) | 2 (22%) | 26 (17%) | 35 (26%) | ||
| cT2 | 65 (61%) | 4 (44%) | 103 (67%) | 67 (50%) | ||
| cT3 | 3 (3%) | 2 (22%) | 4 (3%) | 5 (4%) | ||
| cT4 | 1 (1%) | 0 (0%) | 3 (2%) | 4 (3%) | ||
| Prior intravesical therapy | No | 86 (78%) | 6 (67%) | 113 (71%) | 83 (59%) | 0.018 |
| Yes | 25 (22%) | 3 (33%) | 47 (29%) | 57 (41%) | ||
| Prior neoadjuvant chemotherapy | No | 100 (90%) | 9 (100%) | 150 (94%) | 129 (92%) | 0.478 |
| Yes | 11 (10%) | 0 (0%) | 9 (6%) | 11 (8%) | ||
| Race | Caucasian | 105 (92%) | 9 (100%) | 151 (94%) | 134 (94%) | 0.492 |
| Hispanic | 3 (3%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Black | 3 (3%) | 0 (0%) | 6 (4%) | 7 (5%) | ||
| Asian | 1 (1%) | 0 (0%) | 2 (1%) | 0 (0%) | ||
| Unknown | 1 (1%) | 0 (0%) | 1 (1%) | 1 (1%) | ||
| Hypertension | No | 52 (46%) | 2 (22%) | 73 (46%) | 33 (23%) | < 0.001 |
| Yes | 60 (54%) | 7 (78%) | 87 (54%) | 108 (77%) | ||
| Diabetes mellitus, type II | No | 99 (88%) | 8 (89%) | 132 (82%) | 100 (71%) | 0.006 |
| Yes | 13 (12%) | 1 (11%) | 28 (18%) | 40 (29%) | ||
| Coronary artery disease | No | 89 (80%) | 9 (100%) | 125 (78%) | 103 (74%) | 0.248 |
| Yes | 23 (20%) | 0 (0%) | 35 (22%) | 37 (26%) | ||
| Cardiac arrhythmia | No | 102 (91%) | 7 (78%) | 143 (89%) | 129 (92%) | 0.491 |
| Yes | 10 (9%) | 2 (22%) | 17 (11%) | 11 (8%) | ||
| History of coronary vascular accident | No | 106 (95%) | 8 (89%) | 156 (97%) | 139 (99%) | 0.068 |
| Yes | 6 (5%) | 1 (11%) | 4 (3%) | 1 (1%) | ||
| Pulmonary disease | No | 94 (84%) | 7 (78%) | 147 (92%) | 115 (82%) | 0.063 |
| Yes | 18 (16%) | 2 (22%) | 13 (8%) | 25 (18%) | ||
| Liver disease | No | 110 (98%) | 8 (89%) | 159 (99%) | 138 (99%) | 0.077 |
| Yes | 2 (2%) | 1 (11%) | 1 (1%) | 1 (1%) | ||
| Nephrolithiasis | No | 101 (91%) | 9 (100%) | 149 (93%) | 126 (90%) | 0.606 |
| Yes | 10 (9%) | 0 (0%) | 11 (7%) | 14 (10%) | ||
| Preoperative renal function | Normal | 58 (72%) | 7 (88%) | 79 (70%) | 70 (71%) | 0.652 |
| CKD stage 3 | 19 (24%) | 1 (12%) | 31 (28%) | 26 (27%) | ||
| CKD stage 4 | 2 (2%) | 0 (0%) | 2 (2%) | 2 (2%) | ||
| CKD stage 5 | 2 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Smoking history | No | 27 (24%) | 1 (11%) | 42 (27%) | 36 (26%) | 0.76 |
| Yes | 84 (76%) | 8 (89%) | 116 (73%) | 103 (74%) |
BMI subgroups were evaluated for perioperative, pathologic, and long term outcomes including estimated blood loss, need for blood product transfusion, number of lymph nodes resected, admission length of stay, 30-d readmission rate, rate of continent diversions (ileal neobladder or continent catheterizable stoma), rate of node positive disease, and overall and disease specific survival (months). Follow-up was computed from the date of surgery to last clinic appointment with urologic or medical oncologist. Dates of death were confirmed by the Social Security Death Index[6].
Overall and disease specific survival was estimated by Kaplan-Meier method. Medians were compared using the Mann-Whitney U Test and categorical variables were compared using the χ2 test. A P-value of < 0.05 was considered statistically significant. Statistical analyses were performed using the Software Package for the Social Sciences (SPSS), Version 20 (International Business Machines SPSS, Chicago, IL USA).
Perioperative and pathologic characteristics are demonstrated in Table 2. Of the 424 patients who underwent radical cystectomy for urothelial carcinoma and for whom BMI data was available, the median age was 69 years (range 60-75 years). There was a median follow-up of 23.4 mo (8.7-55.1). Of these 424 patients, there were 9 (2.1%) underweight, 113 (26.7%) normal BMI, 160 (37.8%) overweight, and 142 (33.5%) obese.
| Normal weight(BMI 18.5-24.9) | Underweight(BMI < 18.5) | Overweight(BMI 25.0-29.9) | Obese(BMI > 30) | P value | ||
| Length of stay (median, IQR), d | 8 (7-10) | 13 (10-16) | 8 (7-11) | 9 (7-14) | 0.054 | |
| Prolonged length of stay (> 7 d) | No | 39 (35%) | 0 (0%) | 61 (38%) | 49 (35%) | 0.137 |
| Yes | 73 (65%) | 9 (100%) | 99 (62%) | 93 (65%) | ||
| Estimated blood loss (median, IQR), mL | 500 (400-700) | 600 (450-925) | 700 (500-1000) | 800 (600-1100) | < 0.001 | |
| Urinary diversion | Ileal conduit | 70 (61.9%) | 8 (88.9%) | 88 (55%) | 68 (47.9%) | 0.160 |
| Orthotopic ileal neobladder | 27 (23.9%) | 1 (11.1%) | 58 (26.2%) | 59 (41.5%) | ||
| Continent cutaneous diversion | 14 (12.3%) | 0 (0%) | 12 (7.4%) | 11 (7.7%) | ||
| Cutaneous ureterostomy | 0 (0%) | 0 (0%) | 1 (0.6%) | 0 (0%) | ||
| None | 4 (3.5%) | 0 (0%) | 2 (1.2%) | 1 (0.7%) | ||
| Pathologic tumor/nodal stage | No evidence of disease (pT0) | 8 (7%) | 0 (0%) | 2 (1%) | 9 (6%) | 0.01 |
| Localized disease (pT0-2bN0) | 45 (40%) | 2 (22%) | 73 (46%) | 76 (53%) | ||
| Locally advanced disease (pT3-4bN0) | 25 (22%) | 6 (67%) | 49 (30%) | 33 (23%) | ||
| Solitary nodal metastasis (pT × N1) | 12 (11%) | 0 (0%) | 14 (9%) | 12 (9%) | ||
| Multiple nodal metastases (pT × N2-3) | 23 (20%) | 1 (11%) | 22 (14%) | 12 (9%) | ||
| Readmission within 30 d | No | 83 (75%) | 6 (67%) | 113 (71%) | 97 (70%) | 0.85 |
| Yes | 28 (25%) | 3 (33%) | 46 (29%) | 41 (30%) | ||
| Adjuvant chemotherapy | No | 74 (67%) | 7 (78%) | 105 (66%) | 93 (67%) | 0.907 |
| Yes | 36 (33%) | 2 (22%) | 54 (34%) | 45 (33%) | ||
| Recurrence of disease | No | 81 (72%) | 5 (56%) | 113 (71%) | 97 (69%) | 0.031 |
| Yes | 31 (28%) | 4 (44%) | 47 (29%) | 44 (31%) | ||
| Deceased | No | 59 (52%) | 2 (22%) | 95 (59%) | 91 (64%) | 0.727 |
| Yes | 54 (48%) | 7 (78%) | 65 (41%) | 50 (36%) |
The median estimated blood loss was higher in the obese patients compared with the normal BMI group (500 mL and 800 mL, respectively). The median number of lymph nodes resected was comparable between the normal BMI and obese, at 30 and 32, respectively. The need for transfusion (52.1% and 47.7%), admission length of stay [8 d (7-10) and 9 d (7-14)], and 30 d readmissions (25.2% and 29.7%) also showed no significant differences.
Rates of orthotopic ileal neobladder urinary diversion were higher in obese patients than in normal weight and obese patients (41.5% vs 23.9%, respectively, P = 0.003). At 2 years follow-up, there was no statistical differences in rates of urinary incontinence (21.4% vs 25.6%, P = 0.343) or need for intermittent catheterization (14.3% vs 5.2%, P = 0.685) between normal BMI and obese patients with an ileal neobladder urinary diversion.
While overall survival was improved in obese patients when compared with normal BMI patients [median survival of 67 mo (57-77) and 37 mo (16-58), respectively], it was significantly decreased in the underweight population with a median survival of 19 mo (2-35), P < 0.001 (Figure 1). A similar trend was seen in disease specific survival, with patients in the underweight group having a decreased DSS when compared with other weight groups. DSS in the underweight group, when compared to the obese group was significantly lower, P = 0.007 (Figure 2, Table 3).
On multivariate analysis (Table 4) underweight patients had an increased risk of death (HR = 3.129, P = 0.006) compared to normal BMI patients, as did older patients (age ≥ 65 years) (HR = 1.622, P = 0.006), patients with > 1 nodal metastasis (HR = 3.730, P = 0.007), and patients who had received neoadjuvant chemotherapy (HR = 2.017, P = 0.015).
| HR (95%CI) | P value | ||
| Age at surgery ≥ 65 yr | 1.62 (1.15-2.29) | 0.006 | |
| Gender | 0.330 | ||
| BMI | Normal weight (BMI 18.5-24.9) | Referent | 0.015 |
| Underweight (BMI ≤ 18.5) | 3.13 (1.39-7.07) | 0.006 | |
| Overweight (BMI 25.0-29.9) | 0.94 (0.64-1.38) | 0.764 | |
| Obese (BMI ≥ 30) | 0.83 (0.56-1.24) | 0.370 | |
| Coronary artery disease | 0.808 | ||
| Cardiac arrhythmia | 0.722 | ||
| Pulmonary disease | 0.192 | ||
| Hypertension | 0.110 | ||
| Diabetes mellitus, type II | 0.217 | ||
| Smoking history | 0.657 | ||
| Intravesical therapy | 0.560 | ||
| Neoadjuvant chemotherapy | 2.02 (1.14-3.56) | 0.015 | |
| Adjuvant chemotherapy | 0.251 | ||
| Pathologic stage | pT0 | Referent | < 0.001 |
| pT1-2N0 | 0.78 (0.30-1.99) | 0.602 | |
| pT3-4N0 | 1.47 (0.56-3.80) | 0.430 | |
| pT × N1 | 2.48 (0.91-6.77) | 0.076 | |
| pT × N2-3 | 3.73 (1.43-9.74) | 0.007 |
Radical cystectomy appears to be safe in the obese population, with perioperative and overall survival outcomes comparable to the normal BMI population. We find no clinically or statistically significant differences in rate of blood product transfusion, length of hospital stay, or 30-d readmission. This echoes a NSQIP population-based comparison between these groups, which demonstrated no increase in 30-d mortality or in perioperative complications[7]. Other prior studies have demonstrated an increase in operative time in the obese[4,7].
Further, 59 (42%) obese patients in our series received orthotopic ileal neobladder urinary diversion. While there is a theoretical concern for increased urinary incontinence given the increased intra-abdominal pressure, this was not demonstrated (26.7% vs 26.3%, obese vs normal BMI). Compared to normal BMI patients with orthotopic neobladder, obese patients had a trend toward a lower rate of intermittent catheterization (2% vs 11%, P = NS). Given that orthotopic neobladder patients have been shown to have improved physical functioning[8] without the same body image concerns present in patients with ileal conduits[9], orthotopic neobladder stands out as an appropriate option in the obese population.
An unexpected finding was the significant decrease in overall and disease specific survival in the underweight group. We hypothesize that this tendency toward a worse outcome is the result of a systemic manifestation of cancer-related nutritional deficiency and sarcopenia, resulting in a lower physiologic reserve in the underweight patients. This group did notably have a high proportion of pT3-4N0 disease compared to patients in other BMI distributions, however less incidence of nodal involvement. On multivariate analysis underweight patients had an increased risk of death compared to normal BMI patients (HR = 3.1, P = 0.006). As such, underweight BMI may serve as a surrogate marker for poor outcome following radical cystectomy.
Radical cystectomy patients are known to be nutritionally deficient. Jensen et al[8] reported that 26% of patients are at a preoperative nutritional risk prior to cystectomy. Further, Gregg et al[9] demonstrated that in 538 patients, 19% were nutritionally deficient (defined as BMI < 18.5, albumin < 3.5, pre-surgical weight loss > 5% of body weight). The 90-d mortality in this population was 16.5%, and their 3-year overall survival was decreased compared to nutritionally normal patients (44% vs 68%, respectively)[10].
Radical cystectomy patients are also at increased risk for nutritional deficiency postoperatively. Following surgery these patients are in a catabolic state secondary to the stress response to surgery and wound healing. They may develop ileus and also suffer a loss of lymphatic fluid intra- and post-operatively that can contribute further to their nutritional deficiency[11]. In a case series reported by Mathur et al[12], it was demonstrated that there are significant decreases in mean protein levels and water in the first 2 wk following cystectomy. What is most striking is that it required greater than 6 mo to regain 67% of the protein lost following surgery. In a prospective, randomized trial, Roth et al[10] demonstrated that while patients who received parenteral nutrition had earlier improvements in serum prealbumin (mg/L) and total protein (g/L), there was no improvement in time to gastrointestinal recovery or length of stay. Furthermore, there were increased postoperative infectious complications in this group.
All of the above data suggest that preoperatively underweight and nutritionally deficient patients are at a disadvantage following radical cystectomy. A recent review of the colorectal surgery literature found that the use of preoperative nutritional supplements improved time to return of gastrointestinal function, and decreased time to discharge and postoperative muscle mass loss. It follows that preoperative nutritional intervention may lead to improved outcomes. More will be known in the radical cystectomy population following the results of a pilot study that is currently in enrollment to assess the impact of an enriched oral nutritional shake to improve preoperative nutritional status on patient’s outcomes following radical cystectomy.
Limitations of our study include retrospective design with its inherent bias, as well as small sample size, particularly in the underweight patient group.
While obesity may increase the technical difficulty of surgical management of invasive bladder cancer, perioperative outcomes and survival following radical cystectomy appear better than non-obese patients. Obese patients with orthotopic neobladders have comparable urinary function to patients with a normal BMI, making continent diversion a reasonable option in this population. A paradigm shift may be warranted to incorporate preoperative nutritional assessment and supplementation, particularly in underwent patients, to improve radical cystectomy outcomes. Prospective studies evaluating the effect of nutritional supplementation or hyperalimentation prior to radical cystectomy are necessary to determine how to best improve the nutritional status and outcomes in this nutritionally compromised population.
Stephanie Kliethermes, PhD, biostatistical analysis, for assistance with statistical analysis.
Radical cystectomy is the gold standard for treatment of muscle invasive bladder cancer. As obesity rates in the Unites States increase, so will the rates of obese patients that require radical cystectomy. Currently, literature in other surgical fields note increased operating times in the obese population. However, these studies did not evaluate how these patients tolerated the surgery and how well they did postoperatively. In this study the authors evaluated perioperative outcomes and survival, both overall and disease specific, of patients undergoing radical cystectomy according to their body mass index (BMI). The authors’ hypothesis was that obese patients would have more technically difficult operations leading to increased need for transfusion, less complete oncologic outcomes (specifically evaluated by number of lymph nodes resected), longer hospital stay, and increased 30-d readmissions. However, no significant difference was found between obese and normal weight patient groups with regards to these perioperative factors. An unexpected finding was underweight patients had a significantly decreased overall and disease specific survival when compared with other weight groups.
Given the high complication rate of radical cystectomy of up to 30% in the literature, increased hospital length of stay, and high rate of readmission there is a need for information on preoperative optimization for these patients. The finding that underweight patients have a decreased survival could point to the fact that they are nutritionally deficient prior to surgery. Further studies are necessary to determine if, and by what method, preoperative nutritional supplementation would benefit this patient group.
This study provides information on perioperative factors and survival. Other studies that have looked at BMI and its relation to surgical outcomes have focused on the technical aspects of the surgery alone. Those that have evaluated perioperative outcomes and survival as a function of BMI did not specifically evaluate how underweight patients fared, as this study does.
This study highlights the need for future prospective studies evaluating preoperative nutritional optimization for patients undergoing radical cystectomy.
This study is the first and most extensive study. The presented article is well written and can contribute to current data. The paper describes the relationship between preoperative BMI and long-term outcome. The results are very interesting, showing a substantially decreased overall survival in the underweight group. Separate analysis of cancer-specific survival would improve the manuscript.
P- Reviewer: Behzatoglu K, Sandblom G S- Editor: Kong JX L- Editor: A E- Editor: Li D
| 1. | Sharpe L. U.S. Obesity Rate Climbing in 2013. Gallup Well-Being. [Updated. 2013;Nov 1] Available from: http://www.Gallup.com/poll/165671/obesity-rate-climbing-2013.aspx. |
| 2. | Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009-2010. NCHS Data Brief. 2012;82:1-8. [PubMed] |
| 3. | Hawn MT, Bian J, Leeth RR, Ritchie G, Allen N, Bland KI, Vickers SM. Impact of obesity on resource utilization for general surgical procedures. Ann Surg. 2005;241:821-826; discussion 826-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 105] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 4. | Maurer T, Maurer J, Retz M, Paul R, Zantl N, Gschwend JE, Treiber U. Influence of body mass index on operability, morbidity and disease outcome following radical cystectomy. Urol Int. 2009;82:432-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | WHO Global Database on Body Mass Index. Available from: http://apps.who.int/bmi/. |
| 6. | Social Security Death Index. Available from: http://go.fold3.com/ssdi/. |
| 7. | Butt ZM, Fazili A, Tan W, Wilding GE, Filadora V, Kim HL, Mohler JL, O’Leary KA, Guru KA. Does the presence of significant risk factors affect perioperative outcomes after robot-assisted radical cystectomy? BJU Int. 2009;104:986-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Jensen BT, Laustsen S, Petersen AK, Borre M, Soendergaard I, Ernst-Jensen KM, Lash TL, Borre M. Preoperative risk factors related to bladder cancer rehabilitation: a registry study. Eur J Clin Nutr. 2013;67:917-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Gregg JR, Cookson MS, Phillips S, Salem S, Chang SS, Clark PE, Davis R, Stimson CJ, Aghazadeh M, Smith JA. Effect of preoperative nutritional deficiency on mortality after radical cystectomy for bladder cancer. J Urol. 2011;185:90-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 155] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 10. | Roth B, Birkhäuser FD, Zehnder P, Thalmann GN, Huwyler M, Burkhard FC, Studer UE. Parenteral nutrition does not improve postoperative recovery from radical cystectomy: results of a prospective randomised trial. Eur Urol. 2013;63:475-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Jones C, Badger SA, Hannon R. The role of carbohydrate drinks in pre-operative nutrition for elective colorectal surgery. Ann R Coll Surg Engl. 2011;93:504-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Mathur S, Plank LD, Hill AG, Rice MA, Hill GL. Changes in body composition, muscle function and energy expenditure after radical cystectomy. BJU Int. 2008;101:973-977; discussion 977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |