Published online Jul 24, 2014. doi: 10.5410/wjcu.v3.i2.139
Revised: May 11, 2014
Accepted: June 18, 2014
Published online: July 24, 2014
Processing time: 130 Days and 6.4 Hours
AIM: To investigate the relationship between clinicopathological features and bone turnover markers in castration-resistant prostate cancer (CRPC) patients treated with docetaxel.
METHODS: Thirty-three patients were enrolled in this study. Serum levels of carboxyterminal cross-linked telopeptide of type 1 collagen generated by metalloproteinases (1CTP) and alkaline phosphatase (ALP) were measured at the start of docetaxel chemotherapy. We examined the relationship between clinicopathological features and serum levels of 1CTP and ALP levels in CRPC patients treated with docetaxel.
RESULTS: For the total patient group, the mean ± standard deviation (SD) values for docetaxel chemotherapy dose, dose intensity, dosage interval, and number of cycles were 59.3 ± 10.6 mg/m2, 13.9 ± 5.2 mg/m2 per week, 4.7 ± 1.2 wk, and 11.2 ± 7.4, respectively. Fourteen patients died from prostate cancer. Patients were divided into two groups according to mean + SD of serum 1CTP (8.2 ng/mL) and ALP (538.2 IU/L) levels at the start of docetaxel chemotherapy. Patients with lower levels of serum 1CTP and ALP had significantly better survivals than those with higher serum levels (P < 0.05).
CONCLUSION: Serum levels of 1CTP and ALP are predictors of survival in patients with CRPC who are treated with docetaxel.
Core tip: This study examined the relationship between clinicopathological features and serum levels of carboxy-terminal pyridinoline cross-linked telopeptide parts of type-1 collagen (1CTP) and alkaline phosphatase (ALP) in castration-resistant prostate cancer patients treated with docetaxel. Patients were divided into two groups according to mean + SD of serum 1CTP (8.2 ng/mL) and ALP (538.2 IU/L) levels at the start of docetaxel chemotherapy. Patients with lower levels of serum 1CTP and ALP had significantly better survivals than those with higher serum levels. Serum levels of 1CTP and ALP are predictors of survival in patients with CRPC who are treated with docetaxel.
- Citation: Endo T, Kamiya N, Suzuki H, Oka R, Lee FC, Utsumi T, Yano M, Kamijima S, Kawamura K, Imamoto T, Ichikawa T. Bone markers predict survival in castration-resistant prostate cancer patients treated with docetaxel. World J Clin Urol 2014; 3(2): 139-143
- URL: https://www.wjgnet.com/2219-2816/full/v3/i2/139.htm
- DOI: https://dx.doi.org/10.5410/wjcu.v3.i2.139
Prostate cancer (PCa) is the most common cancer among men in Western countries[1] and is the second leading cause of death in men[2]. In Japan, the incidence of PCa is rapidly increasing [3]. The clinical course of this cancer varies markedly due to its biological heterogeneity[4]. PCa is androgen-dependent, so androgen deprivation therapy (ADT) is generally used for patients with locally advanced and metastatic PCa[5,6]. ADT is effective for several years, but the disease may ultimately evolve into castration-resistant prostate cancer (CRPC)[7,8].
Docetaxel chemotherapy is the first chemotherapy regimen to demonstrate a survival benefit in CRPC patients[9,10], but not all patients gain benefit from this therapy. Thus, identification of factors that can predict favorable responses to docetaxel would be of benefit for rational selection of therapy for patients with CRPC.
PCa commonly metastasizes to bone, and these types of metastases are associated with various complications and significant morbidity, including severe bone pain, prolonged hospital stay, reduced mobility, hypercalcemia, and pathologic fractures. Furthermore, skeletal-related events correlate with reduced overall and median survival and quality of life of patients with PCa. Biochemical markers of bone metabolism may be useful, non-invasive, and sensitive surrogates of skeletal health. Carboxyterminal cross-linked telopeptide of type 1 collagen generated by metalloproteinases (1CTP) is a marker of bone resorption and is a metabolic product of mature type 1 collagen resorption[11]. Several studies have reported that serum 1CTP levels were significantly higher in PCa patients with bone metastasis than in PCa patients without bone metastasis[12]. Alkaline phosphatase (ALP) is a marker of bone formation and is widely used for assessing bone metastases[13]. We previously reported that serum 1CTP and ALP levels were reliable for the detection of bone metastatic spread and for the prediction of survival in PCa patients with bone metastasis[14].
The goal of the present study was to focus on bone resorption and formation markers by examining the relationship between clinicopathological features and serum levels of 1CTP and ALP in patients with CRPC treated with docetaxel.
A total of 33 patients treated at our medical institutions between 2005 and 2009 were investigated in this study. All patients had histologically confirmed PCa. They all eventually became resistant to ADT, progressed to metastatic CRPC, and received docetaxel chemotherapy. The dose of docetaxel was 88.1 ± 24.7 (range, 40-130) mg/body, and the median number of docetaxel chemotherapy cycles was 11 ± 8.5 (range, 1-33). Other patient characteristics are described in Table 1.
| Variable | Value |
| Patients, n | 33 |
| Median (range) | |
| Age at start of chemotherapy, years | 71.5 ± 7.4 (55-83) |
| PSA levels at start of chemotherapy, ng/mL | 93.3 ± 131.3 (6.3-744.5) |
| PSA levels (initial diagnosis), ng/mL | 877.8 ± 2259.1 (21.0-12490) |
| Dose of docetaxel, mg/body | 88.1 ± 24.7 (40-130) |
| Dose of docetaxel, mg/m2 | 53.3 ± 13.1 (30-70) |
| Number of cycles, n | 11 ± 8.5 (1-33) |
| Survival period after chemotherapy, mo | 16.2 ± 12.0 (0.2-38.6) |
| Clinical T stage | |
| 3 | 28 (84.8) |
| 4 | 5 (15.2) |
| Lymph node status | |
| 0 | 15 (45.5) |
| 1 | 18 (54.5) |
| Gleason sum (initial diagnosis) | |
| < 6 | 3 (10.0) |
| 7 | 7 (23.3) |
| 8 | 5 (16.7) |
| 9 | 11 (36.7) |
| 10 | 4 (13.3) |
| EOD score (initial diagnosis) | |
| 0 | 12 (36.4) |
| 1 | 3 (9.1) |
| 2 | 10 (30.3) |
| 3 | 8 (24.2) |
Blood samples were taken with informed consent. Serum samples, such as prostate specific antigen (PSA), C-reactive protein (CRP), 1CTP, and ALP, were measured at the start of docetaxel chemotherapy. All serum samples were immediately frozen and stored at -20 °C until analysis. Serum PSA (ARCHITECT, Abbott Laboratories, Abbott Park, IL, United States) and serum CRP (Alpha Diagnostic Intl. Inc., San Antonio, TX, United States) were measured. Serum 1CTP levels were measured by radioimmunoassay (Immundiagnostik, Bensheim, Germany) to avoid the instability of radioiodinated reagents. Serum ALP levels were measured with IATROLQ ALP (Mitsubishi Chemical Medience Corporation, Tokyo, Japan). The rate of decrease in PSA was stratified according to > decline in 30%PSA and < decline in PSA. AEs were classified using common terminology criteria for adverse events (CTCAE) v4.0.
Statistical significance was examined using the Mann-Whitney U-test and Student’s t-test, and cause-specific survival curves were created using the Kaplan-Meier method with the log-rank test. For all analyses, differences were considered statistically significant at P < 0.05. All statistical analyses were performed using SPSS version 11.0 (SPSS Inc., Chicago, IL, United States).
For the total patient group, the mean ± SD values for docetaxel chemotherapy dose, dose intensity, dosage interval, and number of cycles were 59.3 ± 10.6 mg/m2 (range 20-75 mg/m2; median 60 mg/m2), 13.9 ± 5.2 mg/m2 per week (range 3.3-25 mg/m2 per week; median 13.8 mg/m2 per week), 4.7 ± 1.2 wk (range 3-8 wk; median 4 wk), and 11.2 ± 7.4 (range 4-37; median 9), respectively. Mean follow-up time after the start of docetaxel chemotherapy was 16.2 ± 12.0 mo (range, 0.2-38.6 mo). During the follow-up period, 14 patients (42%) died from PCa. The median survival period of all patients was 16.2 ± 12.0 mo (range, 0.2-38.6 mo).
Fourteen patients had a PSA decrease > 30%, and 19 patients had a PSA decrease < 30%; thus, the PSA response rate was calculated to be 42.4%. The age at the start of chemotherapy, PSA level at the start of docetaxel chemotherapy, with or without PSA flare , Gleason sum and Extent of disease (EOD) score were not statistically related to survival (data was not shown). However, the number of chemotherapy cycles was significantly related to survival (data was not shown).
A number of adverse events (AEs) were reported in this study. Nausea was reported in seven (21%) cases, including two (6%) cases with grade 2 nausea, and three (9%) cases with grade 3 nausea. Grade 2 constipation was reported in three (9%) cases. Grade 3 lymphedema of the limb was seen in two (6%) cases; this complication interfered with activities of daily living and resulted in a decreased quality of life. Grade 3 leukopenia was found in five (15%) cases.
During docetaxel chemotherapy up to five cycles, PSA flare was found in five (15%) cases. However, statistical analysis using the Kaplan-Meier method showed that PSA flare was not related to survival.
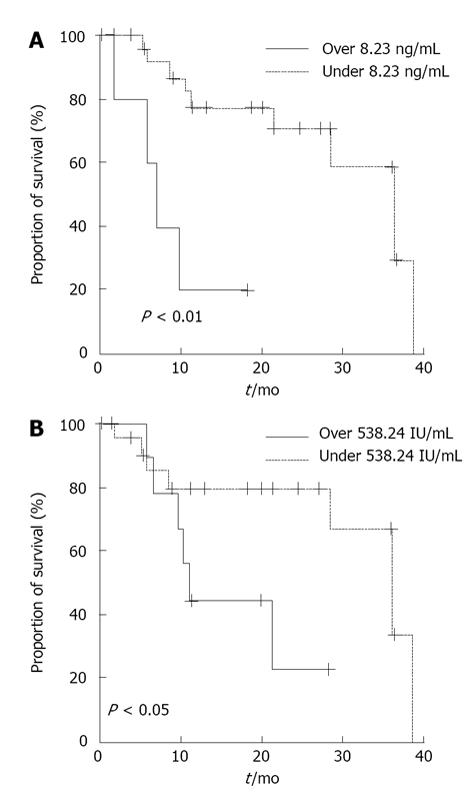
CRPC patients were stratified according to higher versus lower serum 1CTP levels using the mean + SD (8.23 ng/mL). Six patients had higher levels, and 27 patients had lower levels. Using the Kaplan-Meier method to construct cause-specific survival curves, we established that patients with higher serum 1CTP levels showed statistically significantly worse survival when compared with those with lower levels of 1CTP (P < 0.01) (Figure 1A).
CRPC patients were also stratified according to higher versus lower serum ALP levels using the mean + SD (538.24 IU/L). Nine patients had higher ALP levels, and 24 patients had lower ALP levels. Patients with higher serum ALP levels showed statistically significantly worse survival than those with lower ALP levels (P < 0.05) (Figure 1B).
Finally, CRPC patients were stratified according to higher (≥ 1 mg/dL) versus lower (< 1 mg/dL) serum CRP levels. Twenty-four patients had higher levels of CRP, and four patients had lower levels of CRP. Statistical analysis using the Kaplan-Meier method to construct cause-specific survival curves showed that patients with higher serum CRP levels had significantly worse survival than those with low CRP levels (P < 0.01) (data not shown).
There was no significant difference in the dose of docetaxel when comparing patients with higher and lower levels of 1CTP, ALP, or CRP, respectively.
Two large randomized phase III studies (SWOG 99-16 and TAX 327) showed that docetaxel, when combined with either estramustine phosphate (EMP) or prednisone, significantly prolongs overall survival in men with metastatic CRPC when compared with the former standard treatment[9,10,15]. The SWOG 99-16 study using EMP in combination with docetaxel confirmed the survival advantage of docetaxel-based chemotherapy[15]. The TAX-327 study randomized 1006 patients to receive either docetaxel or mitoxantrone, each given with low-dose prednisone, and showed an extension of overall survival, improvement in quality of life, pain control, PSA decline, and objective tumor response[9]. Data from these two landmark trials and from other studies demonstrating promising activity for docetaxel against CRPC have resulted in the use of docetaxel-based chemotherapy as a standard treatment for metastatic CRPC[16,17]. Indeed, docetaxel-based chemotherapy against metastatic CRPC is recommended by the National Comprehensive Cancer Network (NCCN) and the European Association of Urology (EAU) guidelines and is widely used in Japan.
Since the data described above were published, further data have become available from TAX-327, and prognostic factors and nomograms for docetaxel therapy have been proposed[18-21]. In 2010, Armstrong et al[20] reported that four independent risk factors predicted a ≥ 30% increase in PSA within 3 mo of starting chemotherapy, pain, as well visceral metastases, anemia, and bone scan progression following treatment with docetaxel. Furthermore, a nomogram integrating several pretreatment factors (e.g., pain, performance status, ALP, number of sites of metastatic disease, liver metastases, hemoglobin, PSA, and time since diagnosis) was validated for the prediction of post-progression survival. Armstrong et al[21] described evidence for the benefit of continuation of chemotherapy beyond progression only for men who had isolated worsening of pain. We similarly demonstrated that serum ALP level was a prognostic factor and that serum 1CTP level was also a useful prognostic factor for cause-specific survival in patients with CRPC who are treated with docetaxel chemotherapy.
Bone metastases are present in almost all CRPC patients receiving docetaxel therapy. In our previous study, we reported that serum ALP levels were useful for the detection of bone metastatic spread and for predicting survival probability in PCa patients with bone metastasis[14]. Sonpavde et al[22] investigated patients with bone metastasis and high baseline ALP who were treated with docetaxel and reported that normalization of ALP by day 90 was predictive of better survival independent of whether or not a 30% decline in PSA was achieved. An increase in ALP by day 90 was also predictive of poor survival independent of whether or not a 50% increase in PSA occurred.
Serum ALP level is a useful biomarker in patients with prostate cancer that is characterized by osteosclerotic bone metastasis. Based on data from the present study, serum 1CTP (a bone formation marker) may also be a reliable biomarker in CRPC patients. Indeed, serum 1CTP level was an independent predictor of bone metastasis according to univariate and multivariate analysis in our previous paper. Furthermore, as the EOD score increased, serum levels of 1CTP also significantly increased. Serum 1CTP level was also a significant independent predictor of cause-specific survival according to univariate and multivariate analysis[13]. Patients with higher levels of 1CTP showed worse prognosis, and our analysis suggests that therapies other than docetaxel should be considered for patients with higher levels of 1CTP.
A previous study reported that CRP is an independent prognostic factor for overall survival in patients with CRPC treated with docetaxel[23]. Similarly, we found that patients with lower CRP levels (< 1 mg/dL) had better outcomes than those with higher CRP levels (≥ 1 mg/dL) (P < 0.01). These data suggest that CRP level can predict outcomes in patients with CRPC treated with docetaxel. Narita et al[24] used multivariate analysis to demonstrate that serum lactate dehydrogenase (LDH) was an independent prognostic factor for overall survival. Another analysis suggested that levels of serum markers of angiogenesis [e.g., endothelin-1 (ET-1) and tissue factor (TF)] and/or markers of vascular damage [e.g., circulating endothelial cells] could predict overall survival in CRPC patients treated with docetaxel[25].
Measurement of serum bone turnover markers, such as 1CTP or ALP, is useful when PCa patients are diagnosed with CRPC. If high serum bone turnover marker levels are observed, the patient will need to be treated by immediate and adequate intervention (i.e., docetaxel chemotherapy or bone targeted therapy). This study has several limitations, further investigation is necessary to confirm our results.
In conclusion, the present study demonstrates that serum levels of 1CTP and ALP are predictors of survival in patients with CRPC who are treated with docetaxel. The novel agents (i.e., abiraterone acetate, enzalutamide, radium-223, and cabozantinib) offer new options for the treatment of patients with CRPC, including those with disease that is resistant to docetaxel chemotherapy in Western countries.
Docetaxel chemotherapy has been widely administered to men with metastatic castration resistant prostate cancer (CRPC). To investigate the relationship between clinicopathological features and serum levels of bone turnover markaers in CRPC patients treated with docetaxel.
Serum bone turnover markers can be determined frequently and easily, with negligible disturbance to the patient. This present study suggested that both bone formation markers [alkaline phosphatase (ALP)] and bone resorption markers [type 1 collagen generated by metalloproteinases (1CTP)] might be useful predictors of survival in patients with CRPC who are treated with docetaxel.
The authors might help the treatment strategy for CRPC in using a novel agents (i.e., abiraterone acetate, enzalutamide, radium-223, and cabozantinib), and avoid the need for frequent electronic computer X-ray tomography technique or bone scintigraphy by using bone formation markers (ALP) and bone resorption markers (1CTP).
The article supplements the known literature on risk factors for progression on chemotherapy in patients with hormone refractory prostate cancer.
P- Reviewer: Desai DJ S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10002] [Cited by in RCA: 10453] [Article Influence: 696.9] [Reference Citation Analysis (0)] |
| 2. | Eaton CL. Aetiology and pathogenesis of benign prostatic hyperplasia. Curr Opin Urol. 2003;13:7-10. [PubMed] |
| 3. | Cancer Registration Committee of the Japanese Urological Association. Clinicopathological statistics on registered prostate cancer patients in Japan: 2000 report from the Japanese Urological Association. Int J Urol. 2005;12:46-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Isaacs JT. Molecular markers for prostate cancer metastasis. Developing diagnostic methods for predicting the aggressiveness of prostate cancer. Am J Pathol. 1997;150:1511-1521. [PubMed] |
| 5. | Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. J Urol. 2002;168:9-12. [PubMed] |
| 6. | Suzuki H, Kamiya N, Imamoto T, Kawamura K, Yano M, Takano M, Utsumi T, Naya Y, Ichikawa T. Current topics and perspectives relating to hormone therapy for prostate cancer. Int J Clin Oncol. 2008;13:401-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Hinotsu S, Akaza H, Usami M, Ogawa O, Kagawa S, Kitamura T, Tsukamoto T, Naito S, Namiki M, Hirao Y. Current status of endocrine therapy for prostate cancer in Japan analysis of primary androgen deprivation therapy on the basis of data collected by J-CaP. Jpn J Clin Oncol. 2007;37:775-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Cooperberg MR, Hinotsu S, Namiki M, Ito K, Broering J, Carroll PR, Akaza H. Risk assessment among prostate cancer patients receiving primary androgen deprivation therapy. J Clin Oncol. 2009;27:4306-4313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 108] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 9. | Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4284] [Cited by in RCA: 4356] [Article Influence: 207.4] [Reference Citation Analysis (0)] |
| 10. | Berthold DR, Pond GR, Soban F, de Wit R, Eisenberger M, Tannock IF. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol. 2008;26:242-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 803] [Cited by in RCA: 834] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 11. | Coleman R, Brown J, Terpos E, Lipton A, Smith MR, Cook R, Major P. Bone markers and their prognostic value in metastatic bone disease: clinical evidence and future directions. Cancer Treat Rev. 2008;34:629-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Koopmans N, de Jong IJ, Breeuwsma AJ, van der Veer E. Serum bone turnover markers (PINP and ICTP) for the early detection of bone metastases in patients with prostate cancer: a longitudinal approach. J Urol. 2007;178:849-853; discussion 853; quiz 1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Garnero P, Buchs N, Zekri J, Rizzoli R, Coleman RE, Delmas PD. Markers of bone turnover for the management of patients with bone metastases from prostate cancer. Br J Cancer. 2000;82:858-864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 172] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 14. | Kamiya N, Suzuki H, Yano M, Endo T, Takano M, Komaru A, Kawamura K, Sekita N, Imamoto T, Ichikawa T. Implications of serum bone turnover markers in prostate cancer patients with bone metastasis. Urology. 2010;75:1446-1451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2824] [Cited by in RCA: 2815] [Article Influence: 134.0] [Reference Citation Analysis (0)] |
| 16. | Di Lorenzo G, Buonerba C, Autorino R, De Placido S, Sternberg CN. Castration-resistant prostate cancer: current and emerging treatment strategies. Drugs. 2010;70:983-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Oudard S, Banu E, Beuzeboc P, Voog E, Dourthe LM, Hardy-Bessard AC, Linassier C, Scotté F, Banu A, Coscas Y. Multicenter randomized phase II study of two schedules of docetaxel, estramustine, and prednisone versus mitoxantrone plus prednisone in patients with metastatic hormone-refractory prostate cancer. J Clin Oncol. 2005;23:3343-3351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 105] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Armstrong AJ, Garrett-Mayer E, Ou Yang YC, Carducci MA, Tannock I, de Wit R, Eisenberger M. Prostate-specific antigen and pain surrogacy analysis in metastatic hormone-refractory prostate cancer. J Clin Oncol. 2007;25:3965-3970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 231] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 19. | Armstrong AJ, Garrett-Mayer ES, Yang YC, de Wit R, Tannock IF, Eisenberger M. A contemporary prognostic nomogram for men with hormone-refractory metastatic prostate cancer: a TAX327 study analysis. Clin Cancer Res. 2007;13:6396-6403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 297] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 20. | Armstrong AJ, Tannock IF, de Wit R, George DJ, Eisenberger M, Halabi S. The development of risk groups in men with metastatic castration-resistant prostate cancer based on risk factors for PSA decline and survival. Eur J Cancer. 2010;46:517-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 21. | Armstrong AJ, Garrett-Mayer E, de Wit R, Tannock I, Eisenberger M. Prediction of survival following first-line chemotherapy in men with castration-resistant metastatic prostate cancer. Clin Cancer Res. 2010;16:203-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 168] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 22. | Sonpavde G, Pond GR, Berry WR, de Wit R, Armstrong AJ, Eisenberger MA, Tannock IF. Serum alkaline phosphatase changes predict survival independent of PSA changes in men with castration-resistant prostate cancer and bone metastasis receiving chemotherapy. Urol Oncol. 2012;30:607-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 23. | Ito M, Saito K, Yasuda Y, Sukegawa G, Kubo Y, Numao N, Kitsukawa S, Urakami S, Yuasa T, Yamamoto S. Prognostic impact of C-reactive protein for determining overall survival of patients with castration-resistant prostate cancer treated with docetaxel. Urology. 2011;78:1131-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Narita S, Tsuchiya N, Yuasa T, Maita S, Obara T, Numakura K, Tsuruta H, Saito M, Inoue T, Horikawa Y. Outcome, clinical prognostic factors and genetic predictors of adverse reactions of intermittent combination chemotherapy with docetaxel, estramustine phosphate and carboplatin for castration-resistant prostate cancer. Int J Clin Oncol. 2012;17:204-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Strijbos MH, Gratama JW, Schmitz PI, Rao C, Onstenk W, Doyle GV, Miller MC, de Wit R, Terstappen LW, Sleijfer S. Circulating endothelial cells, circulating tumour cells, tissue factor, endothelin-1 and overall survival in prostate cancer patients treated with docetaxel. Eur J Cancer. 2010;46:2027-2035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |