Published online Jun 9, 2025. doi: 10.5409/wjcp.v14.i2.101543
Revised: February 21, 2025
Accepted: March 5, 2025
Published online: June 9, 2025
Processing time: 180 Days and 18.3 Hours
Hypertriglyceridemia thalassemia syndrome is a rare condition that occurs in patients with thalassemia. It typically presents with a combination of profound anemia and milky serum. Although previous case series have demonstrated the benefit of blood transfusions in reducing serum triglycerides, information regar
To identify the clinical course, treatment strategies, and outcomes of patients with hypertriglyceridemia thalassemia syndrome.
We performed a comprehensive search of the Scopus, PubMed, and Embase databases. We included only English-language articles and did not apply any publication date limits. The databases were last accessed on September 1, 2024. This study was registered under number CRD420250587918 and included studies involving children and adults with thalassemia, hypertriglyceridemia, and available data on clinical course.
A total of 14 publications were included in the analysis, all of which were case reports or case series. No higher-quality evidence was available. Among 28 children with hypertriglyceridemia thalassemia syndrome, there were 22 cases of β-thalassemia major and 6 cases of hemoglobin E/β-thalassemia, including our illustrative case. The median age of onset was 11 months, and 92.3% of cases presented prior to the first blood transfusion. The common clinical manifestations included pallor (100%) and hepatosplenomegaly (67.9%). For hypertriglyceridemia-related symptoms, lipemia retinalis and xanthomas were observed in 25.0% and 10.7% of cases, respectively. The median hemoglobin level was 5.5 g/dL, while the median triglyceride level was 935 mg/dL. For management, 92.9% of cases received blood transfusions with or without other interventions. At a median of 12 months’ follow-up, all patients responded to the treatment without lipid-lowering agents, and 85.7% of cases were alive.
Hypertriglyceridemia thalassemia syndrome occurs exclusively in young children and usually presents with anemia and severe hypertriglyceridemia prior to the first transfusion. Management with blood transfusions provides a favorable response. However, long-term regular monitoring is warranted.
Core Tip: Secondary hypertriglyceridemia due to thalassemia should be considered in infants and young children who present with profound anemia and milky serum. Hemoglobin analysis and lipid profiling should be performed on both the patient and the parents to confirm the diagnosis of thalassemia and to rule out primary hypertriglyceridemia in the family. Management of patients with hypertriglyceridemia thalassemia syndrome using blood transfusions according to thalassemia guidelines has provided good outcomes. Although no hypertriglyceridemia-related complications have been observed in this review, regular monitoring of lipid profiles and potential complications - including pancreatitis, atherosclerosis, and non-alcoholic fatty liver disease - is warranted.
- Citation: Choed-Amphai C, Kusontammarat P, Chanthong S, Arkarattanakul N, Rodchaprom P, Sathitsamitphong L, Natesirinilkul R, Charoenkwan P. Clinical course and management of hypertriglyceridemia thalassemia syndrome: A case-based systematic review. World J Clin Pediatr 2025; 14(2): 101543
- URL: https://www.wjgnet.com/2219-2808/full/v14/i2/101543.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v14.i2.101543
Thalassemia is one of the most common inherited chronic hemolytic anemias worldwide, with a higher prevalence in malaria-endemic regions, including Thailand. Tuo et al[1] recently reported the highest age-standardized prevalence rate of 7.35 per 100000 persons in East Asia and the highest age-standardized mortality rate of 0.37 per 100000 persons in Southeast Asia. Patients with the severe form of thalassemia, currently classified as transfusion-dependent thalassemia, often present with anemia, hepatosplenomegaly, and bony changes as their clinical manifestations[2,3].
Hypertriglyceridemia is relatively uncommon in the pediatric population. Its prevalence ranges from 3.5% to 12%, compared with 29.6% in the global adult population[4,5]. It can occur as a primary inherited disorder or as a secondary condition associated with diseases such as metabolic syndrome, and hypothyroidism. However, there have been reports of severe hypertriglyceridemia in patients with thalassemia, a condition referred to as “hypertriglyceridemia thalassemia syndrome”, first described by Rao et al[6]. Since then, only a few cases have been identified with this condition. The largest case series revealed that hypertriglyceridemia thalassemia syndrome occurred in only 12 out of 1482 β-thalassemia patients[7]. Several case reports and case series have detailed the initial presentation and acute management of this syndrome. However, no systematic reviews of the clinical course have been conducted to date. In this study, we aim to demonstrate the long-term outcomes of this condition and provide a systematic review of the clinical course and management of hypertriglyceridemia thalassemia syndrome.
A 6-month-old female infant presented with a one-month history of anemia. Her parents reported increased fatigue, worsening anemia, and jaundice over the past two days. There was no history of recent infection. Her perinatal history included neonatal jaundice, which required phototherapy. Retrospective review of routine prenatal thalassemia screening revealed that her father’s hematocrit was 43%, mean corpuscular volume (MCV) was 63 fL, and dichlorophenolindophenol test was negative. Her mother’s hematocrit was 34%, MCV was 62 fL, and dichlorophenolindophenol test was also negative. There was no family history of dyslipidemia, including hypertriglyceridemia, pancreatitis, atherosclerosis, or cardiovascular disease. On physical examination, her weight was 6020 g (10th-25th percentile), and her length was 64 cm (50th-75th percentile). She exhibited no dysmorphic features, xanthomas, or lipemia retinalis. She had markedly pale conjunctivae and icteric sclerae. Her liver and spleen were both palpable 2 cm below the costal margin.
During initial blood work, milky serum was incidentally noted. Her lipid profile showed a cholesterol level of 129 mg/dL (normal range: 0-200 mg/dL), triglycerides of 662 mg/dL (normal range: 0-100 mg/dL), high-density lipoprotein of 14 mg/dL (normal range: ≥ 40 mg/dL), and low-density lipoprotein of 36 mg/dL (normal range: 0-130 mg/dL). Pancreatic enzymes, including amylase and lipase, as well as thyroid function tests (thyroid stimulating hormone, free T3, and free T4), were within normal limits. Her initial hemoglobin was 4.9 g/dL with an MCV of 62.7 fL. White blood cell and platelet counts were 22500/mm³ and 301000/mm³, respectively. Hemoglobin analysis using the Variant II HPLC system (Bio-Rad Laboratories, CA, United States) revealed 100% hemoglobin F, confirming a diagnosis of β-thalassemia major. Subsequent mutation analysis revealed a homozygosity for HBB: c.126_129del (p.Phe42fs), while polymerase chain reaction for common α-thalassemia 1 deletions was negative.
The patient was started on regular blood transfusions every 3 weeks to 4 weeks to maintain a pre-transfusion hemoglobin level between 9.0 g/dL and 10.5 g/dL. After the first transfusion, her serum triglyceride level decreased to 464 mg/dL. Follow-up non-fasting triglyceride levels at 8 months, 1.5 years, and 2 years were 134 mg/dL, 111 mg/dL, and 150 mg/dL, respectively. She demonstrated normal growth and development, with no facial changes or progressive hepatosplenomegaly. She did not develop any complications related to hypertriglyceridemia. Her treatment consisted solely of transfusion-dependent thalassemia management, including regular blood transfusions, folic acid supplementation, and the subsequent initiation of deferasirox due to secondary iron overload, without the use of lipid-lowering agents. She has not experienced rebound severe hypertriglyceridemia up to her last clinic visit.
This study was registered under number CRD420250587918. Three authors (Choed-Amphai C, Chanthong S, and Arkarattanakul N) independently conducted comprehensive searches of the Scopus, PubMed, and Embase databases without applying time restrictions. The search used the following keywords: “hypertriglyceridemia” and “thalassemia.” The specific search strategy for Scopus was: [TITLE-ABS-KEY (“hypertriglyceridemia”) AND TITLE-ABS-KEY (“thalassemia”)]. For PubMed, the search terms were: [“thalassaemia” (All Fields) OR “thalassemia” (MeSH Terms) OR “thalassemia” (All Fields) OR “thalassaemias” (All Fields) OR “thalassemias” (All Fields)] AND [“hypertriglyceridaemia” (All Fields) OR “hypertriglyceridemia” (MeSH Terms) OR “hypertriglyceridemia” (All Fields) OR “hypertriglyceridaemias” (All Fields) OR “hypertriglyceridemias” (All Fields)]. In Embase, the search terms were: (“thalassemia”/exp OR thalassemia) AND (“hypertriglyceridemia”/exp OR hypertriglyceridemia). Only articles published in English were included.
The inclusion criteria were as follows: (1) Studies involving children and adults with thalassemia; (2) Evidence of hypertriglyceridemia, defined as a serum triglyceride level of ≥ 100 mg/dL for children aged 0 year to 9 years and ≥ 130 mg/dL for children aged 10 years to 19 years[8]; and (3) Availability of data on clinical course, treatment strategies, and outcomes. Exclusion criteria included: (1) Studies where hypertriglyceridemia was attributed to causes other than thalassemia; (2) Studies lacking information on management and clinical course; and (3) Articles without full-text availability. The detailed inclusion and exclusion criteria were presented in Table 1. The study adhered to the PRISMA 2020 guidelines[9]. Chanthong S and Arkarattanakul N participated in the selection process, which was conducted independently by two authors. Choed-Amphai C supervised the process and resolved any disagreements that arose during selection.
| Criteria | Classifications |
| Inclusion criteria | Diagnosis of thalassemia disease, including both α- and β-thalassemia |
| Evidence of hypertriglyceridemia1 | |
| Availability of data on clinical course, treatment, and outcome data | |
| Exclusion criteria | Diagnosis of other hemoglobinopathies or sickle cell disease |
| Presence of other causes of hypertriglyceridemia | |
| Incomplete information regarding clinical courses, treatment, or outcomes | |
| Unavailability of full-text articles |
Extracted data included clinical characteristics (age, sex, ethnicity, diagnosis, clinical manifestations, comorbidities, and family history of dyslipidemia or hypertriglyceridemia), laboratory parameters (specific globin mutations, initial hemoglobin level, triglyceride level, and cholesterol level), clinical course (triglyceride levels after treatment), management strategies (treatment options), and outcomes (survival, follow-up time, thalassemia-related complications, and hypertriglyceridemia-related complications).
A qualitative systematic analysis was performed using descriptive statistics. All statistical analyses were conducted using Stata version 16.1 (StataCorp LLC, College Station, TX, United States). Categorical variables were summarized as counts (n) and percentages (%). Continuous variables were reported as the mean ± SD or median with interquartile range (IQR), depending on data distribution. However, due to variations among individual cases and the small sample sizes, a meta-analysis was not feasible.
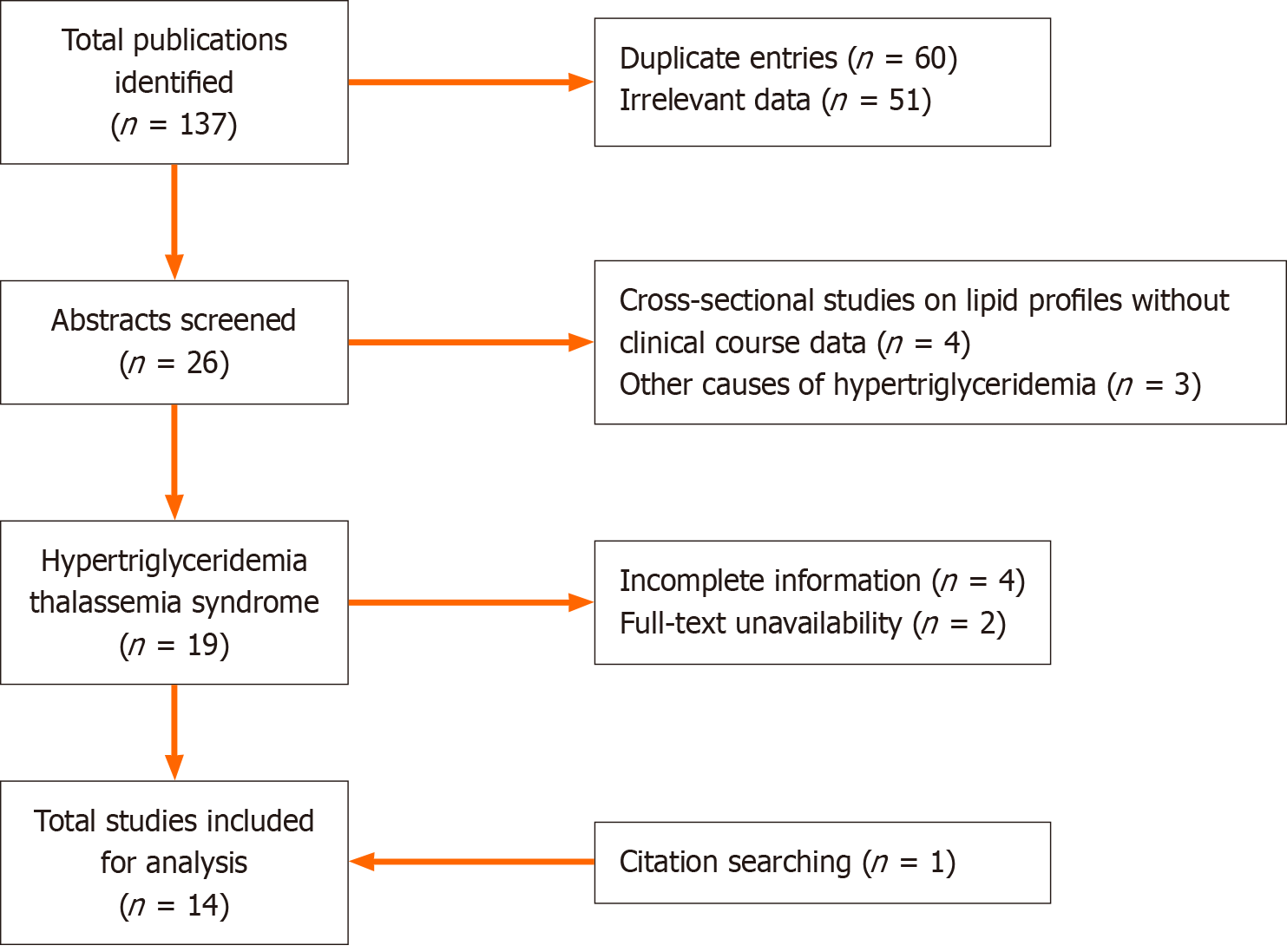
The databases were last accessed on September 1, 2024. The initial search yielded 137 publications, including 60 duplicate entries, resulting in a total of 77 publications for screening. After screening, 26 abstracts were reviewed. The selection was narrowed to English-language articles that provided detailed information on the clinical course of hypertriglyceridemia thalassemia syndrome and had full-text availability, resulting in the inclusion of 13 articles. An additional publication was identified through manual reference searches of prior articles (Figure 1). The final 14 included publications consisted of case reports and case series, as no higher-quality evidence was available for this rare clinical manifestation. Table 2 summarizes the clinical and laboratory parameters, treatments, and outcomes of the previously reported cases[6,7,10-21].
| Ref. | Age (months), sex | Ethnicity | Diagnosis, mutation(s) | Clinical manifestations, comorbidity | Hb (g/dL) | TG (mg/dL) | Treatment | Clinical course | |
| Hyper TG | Outcome, follow up (months) | ||||||||
| Rao et al[6], 1972 | 12, male | India | BTM | Pallor, lipemia retinalis | NA | 1057 | Transfusion | I | Alive, 0.3 |
| Desai et al[10], 1973 | 5, female | India | BTM | Pallor, xanthomas, lipemia retinalis, HSM | 3.5 | 2520 | Transfusion, diet control | I | Alive, 0.2 |
| 6, male | India | BTM | Pallor, xanthomas, lipemia retinalis, HSM | 5.8 | 2140 | Transfusion, diet control | I | Dead (infection), 0.8 | |
| Jaiswal et al[11], 1974 | 11, female | India | BTM | FG, pallor, xanthomas, lipemia retinalis, HSM | 4.5 | 2200 | Diet control | I | Dead (infection), 3 |
| 30, male | India | BTM | FG, pallor, lipemia retinalis, HSM | 6.5 | 2400 | Diet control | I | Dead (infection), 2 | |
| Ameri et al[12], 1975 | 11, female | Iran | BTM | FG, pallor, hip subluxation | 4.4 | 633 | Transfusion, diet control, UFH | R | Alive, 0.9 |
| Ameri et al[13], 1977 | |||||||||
| Seervai et al[14], 1976 | 15, female | India | BTM | Pallor, HSM | 5.5 | 1875 | Transfusion, diet control | I | Alive |
| 5, female | India | BTM | Pallor, jaundice, lipemia retinalis, HSM, rickets | 5.2 | 1633 | Transfusion, diet control | I | Alive | |
| Gomber et al[15], 1996 | 4, male | India | BTM | Fever, dyspnea, pallor, lipemia retinalis, HSM | 6 | 1137 | Transfusion, antibiotics | I | Dead (anemia, CHF), 1 |
| Das et al[16], 2016 | 7, male | India | BTM, hmz. HBB: c.92 + 5G>C | FG, pallor, HSM | 5.3 | 1805 | Transfusion | I | Alive, 0.4 |
| Mohan et al[17], 2017 | 7, female | India | BTM | FG, pallor, jaundice, HSM | 6.6 | 871 | Transfusion | R | Alive, TD |
| Jain et al[18], 2018 | 8, female | India | BTM | FG, pallor, SM | 7.7 | 1149 | Transfusion | R | Alive |
| Mahajan and Dewan[19], 2020 | 6, female | India | BTM | Pallor, HSM | 7.8 | 823 | Transfusion, folic acid | R | Alive, TD |
| Thatikonda et al[20], 2021 | 9, male | India | BTM | Dyspnea, pallor, jaundice, HSM, AIHA | 3.2 | 874 | Transfusion, MP | I | Alive, TD, 2.7 |
| Mohd Kasim et al[21], 2023 | 12, female | Malaysia | β/E | FG, pallor, HSM | 7.2 | 802 | Transfusion | I | Alive |
| Kapat et al[7], 2024 | 6, male | India | β/E, HBB: c.92 + 5G>C and HBB: c.79G>A (p.Glu27 Lys) | FG, pallor | 4.7 | 890 | Transfusion | R | Alive, TD, 12 |
| 9, male | India | BTM, hmz. HBB: c.92G>C (p.Arg31Thr) | FG, pallor, SM | 6.1 | 750 | Transfusion | R | Alive, TD, 12 | |
| 24, female | India | BTM, hmz. HBB: c.92 + 5G>C | Pallor, jaundice, HSM | 5.7 | 850 | Transfusion | R | Alive, TD, 12 | |
| 14, male | India | BTM, hmz. HBB: c.126_129del (p.Phe42fs) | Pallor, HSM | 4.6 | 1150 | Transfusion | R | Alive, TD, 12 | |
| 11, female | India | β/E, HBB: c.92 + 5G>C and HBB: c.79G>A (p.Glu27 Lys) | Pallor, HSM | 2.9 | 660 | Transfusion | R | Alive, TD, 12 | |
| 13, female | India | BTM, hmz. HBB: c.126_129del (p.Phe42fs) | Pallor, SM | 5.5 | 1200 | Transfusion | R | Alive, TD, 12 | |
| 14, male | India | β/E, HBB: c.92 + 5G>C and HBB: c.79G>A (p.Glu27 Lys) | Pallor, HSM | 5.9 | 980 | Transfusion | R | Alive, TD, 12 | |
| 14, male | India | β/E, HBB: c.92 + 5G>C and HBB: c.79G>A (p.Glu27 Lys) | FG, pallor, HSM | 4.1 | 1040 | Transfusion | R | Alive, TD, 12 | |
| 19, male | India | β/E, HBB: c.92 + 5G>C and HBB: c.79G>A (p.Glu27 Lys) | Pallor, jaundice, HSM | 3.3 | 880 | Transfusion | R | Alive, TD, 12 | |
| 11, male | India | BTM, hmz. HBB: c.92 + 5G>C | Pallor, jaundice, HM | 3.1 | 650 | Transfusion | R | Alive, TD, 12 | |
| 12, female | India | BTM, hmz. HBB: c.92 + 5G>C | FG, pallor, SM | 6 | 720 | Transfusion | R | Alive, TD, 12 | |
| 8, female | India | BTM, hmz. HBB: c.126_129del (p.Phe42fs) | Pallor, SM | 5.9 | 530 | Transfusion | R | Alive, TD, 12 | |
| This study | 6, female | Thailand | BTM, hmz. HBB: c.126_129del (p.Phe42fs) | Pallor, HSM | 4.9 | 662 | Transfusion, folic acid | I | Alive, TD, 48 |
Among the 28 cases of hypertriglyceridemia thalassemia syndrome, all involved β-thalassemia. This included 22 cases of β-thalassemia major and 6 cases of hemoglobin E/β-thalassemia, with no cases of α-thalassemia reported. Molecular testing was conducted in 14 cases, revealing 5 cases of compound heterozygosity for HBB: c.92 + 5G>C and HBB: c.79G>A (p.Glu27 Lys), 4 cases of homozygosity for HBB: c.92 + 5G>C, 4 cases of homozygosity for HBB: c.126_129del (p.Phe42fs), and 1 case of homozygosity for HBB: c.92G>C (p.Arg31Thr). The median age was 11 months (IQR: 6.5-13.5 months), and 53.6% were female. The majority of patients (89.2%) were of Indian origin, with one case each from Iran (3.6%), Malaysia (3.6%), and Thailand (3.6%). A family history of hypertriglyceridemia was presented in only 7.4% of cases.
The most common clinical manifestation was pallor (100%), followed by hepatosplenomegaly (67.9%), faltering growth (39.3%), jaundice (21.4%), splenomegaly (17.9%), dyspnea (7.1%), hepatomegaly (3.6%), and fever (3.6%). Hypertriglyceridemia-related symptoms were also observed, including lipemia retinalis (25.0%) and xanthomas (10.7%). No other hypertriglyceridemia-related complications, such as pancreatitis or atherosclerosis, were reported in this population. Hypertriglyceridemia onset occurred prior to the first blood transfusion in 92.3% of cases. Three cases had co-morbidities at the time of presentation: One with autoimmune hemolytic anemia, one with hip subluxation, and one with rickets. Initial laboratory investigations revealed a median hemoglobin level of 5.5 g/dL (IQR: 4.4-6.0 g/dL), a median triglyceride level of 935 mg/dL (IQR: 776-1416.5 mg/dL), and a median cholesterol level of 127.5 mg/dL (IQR: 113-147 mg/dL).
Of the 28 cases, 26 (92.9%) received blood transfusions. Treatment details for these patients were as follows: 17 cases received transfusion alone; 4 cases received transfusion combined with dietary control; 2 cases received transfusion and folic acid supplementation; 1 case received transfusion, dietary control, and unfractionated heparin; 1 case received transfusion and methylprednisolone; and 1 case received transfusion and antibiotics. The remaining 2 cases (7.1%) did not receive blood transfusions and were managed with dietary control alone. No lipid-lowering agents were used in any case.
All previous cases, including this illustrative case, responded to treatment. Hypertriglyceridemia was completely resolved in 57.1% of cases, while triglyceride levels improved in the remainder. No hypertriglyceridemia-related complications developed during follow-up. With a median follow-up of 12 months, 85.7% of cases were alive; 16 of them received regular blood transfusions, while the subsequent transfusion status was not mentioned for the other 8 cases. All four non-surviving cases presented before the year 2000. Three of them died from infection, and the other developed congestive heart failure due to anemia.
Hypertriglyceridemia in children can arise from genetic (primary) or acquired (secondary) causes. Primary causes include familial hypertriglyceridemia, lipoprotein lipase deficiency, and dysbetalipoproteinemia, where genetic defects impair triglyceride metabolism. Elevated triglyceride levels are also observed in traditional Fredrickson classification types I to V, except type IIa[8]. Secondary causes, which are more common, include obesity, metabolic syndrome, diabetes, hypothyroidism, medications, and thalassemia. While the incidence of primary hypertriglyceridemia is less than 0.2%, that of secondary causes is up to 10.7%[22]. Major complications in patients with hypertriglyceridemia include pancreatitis, increased cardiovascular risk, and non-alcoholic fatty liver disease. General management in the pediatric population primarily involves lifestyle modifications. Pharmacological interventions, such as fibrates, are reserved for severe cases, particularly when triglyceride levels exceed 500 mg/dL, to prevent pancreatitis[8].
Previous studies in children and adolescents with thalassemia have revealed significantly higher triglyceride levels compared to normal controls[23-26]. Saki et al[23] reported hypertriglyceridemia in up to 23% of adolescent thalassemia patients. However, cases with hypertriglyceridemia thalassemia syndrome commonly demonstrate visualization of milky or chylous serum in blood samples and have severe hypertriglyceridemia with serum triglyceride levels exceeding 500 mg/dL. The case series by Kapat et al[7] reported an incidence of hypertriglyceridemia thalassemia syndrome of only 0.8%. To investigate the possibility of coexisting primary hypertriglyceridemia and thalassemia in patients with high triglyceride levels, a recent report performed whole exome sequencing in all 12 cases of hypertriglyceridemia thalassemia syndrome and observed only the HBB mutation, without evidence of monogenic hypertriglyceridemia[7]. Previous studies have shown that triglyceride levels have a negative correlation with hemoglobin levels in thalassemia patients[25]. In children with β-thalassemia, anemic symptoms usually develop after hemoglobin switching, which occurs around 6 months of age[2]. This correlation and the onset of anemia in β-thalassemia may reflect the onset of hypertriglyceridemia thalassemia syndrome, which has a median age of presentation of 11 months and usually develops symptoms prior to the first blood transfusion. This systematic review demonstrated a median hemoglobin level of 5.5 g/dL and a median triglyceride level of 935 mg/dL. Without prenatal diagnosis and proper postnatal monitoring, this period could be when patients develop the most severe anemia in their lives before initiating a regular blood transfusion program. Al-Quobaili and Abou Asali[27] proposed that reduced extrahepatic lipolytic activity could account for the rise in triglyceride levels observed in this population. Although most cases have been reported from India, previous whole exome sequencing results showed no other genetic predisposition except for HBB mutations[7]. The incidence of this condition might be underestimated in other ethnicities and should be further evaluated.
The clinical manifestations of hypertriglyceridemia in this population were limited to lipemia retinalis and xanthomas, without other serious complications such as pancreatitis. The general management of this condition is blood transfusion, which has provided very good responses. Of the cases who subsequently received regular blood transfusions, 87.5% had normal triglyceride levels at their last follow-up. Despite four deaths occurring between 1972 and 1996, there has been an excellent outcome with 100% survival after the year 2000, when the Thalassemia International Federation launched their first guidelines on the management of thalassemia, including regular blood transfusion programs[28]. Furthermore, patients previously diagnosed with hypertriglyceridemia thalassemia syndrome have not experienced any complications related to hypertriglyceridemia at their last follow-up. However, these patients are known to have a higher risk of atherosclerosis than the general population[29]. Routine follow-up of lipid profiles and assessment of further complications should be considered starting from childhood. The current role of pharmacological interventions, including fibrates, is also controversial in children, since hypertriglyceridemia in this population responds well to blood transfusion. Further research to explore the benefit of lipid-lowering agents on short- and long-term outcomes in patients with thalassemia is warranted.
This systematic review is the first to address the clinical course and outcomes of patients with hypertriglyceridemia thalassemia syndrome, providing new insights into management and post-treatment monitoring. However, the rarity of this condition limits the available data to a few case reports and case series. Additionally, certain laboratory parameters, such as hemoglobin and triglyceride levels, were not reported during post-treatment follow-up in some articles.
The incidence of hypertriglyceridemia thalassemia syndrome may be underreported in various regions, highlighting the need for prospective studies. Because most patients present at a young age, often before receiving their first blood transfusion, lipid profile screening should be considered during this early stage. Although frontline management with blood transfusions has demonstrated favorable outcomes, regular long-term monitoring remains essential. Further research is needed to clarify the role of pharmacological interventions in treating this condition.
Hypertriglyceridemia thalassemia syndrome is a rare clinical manifestation that occurs exclusively in young children and usually presents with anemia and severe hypertriglyceridemia prior to the first transfusion. Management with blood transfusions provides a favorable response. At a median follow-up of one year, no clinical complications of hypertriglyceridemia have occurred.
| 1. | Tuo Y, Li Y, Li Y, Ma J, Yang X, Wu S, Jin J, He Z. Global, regional, and national burden of thalassemia, 1990-2021: a systematic analysis for the global burden of disease study 2021. EClinicalMedicine. 2024;72:102619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 60] [Reference Citation Analysis (0)] |
| 2. | Taher AT, Weatherall DJ, Cappellini MD. Thalassaemia. Lancet. 2018;391:155-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 514] [Article Influence: 73.4] [Reference Citation Analysis (0)] |
| 3. | Charoenkwan P, Tantiworawit A. Treatment strategies for haemoglobin E thalassaemia. Lancet Glob Health. 2022;10:e18-e19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 4. | Balder JW, Lansberg PJ, Hof MH, Wiegman A, Hutten BA, Kuivenhoven JA. Pediatric lipid reference values in the general population: The Dutch lifelines cohort study. J Clin Lipidol. 2018;12:1208-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 5. | Ruiz-García A, Arranz-Martínez E, López-Uriarte B, Rivera-Teijido M, Palacios-Martínez D, Dávila-Blázquez GM, Rosillo-González A, González-Posada Delgado JA, Mariño-Suárez JE, Revilla-Pascual E, Quintana-Gómez JL, Íscar-Valenzuela I, Alonso-Roca R, Javierre-Miranda AP, Escrivá-Ferrairó RA, Tello-Meco I, Ibarra-Sánchez AM, Gutiérrez Sánchez MI, Iglesias Quintana JR, Hernández-Beltrán MI, Pérez Fernández M, Barrios-Rueda E, Pérez Muñoz R, Prieto Marcos M, Delgado Rodríguez S, Pleite Raposo R, Rodríguez-Cabanillas R, Morales-Chico MR, Fernández-Pacheco Vila D, Remón-Pérez B, Del Villar Redondo MJ, Reguillo-Díaz J, Aguilera Reija P, Rodríguez Rodríguez AO, Gómez-Fernández O, Antón-Sanz MDC, Sánchez-Calso A, Doria-Carlin NA, Frías-Vargas MJ; en representación del Grupo de Investigación del Estudio SIMETAP. Prevalence of hypertriglyceridemia in adults and related cardiometabolic factors. SIMETAP-HTG study. Clin Investig Arterioscler. 2020;32:242-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Rao AV, Bai KI, Ramanujiah D. Hypertriglyceridaemia in thalassaemia major. J Indian Med Assoc. 1972;59:15-17. [PubMed] |
| 7. | Kapat A, Murmu R, Mandal S, Biswas K, Bhakta S, Mandal AK. Clinico-Laboratory Profile of Hypertriglyceridemia Thalassemia Syndrome: A Case Series in a Paediatric Tertiary Care Centre. Cureus. 2024;16:e67936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Valaiyapathi B, Sunil B, Ashraf AP. Approach to Hypertriglyceridemia in the Pediatric Population. Pediatr Rev. 2017;38:424-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 39828] [Article Influence: 9957.0] [Reference Citation Analysis (2)] |
| 10. | Desai M, Akruwala S, Babar ST. Idiopathic hyperlipemia with thalassemia major: report of two cases. Indian Pediatr. 1973;10:511-516. [PubMed] |
| 11. | Jaiswal RB, Bhai I, Parande AS, Wechalekar MD, Nath MC. Hypertriglyceridemia-thalassemia syndrome. Indian Pediatr. 1974;11:385-389. [PubMed] |
| 12. | Ameri MR, Alebouyeh M, Ziai M, Conn RB. Letter: Hypertriglyceridemia in homozygous beta thalassemia. J Pediatr. 1975;87:1002-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Ameri MR, Alebouyeh M, Ziai M, Conn RB. Hypertriglyceridemia in homozygous beta-thalassemia. Helv Paediatr Acta. 1977;32:83-86. [PubMed] |
| 14. | Seervai MH, Merchant SM, Babar ST. Thalassemia major with idiopathic hypertriglyceridemia (type 1). Indian Pediatr. 1976;13:623-628. [PubMed] |
| 15. | Gomber S, Kela K, Abdul Rahiman CL. Hypertriglyceridaemia-thalassaemia syndrome. Ann Trop Paediatr. 1996;16:359-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Das L, Samprathi M, Shukla U, Bandyopadhyay D, Das RR. Hypertriglyceridemia Thalassemia Syndrome: Common Disease, Uncommon Association. Indian J Pediatr. 2016;83:720-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Mohan BP, Prabhalekshmy KK, Letha V, Nisha TR. Idiopathic Hypertriglyceridemia in Thalassemia Major: A Case Report. Natl J Lab Med. 2017;6:PC04-PC06. [DOI] [Full Text] |
| 18. | Jain M, Ali W, Singh BB, Verma N, Kumar A. Hypertriglyceridemia thalassemia syndrome. J Pediatr Endocrinol Metab. 2018;31:821-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Mahajan S, Dewan P. LIPEMIC SERUM: EYES SEE WHAT THE MIND KNOWS. J Paediatr Child Health. 2020;56:185-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Thatikonda KB, Kalra M, Sachdeva P, Ranjan V, Sachdeva A. Uncommon association of hypertriglyceridemia and autoimmune haemolytic anaemia in an infant with transfusion dependent thalassemia. Pediat Hemat Oncol J. 2021;6:110-112. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Mohd Kasim NA, Mohd Nor NS, Wen MT, Syed Kamaruddin SKA, Sheikh Abdul Kadir SH. Lipaemic serum in Hb E-Beta thalassaemia major: A rare case of hypertriglyceridaemia thalassaemia syndrome. Malays J Pathol. 2023;45:293-296. [PubMed] |
| 22. | Stewart J, McCallin T, Martinez J, Chacko S, Yusuf S. Hyperlipidemia. Pediatr Rev. 2020;41:393-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 114] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 23. | Saki F, Bahadori R, Kashkooli NM, Jazayeri A, Ghahremani N, Omrani GHR. Prevalence of metabolic syndrome in beta thalassemia major adolescents in southern Iran: a cross-sectional study. Int J Diabetes Dev Ctries. 2019;39:444-450. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Kumar T, Basu S, Kundu R, Majumdar I, Mukherjee D. Lipid Profile in Children With Thalassemia: A Prospective Observational Study From Eastern India. Indian Pediatr. 2020;57:1072-1073. [PubMed] |
| 25. | Jabbar HK, Hassan MK, Al-Naama LM. Lipids profile in children and adolescents with β-thalassemia major. Hematol Transfus Cell Ther. 2023;45:467-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 26. | Ray S, Saikia D, Vashisht Y, Sharma S, Meena RK, Kumar M. Dyslipidemia and atherogenic indexes in children with transfusion-dependent thalassemia. Hematol Transfus Cell Ther. 2024;46:345-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 27. | Al-Quobaili FA, Abou Asali IE. Serum levels of lipids and lipoproteins in Syrian patients with beta-thalassemia major. Saudi Med J. 2004;25:871-875. [PubMed] |
| 28. | Farmakis D, Porter J, Taher A, Domenica Cappellini M, Angastiniotis M, Eleftheriou A. 2021 Thalassaemia International Federation Guidelines for the Management of Transfusion-dependent Thalassemia. Hemasphere. 2022;6:e732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 119] [Reference Citation Analysis (0)] |
| 29. | Sherief LM, Dawood O, Ali A, Sherbiny HS, Kamal NM, Elshanshory M, Alazez OA, Alhady MA, Nour M, Mokhtar WA. Premature atherosclerosis in children with beta-thalassemia major: New diagnostic marker. BMC Pediatr. 2017;17:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |