INTRODUCTION
Mast cells have normal physiologic functions such as homeostasis, tissue repair, angiogenesis, and their role in the innate/acquired immune system[1]. Mast cell activation and liberation of mediators are needed for the maintenance of normal physiological processes[2]. However, there are also abnormal conditions, in which this process is not regulated and, like mast cell activation syndrome (MCAS), causes manifestations in different organ systems in the body. Therefore, MCAS is a disorder that should be considered and diagnosed clinically with specific signs and symptoms of activation in individuals with skin, gastrointestinal, cardiovascular, respiratory, and neurological system involvement[1,3,4].
MCAS is a rare disease that is mostly diagnosed based on clinical symptoms. Here, we summarize recent developments about this disease, which is rapidly increasing in the frequency of diagnosis today, in the light of current literature data. We also review the epidemiology, pathogenesis, and diagnosis of MCAS in a broader context and to examine the signs and symptoms that occur due to its clinical relationship with other systems.
HISTORICAL BACKGROUND
Mast cells were first described in 1863 as granular cells observed in a study using frog mesenteries and in 1877, following this examination, named the as yet unidentified cells ‘mastzellen’. A few years later, the relationship of mast cells with a pathological condition was first understood when they were found scattered in this area while examining the lesions of a case with urticaria pigmentosa (UP). The certainty that mast cells were associated with any disease only emerged in 1949 discovered the existence of mast cells in many organs in the autopsy of a one-year-old child who passed away from cachexia[5]. This was the first report of systemic mastocytosis (SM). In 1988, Travis et al[6] designed a classification scheme for mastocytosis and this was accepted by the scientific world as the first scheme by the National Institutes of Health of United States in 1991.
In 2010, a conference was organized to elaborate on this topic to modernize the classification and diagnostic criteria for mast cell disorders, especially MCAS. Mastocytosis was already well known, but MCAS also required to be described and some criteria established. The classification into primary, secondary, and idiopathic MCAS was first proposed at this conference for diagnostic purposes[1]. Two years later, Valent et al[7] published an update of the criteria based on learning and observation of the disease. In 2016, the World Health Organization published a second updated classification of mastocytosis[7].
EPIDEMIOLOGY
Epidemiologic studies are not yet sufficient to verify the incidence and prevalence of both mastocytosis and MCAS. The incidence of mastocytosis is currently thought to be 1/10000[8,9]. It is equally distributed by sex and can occur at any age. Epidemiologic studies on MCAS have not yet been conducted sufficiently and the frequency of these syndromes is much more hard to guess[10]. The incidence and prevalence of cases with monoclonal MCAS (MMCAS) and idiopathic MCAS are not well known and there is insufficient data in the literature[11,12].
PATHOGENESIS AND MECHANISMS INVOLVED
An increase in cell number due to decreased apoptosis, an abnormal activation in response to microenvironmental triggers, rather than neoplasia of mast cells have a role in the development and classification of the syndrome. The disease can be categorized into three main groups: Primary, secondary, and idiopathic[13].
In MCAS, the pathological behavior of mast cells is not due to abnormal mast cell proliferation but to chronic abnormal constitutive and reactive activation[5]. This abnormal activation may occur as a result of a change in the activation threshold, abnormal expression of receptors and mediators inducing an allergic immune response, changes in the tissue environment affecting the expression and function of mediators, or mutations in the regulatory genes of the cells[14,15]. Although the genetic basis is not fully understood, mutations and alternative variants have been detected in the c-KIT receptor (CD117+), which is responsible for the proliferation of mast cells in tissue via stem cell factor[12].
The best-defined and most focused physiopathologic role of mast cells is the allergic reaction caused by an abnormal response of mast cells to harmless antigens. Mastocytosis and MMCAS are both described as having clonal mutations in mast cells[16,17]. Although the pathogenic mechanisms of mastocytosis are comparatively well characterized, the mechanism of MMCAS has not been explained. Moreover, no mutations have been found in idiopathic MCAS and not enough is known about its pathogenesis[18,19].
PARAMETERS USED AS MCAS DIAGNOSTIC CRITERIA
The MCAS criteria were first recognized in 2012 and remain to be developed by an international consensus group. Three criteria are considered, all of which must be met for a diagnosis of MCAS to be made[20].
In the case of clinically episodic, recurrent, severe (mostly like anaphylaxis), and systemic (including at least two organ systems) typical MCAS symptoms; the criteria used in the diagnosis are following: (1) Serum tryptase level, one of the markers of MCAS in the laboratory, exceeding 120% + 2 ng/mL above the serum baseline value of the individual; and (2) clinical reaction to anti-mediator drugs that inactivate mast cell mediators or preclude their release, and finally iii) primary (clonal) and/or secondary diseases of mast cell activation ruled out[4].
The standard laboratory diagnostic marker for MCAS is serum tryptase, with a normal serum level defined to be between 0 and 11.4 ng/mL in adults. Studies have shown that blood samples should be taken within 1 to 4 h of the beginning of symptoms and that basal levels should be evaluated in advance during a symptom-free period of at least 24-48 h after complete recovery. However, some studies have suggested that normal tryptase levels do not diagnostically rule out MCAS[1,6,21].
In addition, other mediators such as histamine, prostaglandin D2, chromogranin-A, leukotriene E4, and urinary metabolites of histamine and 11-beta-prostaglandin are not well known about the increased levels required for the diagnosis of MCAS. These mediators are also thought to be less specific for the diagnosis of MCAS[6].
Biologic agents used therapeutically, such as antihistamines, leukotriene modifiers, mast cell stabilizers, cyclooxygenase inhibitors, or omalizumab, have been identified as supporting the diagnosis as a recognized reaction to drugs that act specifically on MCAS[19,22].
MCAS CLASSIFICATION
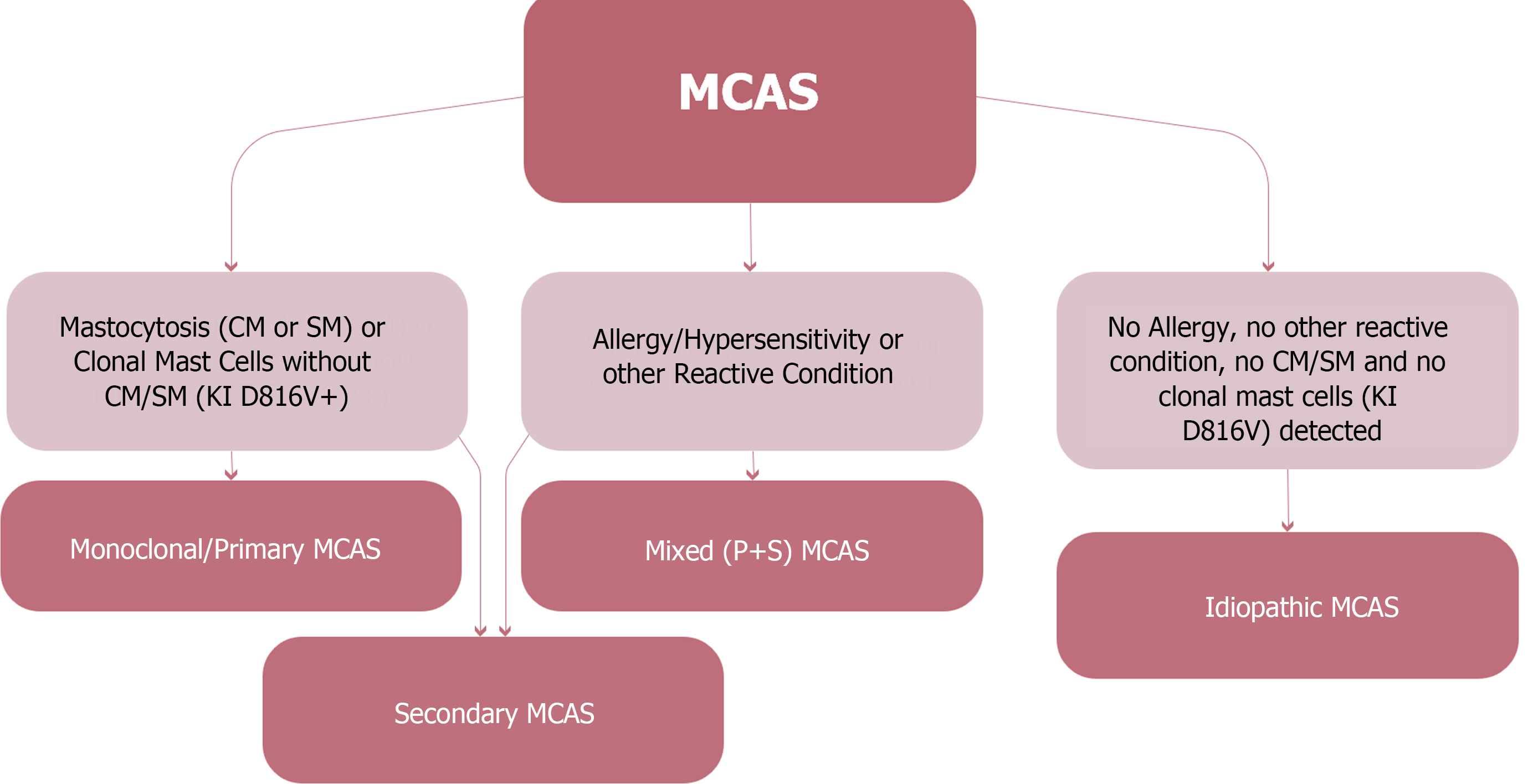
Clonal mast cell disorders are considered primary MCAS if they are due to the existence of c-KIT mutations (generally involving the D816V mutation or expression of CD25, CD2, or CD30 on mast cells). If mast cell activation is owing to an allergic or other hypersensitivity disorder, then it is considered secondary MCAS (non-clonal)[12,23]. In addition, if there is no clonality and no other specific reason can be recognized, then MCAS is accepted as idiopathic (Figure 1). Combined types of MCAS have also been defined in which cases have both primary and secondary MCAS features and are categorized as mixed MCAS[24].
Figure 1 Classification of mast cell activation syndrome.
MCAS: Mast cell activation syndrome; SM: Systemic mastocytosis; CM: Cutaneous mastocytosis.
CLINICAL SYMPTOMS AND SIGNS IN DIFFERENT ORGANS AND SYSTEMS IN MCAS
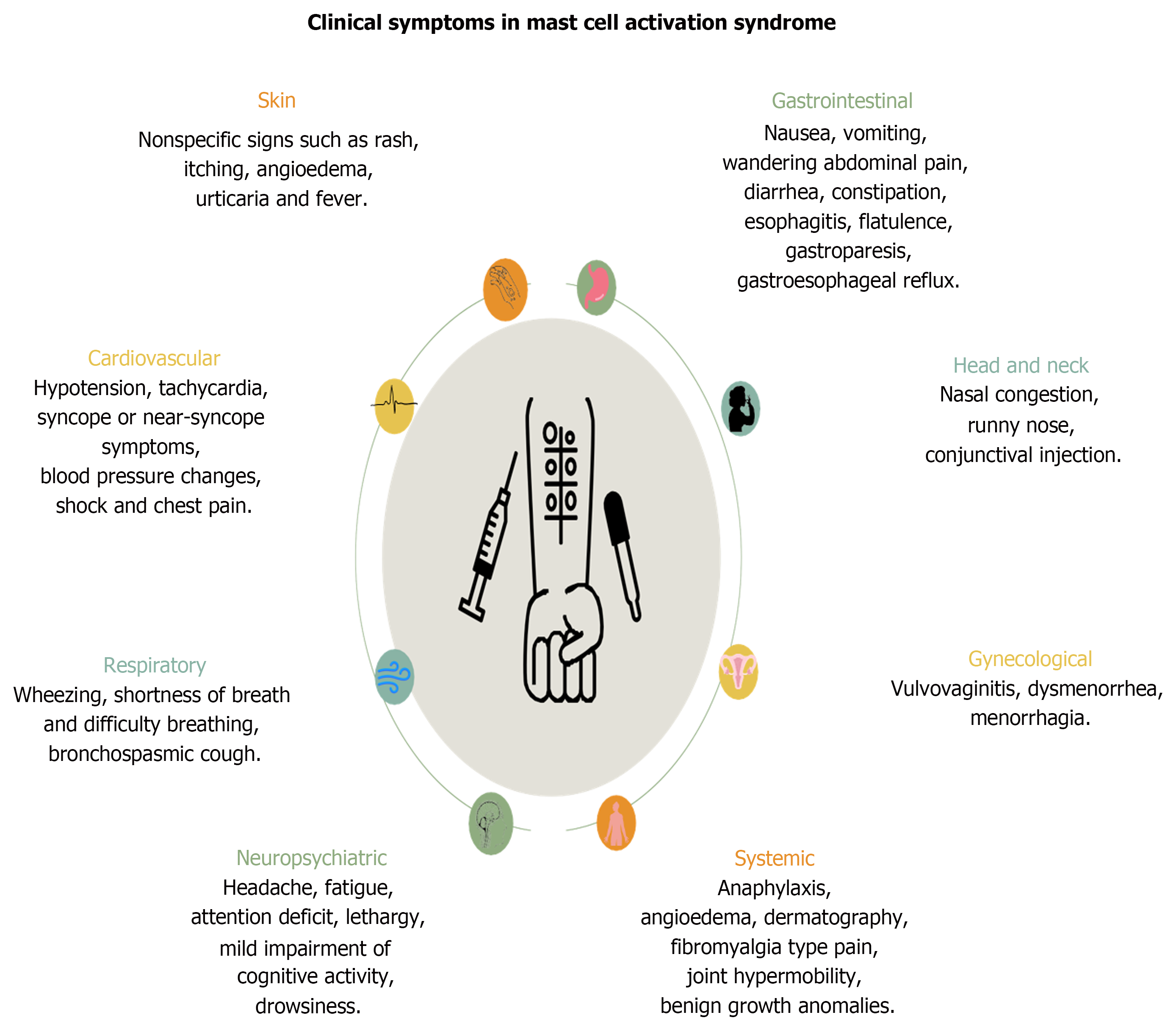
This syndrome involves different systems and organs in the body, resulting in different clinical symptoms and signs (Figure 2)[3]. Common symptoms typically include symptoms suggestive of allergies such as flushing that may increase, decrease, and travel throughout the body, fatigue, cognitive dysfunction, irritation of the eyes, nose, mouth, and throat, lymph node inflammation, nausea, reflux, headache, dyspnea, palpitations, abdominal pain, diarrhea/constipation, anxiety, and mood disorders. In addition, dermatographism, fibromyalgia-type pain, joint hypermobility, benign growth anomalies, interstitial cystitis, menorrhagia, dysmenorrhea, vulvovaginitis, sensory neuropathy, dysautonomia, and various metabolic endocrinologic abnormalities can be seen affecting many organs and systems. Here, different system involvement will be discussed below one by one.
Figure 2
Clinical manifestations in different systems in patients with mast cell activation syndrome.
Cardiovascular system involvement
Common symptoms of MCAS include hypotension, tachycardia, syncope or near syncope, blood pressure changes, shock, and chest pain. In addition, postural orthostatic tachycardia syndrome (POTS) has some similar clinical symptoms as in MCAS, although the etiology is not fully known, suggesting that there may be a relationship between them and they may have a common pathogenesis[25]. Although the pathogenesis is not yet fully known, several pathophysiologic processes have been suggested to be involved in this syndrome. The first likely pathophysiologic mechanism is incomplete sympathetic neuropathy[26]. Some cases have been observed to have incomplete sympathetic denervation and irregular epinephrine reactions in the lower extremities[27]. As a result, inadequate vasoconstriction is thought to increase heart rate with sympathetic activation in response to congestion in the legs. Clinically, orthostatic intolerance symptoms should be present in patients with POTS, which mostly affects women of reproductive age[25].
POTS is characterized by symptoms of palpitations, chest pain, heart discomfort, headache, blurred vision, and dizziness. POTS, which has been described by many researchers with different names until today, is now characterized as 'orthostatic intolerance syndrome'. The pathognomonic feature of orthostatic intolerance is the manifestations of symptoms while upright and relief when in supine positions[25].
The first study targeting to investigate a relationship between MCAS and POTS was conducted in by Shibao et al[26]. Since flushing is a symptom in both POTS and MCAS, it is thought that MCAS may contribute to the pathogenesis of POTS. Since studies on the relationship between these two are very limited, more data are needed to better characterize the mechanisms. As a result, it is believed that more research should be done on patients who meet the criteria for both diseases.
Dermatologic involvement
Cases with MCAS often manifest flushing caused by the vasodilating effects of histamine and other mediators[28]. Flushing may be caused by exercise, alcohol, temperature, and emotional changes. Angioedema may occur especially on the lips and tongue. The flushing seen in mast cell disorders is episodic, lasts longer, and it is usually not accompanied by sweating[29]. Among other clinical findings, nonspecific urticaria, pruritus, etc. may occur[30].
Respiratory system involvement
Upper and lower respiratory tract symptoms are common in patients with MCAS. These symptoms include nasal congestion, nasal itching, shortness of breath, wheezing, bronchoconstriction, bronchospastic cough, throat swelling, and rhinorrhea[1,23]. Angioedema of the upper respiratory tract may also be seen, but it is rare[4].
Gastrointestinal tract involvement
General effects of MCAS on the gastrointestinal tract include nausea, vomiting, wandering abdominal pain, abdominal tenderness, gastroesophageal reflux, dysphagia, atypical chest pain, diarrhea, constipation, esophagitis, intestinal cramps, bloating, malabsorption, mouth sores, gastroparesis, and angioedema[31].
Irritable bowel syndrome (IBS) may be observed in some patients in association with MCAS. IBS is a common gastrointestinal disorder affecting the quality of life in a large quantity of the population. It is characterized by abdominal pain, alternating constipation, and diarrhea. It has been noted that impaired intestinal barrier function in IBS is caused by mast cell activation due to stress response. One of these stress responses is certain components in the food ingested, such as plant-derived substances. These components regulate mast cell activation[32]. Mast cell numbers have been shown to increase in the terminal ileum, jejunum, and colon of patients with IBS. At the same time, biopsies performed on atopic IBS patients showed a higher proportion of mast cells compared to non-atopic patients[33]. Mast cell numbers were found to be directly proportional to the severity of symptoms in IBS and it is thought that mast cell mediators may cause symptoms. The rise in the count of mast cells in the colon is one of the most consistent changes observed in IBS[34]. In addition, the severity of pain observed in patients with IBS is associated with the number of mast cells located close to enteric nerves. The released mediators activate enteric nerves[32,35].
Small intestinal bacterial overgrowth (SIBO) is common in MCAS. SIBO is a condition in which colon bacteria overgrow in the small intestine. It occurs as a result of anatomical abnormalities as well as motility, and metabolic, systemic, and immune system disorders. In this condition, intestinal symptoms include nausea, anorexia, and bloating due to malabsorption and impaired small intestinal motility. These clinical symptoms are probably caused by invasive strains. Studies have shown the presence of 3 main organisms, E. coli, Klebsiella, and Aeromonas species. The diagnosis of SIBO is made when the bacterial count exceeds 10³ organisms /mL in a patient with clinical symptoms[36]. The relationship between SIBO and MCAS is as follows; SIBO causes activation of mast cells and increase in T lymphocytes. T lymphocytes in turn secrete microparticles that again activate mast cells. Activated mast cells and T lymphocytes release cytokines that increase intestinal permeability[37]. This leads to a vicious cycle in which intestinal permeability is constantly impaired and inflammation is constantly increased[36,38].
In mice, abdominal surgeries such as colorectal surgery as well as laparoscopic procedures are known to increase the release of mast cell mediators in peritoneal fluid, extracellular matrix thickness and the risk of intra-abdominal adhesions. This suggests that it may increase the likelihood of intra-abdominal adhesions such as postoperative ileus in humans[38].
Bone-joint system involvement
Ehler-Danlos syndrome (EDS) is frequently associated with MCAS. Patients are increasingly being admitted to hospitals with EDS and MCAS. EDS is primarily presented by skin hyperextensibility, joint hypermobility, and tissue fragility. It is caused by a group of genetic disorders. Extensive gastrointestinal tract involvement also occurs in this syndrome. Esophagitis, gastroesophageal reflux, abdominal pain, and IBS are common gastrointestinal symptoms[35]. Patients with EDS have been reported to have increased gastrointestinal symptoms seen in MCAS. It is also thought that mast cell activation may be the cause of other symptoms seen in patients with EDS[18]. It is recommended that patients with EDS should also be screened for MCAS during evaluation. In addition, it is thought that IL-17A, which is highly produced as a result of mast cell activation disorder in MCAS, causes focal bone loss[27]. Therefore, it has been reported that osteoporosis may occur at an early age in patients with MCAS and EDS[26].
Allergic symptoms and disorders
Anaphylaxis is a severe systemic hypersensitivity reaction and increased levels of mast cell mediators such as tryptase and histamine have been detected during attacks[39]. Mast cells seem to be the primary cells that trigger anaphylaxis in humans[40]. In addition, activation of basophils results in histamine and mediators such as LTC-4 may be secreted and indirectly contribute to the development of symptoms. To date, no specific biomarker has been identified to follow suspected patients, classify the severity of reactions, or manage the disease. The underlying mechanisms leading to idiopathic anaphylaxis are not fully understood, but several theories have been proposed to explain the pathogenesis[41]. Researchers have observed that peripheral blood from patients with idiopathic anaphylaxis has higher mast cell counts in culture compared to healthy controls[42].
In another study, the relationship between MCAS and anaphylaxis was examined through some molecules via the ERK 1/2 pathway. Extracellular signal-regulating kinases (ERK 1/2) contribute to allergic responses by regulating degranulation, eicosanoid creation, and cytokine expression by mast cells, but the mechanisms emphasizing their positive effects on FcεRI-dependent signaling have not been fully elucidated. It has recently been demonstrated that mast cell activation and anaphylaxis are negatively regulated by AMP activated protein kinase (AMPK a type of serine-threonine kinase that is a central regulator of energetic metabolism). However, little was known about the association between ERK1/2-mediated positive and AMPK-mediated negative regulation of FcεRI signaling in mast cells. In one study, ERK1/2 activated FcεRI signaling in mast cells by abolishing the AMPK-dependent negative regulatory axis[43].
In conclusion, the possible relationship between anaphylaxis and MCAS has been examined through many theories and studies, but more clinical trials are needed to say anything definitive[29].
Neuropsychiatric symptoms and disorders
Headache, fatigue, weakness, lethargy, lack of attention, feelings of exhaustion, lethargy, and mild impairment of cognitive activity are common symptoms in cases with primary and secondary mast cell activation (i.e. with mastocytosis and also with allergic disorders). These complaints might be owing to the psychosomatic effects of mast cell-mediated mediators, drugs, or (in adults) having a chronic disease[44]. Subjective neuropsychiatric symptoms alone should not be taken into consideration for mast cell disorders. Fairly, individual complaints must be along with signs and complaints affecting other organ systems before mast cell disorders can be thought of.
According to various studies and investigations, mast cells are considered to make a significant contribution to the pathophysiology of migraine[45]. Mast cells are found in the meninges and are thought to be included in the pathophysiology of migraine through events such as sequential neuropeptide release and vasodilation leading to mast cell degranulation[46,47]. Mast cells discharge hundreds of various mediators such as histamine, tryptase, and leukotrienes, and degranulation of meningeal mast cells contributes to the activation of the trigeminal vascular afferent pathway[48]. This is assumed to be one of the underlying mechanisms of migraine and pain. A recent study has examined the link between the parasympathetic nervous system, mast cells, and migraine, with research suggesting that endogenous acetylcholine activates meningeal mast cells and thus contributes to migraine pathophysiology[49]. However, although the data obtained are enlightening, further studies are required to explain the complex interaction between the autonomic nervous system, mast cells, and connective tissues of the meninges, cerebral vasculature, and other structures significant in the pathophysiology of migraine headaches[50].
MASTOCYTOSIS-MCAS RELATIONSHIP
Mastocytosis is divided into two categories: Systemic (SM) and cutaneous mastocytosis (CM). In SM, abnormally proliferating mast cells affect the skin, bone marrow, and other organs, causing various symptoms. Common symptoms include itching, abdominal cramps, and tachycardia[16]. CM is more common in young children and usually resolves spontaneously in puberty. In CM, mast cells gather in the skin but not in other organs. However, systemic symptoms may still be observed. This is because mediators secreted from activated mast cells accumulated in the skin are released into the circulation[29].
The symptoms of SM and MCAS are similar. Both conditions have symptoms of mast cell activation, such as facial flushing, abdominal cramps, and hypotension due to degranulation. Mastocytosis is mast cell proliferation with infiltration of dermis (CM) or other tissues and organs (SM). MCAS is increased and inappropriate activation of mast cells without clonal proliferation. The differences between these two conditions are as follows: (1) Patients with SM have an elevated baseline serum tryptase level, typically >20 ng/mL. In cases with MCAS, baseline serum tryptase levels are normal or slightly elevated; (2) multifocal mast cell aggregates are observed. It is characteristic to observe these aggregates in the bone marrow of cases with SM, but not in MCAS; and (3) it is characteristic to usually observe UP called maculopapular CM (MPCM) in patients with CM. However, UP/MPCM-like lesions are not observed in MCAS[17].
THE RELATIONSHIP BETWEEN PEOPLE WITH LONG-TERM CORONAVIRUS DISEASE 2019 AND MCAS
Long-term coronavirus disease 2019 (COVID-19) is an outcome of immune dysregulation. T and B cell deficiency, hyperactivity of innate immune cells, and an increase in proinflammatory cytokines are observed[7]. This dysfunction leads to a constant inflammatory reaction, pathogen reactivation, endothelial damage, host-microbiome dysfunction, and autoimmunity. Risk factors for the development of long-term COVID-19 include female gender, type 2 diabetes, autoimmune diseases, connective tissue, and allergic disorders[51,52].
People with long-term COVID-19 disease have major cardiac, neuropsychiatric, and pulmonary complaints. Multi-system disorders such as myocardial inflammation, POTS, dystonia, and myalgic encephalomyelitis/chronic fatigue syndrome can develop. Immune system effects include recurrent infection, autoimmunity, urticaria, allergic rhinitis, and asthma. MCAS is thought to be the possible mechanism underlying these effects[26,53].
Mast cells are the key producers of the inflammatory cytokines of COVID-19. A persistent inflammatory state with prolonged COVID-19 causes abnormal mast cell activation. One of the reasons for this is the maturation of mast cells in the pulmonary perivascular space[54]. Another cause is the discharge of substance P from immune cells due to severe acute respiratory syndrome coronavirus 2 infection. The secretion of this substance increases the stimulation of the G-protein X2 receptor and predisposes to MCAS formation[55,56].
It is thought that symptomatic improvement will be achieved with the treatment of MCAS in long-term COVID-19 cases. It is thought that stabilization of mast cells and reduction in related symptoms will be achieved by histamine blockade[57,58].
DIFFERENTIAL DIAGNOSIS
The differential diagnosis of MCAS includes numerous diseases and nonspecific signs and symptoms: Infectious diseases, gastrointestinal (IBS, gastrinoma, VIPoma, eosinophilic gastroenteritis or esophagitis, inflammatory bowel disease, food intoxication, etc.), cardiovascular (endocarditis or endomyocarditis, pulmonary embolism, aortic stenosis with syncope, myocardial infarction), endocrinological (medullary thyroid carcinoma, pheochromocytoma, carcinoid), neuropsychiatric disorders (anxiety/panic attacks, vasovagal syncope), urticaria and angioedema types, drug-induced itching/rash, vasculitis, and atopic dermatitis[59]. In addition, the differential diagnosis should include two disorders in which there is chronic systemic elevation of mast cell pre-performed mediators in granules and depends on the function of the granules without over activation of mast cells, namely histamine intolerance and hereditary alpha tryptasemia.
A thorough physical examination, together with a thorough history and laboratory evaluation of specific biomarkers, can help differentiate these disorders from MCAS[60].
TREATMENT PLANS OF MCAS
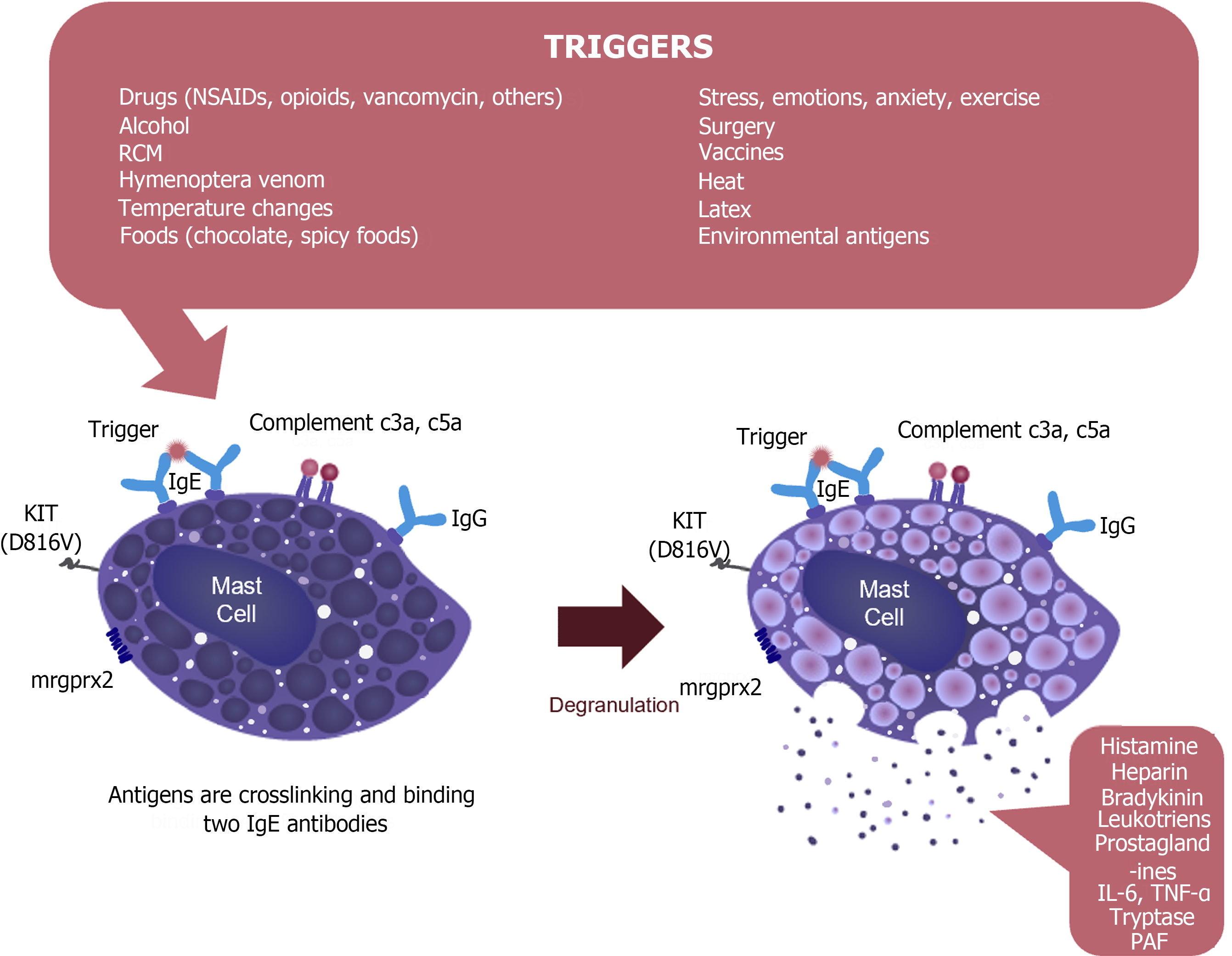
First, it is of paramount importance to explain to patients and their parents to elude any agents or interactions that could trigger anaphylactic or allergic reactions (Figure 3)[18]. MCAS cases should also be counseled to take prophylactic anti-mediator therapy (e.g. histamine receptor blockers) throughout their lives and to carry at least two self-administered epinephrine autoinjectors after being instructed on how to utilize these injectors in case of illness[41].
Figure 3
The mechanism of mast cell activation and release of mast cell mediators.
In cases with reaction-inducing (IgE-dependent) anaphylaxis and thus secondary MCAS, immunotherapy is generally suggested because its potential side effects are better known. The frequency of life-threatening MCAS events can be significantly reduced after inactivation of neoplastic mast cells. Aspirin has formerly been suggested as a potential therapeutic tool for the therapy of anaphylaxis in patients with SM[61], but it has been observed that the doses of aspirin required to prevent MCAS must be high and are not tolerated in many cases. Other medications contain mast cell regulatory agents and corticosteroids[7,62].
In addition, there is also an emerging class of drugs targeting mast cells, namely broad-acting tyrosine kinase inhibitors e.g. midostaurin or avapritinib. Some of these medications, such as midostaurin, not only inhibit mast cell proliferation but also block IgE-dependent allergic stimulation of mast cells. Hence, midostaurin might be an encouraging treatment to subdue the effects of MCAS, especially in cases with primary MCAS[19,63].
Another proposed interpretation of MCAS treatment is the use of specific IgE (e.g., omalizumab) in secondary MCAS cases with underlying IgE-dependent allergy. Furthermore, in cases with mixed MCAS (primary + secondary MCAS), multiple specific treatment modalities may be necessary after an individualized medicine approach[18]. For instance, cases with high levels of neoplastic mast cells, advanced SM, and severe IgE-dependent allergy may require a drug directed against the c-KIT mutation or combined treatment with cladribine and omalizumab to control MCAS events[18,62].
CONCLUSION
Although the mast cell is an obligatory cell for life, issues related to its activation may also be associated with disorders such as MCAS that may present with a wide variety of clinical findings and complaints. Awareness of this disorder should be increased so that MCAS can be easily differentiated from other diseases and treatment plans should be well known.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country of origin: Türkiye
Peer-review report’s classification
Scientific Quality: Grade A
Novelty: Grade A
Creativity or Innovation: Grade A
Scientific Significance: Grade B
P-Reviewer: Poerwosusanta H, Indonesia S-Editor: Qu XL L-Editor: A P-Editor: Cai YX