Published online Jun 9, 2024. doi: 10.5409/wjcp.v13.i2.91587
Revised: April 7, 2024
Accepted: April 18, 2024
Published online: June 9, 2024
Processing time: 159 Days and 8.1 Hours
Over the past 20 years, the incidence and prevalence of type 2 diabetes mellitus (T2DM) in children and adolescents have increased, particularly in racial and ethnic minorities. Despite the rise in T2DM in children and adolescents, the pathophysiology and progression of disease in this population are not clearly understood. Youth-onset T2DM has a more adverse clinical course than is seen in those who develop T2DM in adulthood or those with T1DM. Furthermore, the available therapeutic options are more limited for children and adolescents with T2DM compared to adult patients, mostly due to the challenges of implementing clinical trials. A better understanding of the mechanisms underlying the de-velopment and aggressive disease phenotype of T2DM in youth is important to finding effective prevention and management strategies. This review highlights the key evidence about T2DM in children and adolescents and its current burden and challenges both in clinical care and research activities.
Core Tip: The incidence and prevalence of type 2 diabetes mellitus (T2DM) in children and adolescents have dramatically increased over the past 20 years. Accumulating evidence suggests that youth-onset T2DM presents unique characteristics, demographics, and disease progression compared to adult-onset T2DM. In addition, the available therapeutic options for children and adolescents with T2DM are inadequate. T2DM in children and adolescents is becoming a public health concern worldwide. Research programs should aim to overcome the challenges seen with clinical studies in this population to address the unmet need of optimal management of T2DM in children and adolescents.
- Citation: Pramanik S, Mondal S, Palui R, Ray S. Type 2 diabetes in children and adolescents: Exploring the disease heterogeneity and research gaps to optimum management. World J Clin Pediatr 2024; 13(2): 91587
- URL: https://www.wjgnet.com/2219-2808/full/v13/i2/91587.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v13.i2.91587
Type 2 diabetes mellitus (T2DM) has usually been regarded as a disease with onset in middle age to older ages. However, the incidence and prevalence of T2DM in children, adolescents, and young adults are increasing worldwide. In North America, a study among individuals aged < 20 years confirmed the presence of T2DM in all racial and ethnic populations and observed a high prevalence, especially among American Indian and Indigenous peoples[1]. Over the last few decades, the rising frequency, greater severity, and earlier onset of childhood obesity in conjunction with sedentary lifestyles and an increasing occurrence of intrauterine diabetes exposure are key drivers of young-onset T2DM. T2DM in children and adolescents differs from T1DM and more closely resembles the pathophysiology in adult patients: insulin resistance and non-autoimmune failure of β-cells. In people who develop T2DM during childhood or adolescence, a more aggressive course is frequently noted than those who develop T2DM in adulthood with a faster decline in β-cell function, high incidence of treatment failure, and accelerated development of complications[2,3]. Moreover, the available the-rapeutic options for children and adolescents with T2DM are inadequate. Until recently, pharmacological treatments recommended by the regulatory bodies were limited to metformin and insulin. Taking together, young-onset T2DM is an emerging disorder with unique challenges in clinical care as well as research programs[4], leaving huge knowledge gaps in pathophysiology, clinical course, and optimization of treatment.
In this review, we examine the epidemiology of T2DM in children and adolescents and its complicated course globally, with a focus on disease heterogeneity. The clinical courses of youth who develop T2DM are compared with adults who develop T2DM. The impact of young-onset T2DM on the important microvascular and macrovascular complications is also explored. Finally, we investigate the possible mechanisms to elucidate the observed aggressive metabolic phenotype in individuals with youth-onset T2DM and discuss available treatment strategies and the complexities that hinder their implementation in this population.
T2DM in children and adolescents is often underrecognized because of its prolonged subclinical course and lack of awareness regarding the increasing risk among patients and providers. Consequently, national population-based disease registry data might not adequately reflect the frequency and growing trends of T2DM in this population. Nevertheless, the prevalence of T2DM in the 10- to 19-year-old population has doubled over the past two decades in the United States[5]. It is present in nearly 2 persons per 1000 among Black and American Indian youth. The prevalence of youth-onset T2DM will double to quadruple by 2050 as estimated by the SEARCH for Diabetes in Youth study. The incidence of T2D has similarly doubled among adolescents in the United States over this period, from 9 to 18 cases per 100000 per year, with an incidence that is particularly high in Black and American Indian youth[6]. Similarly, T2DM in youth is increasing in incidence and prevalence worldwide. In a review of country-specific prevalence and incidence of youth-onset T2DM, it was found that some of the more economically developed countries, such as the United States and China, have the highest reported rates worldwide[7]. The analysis also noted that data for T2DM in children and adolescents are still scarce across the globe. The number of incident cases of T2DM in youth is more than 2-fold higher in China and 1.4-fold higher in India than in the United States based on the larger population of these countries, but with the United States showing the highest reported incident rates in a more recent worldwide survey among children and adolescents aged under 20 years[8]. In general, the prevalence of T2DM in youth is lower in Europe than in other developed countries[7]. Data on youth-onset T2DM are scarce in South America and Africa. The escalating prevalence of obesity in children and adolescents has likely triggered an increased rate of T2DM[5]. However, this alone does not seem to fully explain the increase in T2DM.
All of the traditional risk factors for T2DM are also involved in young-onset T2DM but in an amplified manner. Obesity is the single most important risk factor, with more than 90% of patients suffering from young T2DM being obese[9]. Early onset obesity or greater cumulative exposure to obesity are more important risk factors for diabetes[10]; however, the risk is reduced if weight normalizes before puberty[11]. Positive family history and female sex are considered to be non-modifiable risk factors, with more than 80% of United Kingdom adolescents having a family history of T2DM[9]. In cohort studies, the female sex predominates until age 25 years after which it equalizes and the male sex predominates after 40 years of age[12]. Ethnicity and socioeconomic status also play important roles in determining the prevalence. Asian, Middle East and North Africa, Hispanic, and Black ethnic populations have a disproportionately high prevalence of diabetes in adolescents[13], and low middle and middle socioeconomic index countries have the highest age-standardized incidence compared with low socioeconomic index[14]. Other risk factors can be prenatal exposure, maternal malnutrition, and diabetes, which increase the risk of diabetes in the offspring. The SEARCH study showed that maternal obesity (odds ratio [OR]: 2.8, 95% confidence interval [CI]: 1.5–5.2) and maternal gestational diabetes (OR: 5.7, 95%CI: 2.4–13.4) were associated with risk of youth-onset T2DM compared with controls that persisted even after adjustment for age, socioeconomic factor, and ethnicity[15]. The Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) study group reported an association of maternal diabetes history with faster glycemic progression and worsening β-cell function in offspring with youth-onset T2DM over 12 years of follow-up[16]. In the SEARCH Case-Control Study, the odds of T2DM were significantly lower among those who were breastfed in infancy (OR: 0.26, 95%CI: 0.15–0.46). Nevertheless, the association was confounded by current body weight[17]. Additionally, some endocrine-disrupting chemicals, such as perfluoroalkyl and polyfluoroalkyl substances, are associated with an increased risk of T2DM at a young age[18].
Although obesity and insulin resistance are critical initial factors, progression to glycemic failure is heralded by evidence of impaired β-cell function. Adolescents with T2DM have rapid progression to glycemic failure compared with adults[19]. Their pathogenesis involves an accelerated rate of -cell function loss[20] and lower insulin sensitivity than adults[21]. In the RISE study, the rate of loss of β-cell function was 25%–30% in young people vs 7% in those with adult-onset T2DM[22]. The exact etiology for this accelerated β-cell function loss is not known, but excess growth hormone during puberty and obesity-induced insulin resistance and glucolipotoxicity are postulated to have a role[23]. In the TODAY study[24] for T2DM in young people (< 20 years), the initial hemoglobin A1c (HBA1c) and β-cell reserve were independent predictors of glycemic durability; participants with lower β-cell function experienced earlier treatment failure. There were no significant differences in markers of insulin sensitivity between adult and young-onset T2DM. A similar finding was observed in the RISE study[22] and unlike adults, early insulin treatment did not ameliorate β-cell function loss, suggesting that inadequate glycemic control is not contributing to this faster progression. In addition to β-cell dysfunction, higher insulin resistance was noted in young people vs adults with T2DM[21] and also with impaired glucose tolerance, which persisted after adjustment of body mass index (BMI), race, and sex[25].
Based on family and twin studies, the heritability of T2DM in children ranges between 30% and 70%[26]. The first genome-wide association study for T2DM in young people was published in 2021, which included 9067 participants from multiethnic backgrounds and consisted of 3006 young people and 6061 adult controls[27]. Seven genome-wide significant loci were identified, including a novel locus (rs10992863) in PHD finger protein 2. The remaining six loci (transcription factor 7-like 2 [TCF7L2], melanocortin 4 receptor, cell division cycle protein 123, KCNQ1 channel, insulin-like growth factor-binding protein 2, and solute carrier family 16 member 11) were previously reported as adult loci. Among them, TCF7L2 has one of the strongest effects on the risk of T2DM among common variants (odds ratio [OR] of about 1.4). The T2DM liability variance was 3.4 times higher for common variants and five times higher for rare variant associations compared with adult-onset T2DM, indicating higher genetic contribution for young-onset T2DM. These findings suggest that adults and young-onset T2DM persons have overlapping genetic architecture, but the role of genetics is probably higher for disease risk in adolescents than in adults.
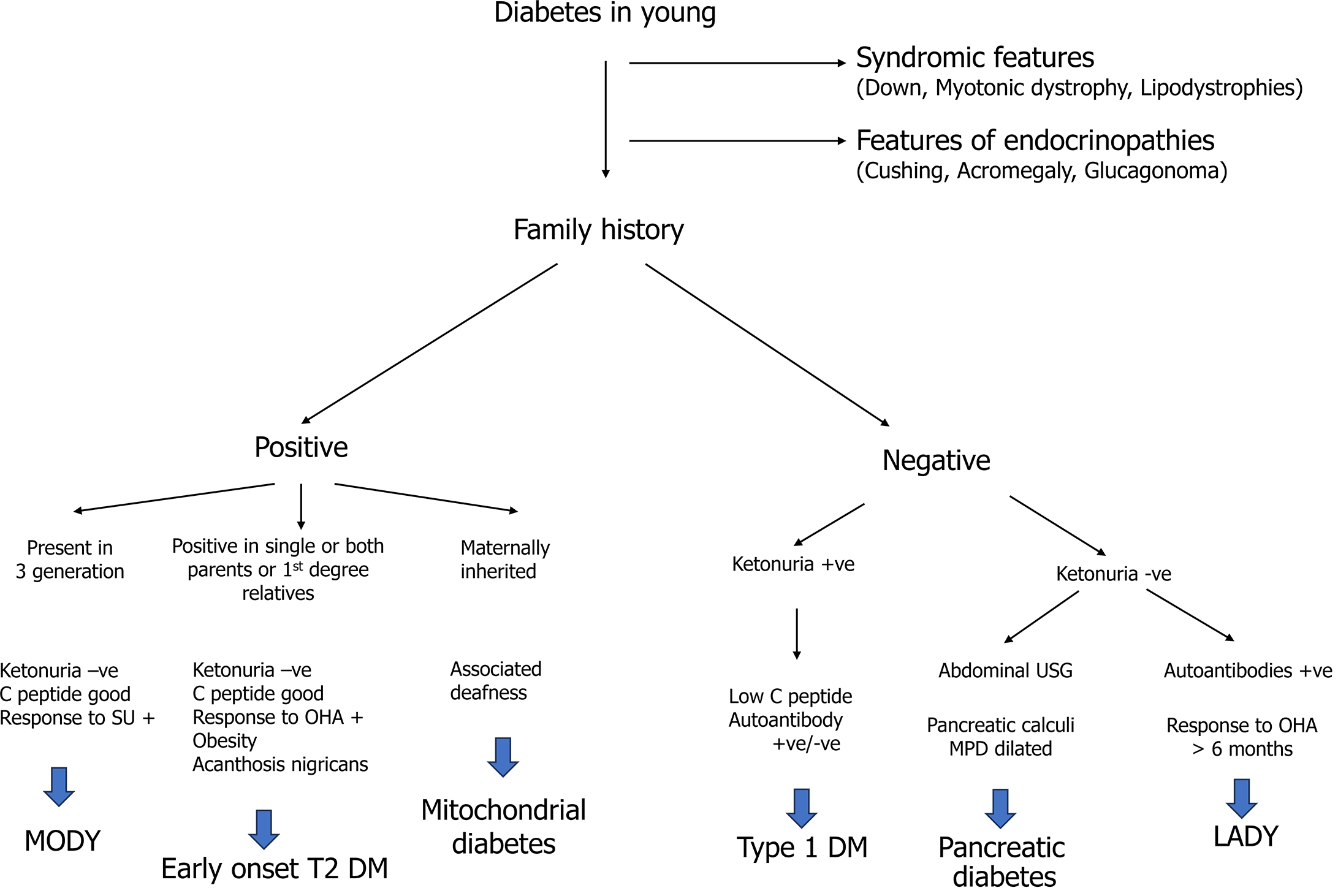
During the adolescent period, elevated blood glucose levels can be due to multiple etiologies. As T2DM is a disease of exclusion, one must reasonably exclude other etiologies before jumping to a diagnosis. Common differentials are T1DM, maturity-onset diabetes in the young, diabetes due to pancreatic disease, latent autoimmune diabetes in the young, endocrinopathies (acromegaly, Cushing’s), lipodystrophies, mitochondrial disease, and syndromic diabetes. Detailed history taking, focused clinical examination, and a few laboratory investigations are required to clinch the diagnosis. T2DM is typically considered in pubertal youth with obesity, a family history of T2DM, features of the metabolic syndrome, and/or absent islet autoantibodies. A detailed approach to the diagnosis of T2DM in adolescents is illustrated in Figure 1.
There is a high prevalence of diabetes-associated complications and comorbidities in those with young-onset T2DM - both at the time of diagnosis and beyond. The implications of these complications are of particular importance in this age group because of their effects on education and jobs, psychological consequences, and increased health-care expenditure. Also, women of reproductive potential with DM need special care and attention. Several studies suggest that for people of the same age, the risks of macrovascular and microvascular complications are higher for people with early-onset vs later onset T2DM[28].The risks are also higher for young-onset T2DM compared to T1DM of the same duration[29]. With improved healthcare, there has been a significant reduction in the complications and mortality of people living with T2DM; these improvements have not been evident in the youngest subgroup. Rather, there has been a resurgence of complications from 1995 to 2015 among people with T2DM between 18 and 44 years of age from 1995 to 2015[30]. After 15 years of follow-up, about 80% of adolescents in the TODAY study had developed a minimum of one microvascular complication[31].
The proposed hypotheses behind the increased risk of complications could be a more aggressive pathophysiology, poorly controlled hyperglycemia, more prolonged exposure to glycemic burden, the concurrent presence of other metabolic risk factors, an unrecognized period of untreated or inadequately treated hyperglycemia before the diagnosis and the effects of obesity and inflammation since a large proportion of those with young-onset TDM are overweight or obese. There are other factors such as socioeconomic factors, mental health issues with reduced self-management capacity, reluctance to undergo repeated screening tests, or reduced engagement with health services with infrequent follow-up.
An Australian study showed that individuals with young-onset T2DM had a higher risk of coronary artery disease and stroke compared to individuals of similar aged individuals with T1DM[31]. Studies have shown that those with young-onset T2DM have up to 50% higher cumulative risk of cardiovascular disease (CVD) than those with later-onset T2DM. Also, at any age, the risk of adverse cardio-renal outcomes was higher than in the late-onset group, but these differences were primarily driven by the longer duration of diabetes in the young-onset T2DM group[32].
Although overt CV events are not very common in youth with T2DM, it is a well-established fact that the process of atherosclerosis starts early during childhood. Studies have revealed the presence of subclinical vascular disease in adolescents with obesity and T2DM in the form of elevated aortic pulse wave velocity or increased carotid intima-media thickness (CIMT) compared with normoglycemic youth. In the SEARCH study, those with young-onset T2DM had greater degrees of arterial stiffness than those with T1DM, which was unrelated to the duration of diabetes or glycemic control[33]. Generalized obesity as well as abdominal adiposity were found to be determinants of coronary artery calcifications in those with young-onset T2DM[34]. In the TODAY study, BMI and blood pressure were associated with adverse cardiac measures[35]. Overall, there appears to be more significant vascular dysfunction and a higher chance of progression to overt CVD among youth with T2DM. However, as in adults, there is no recommendation to routinely screen for subclinical atherosclerotic CVD in young-onset T2DM.
Nephropathy: The SEARCH study revealed greater rates of microvascular complications, including diabetic nephropathy, retinopathy, and peripheral neuropathy, in youth with T2DM compared to those with T1DM[36].
The progression from microalbuminuria to macroalbuminuria is also higher in young-onset T2DM compared to T1DM[37]. The risk is particularly high for Asian, Native American, and Pima Indian people, suggesting a possible influence of ethnicity[38]. A Japanese study showed that among individuals < 20 years of age, the incidence of nephropathy was higher in those with T2DM than in T1DM and the differences persisted even after adjustment for disease duration[39]. Interestingly, in this study, the incidence of nephropathy slowly declined over several decades in T1DM, whereas the incidence remained high in patients with young-onset T2DM. Another study reported worse renal survival (that is, remaining free of end-stage kidney disease) in T2DM compared with T1DM, with 55.0% survival after 20 years in the T2DM group while in the T1DM group, renal survival persisted at 100%[40]. However, there are inadequate data regarding the risk of microvascular complications in early-onset T2DM compared to later-onset T2DM. One study demonstrated a higher risk of microalbuminuria in young-onset T2DM than later or adult-onset T2DM (hazard ratio [HR]: 1.2, 95%CI: 1.1-1.4)[41]. A recent meta-analysis reported the prevalence of albuminuria to be 22.17%, with higher risk among Pacific Islander, Indigenous, and Asian youth compared to White youth[42].
It should be noted that the process of albuminuria and hyperfiltration may start before the onset of diabetes in youth with T2DM and may be related to early onset of vascular dysfunction in the obese[43,44]. In children and adolescents, estimated glomerular filtration rate (eGFR) can be calculated by the Schwartz equation using serum creatinine levels and height of the patient. However, this formula could underestimate hyperfiltration, which is common in young-onset T2DM. eGFR estimation by formula using a combination serum creatinine and serum cystatin C may be preferable[38].
Retinopathy: The prevalence of diabetic retinopathy in young-onset T2DM is reportedly between 2% and 40%, and varies according to the testing method used for the patient and the duration of diabetes[45,46]. Although chances increase with disease duration, retinopathy has also been seen at the time of DM diagnosis. In the TODAY study, retinopathy in young-onset T2DM detected by digital fundus photography was seen in 13.7% of patients at a mean duration of diabetes of 4.9 years, although there was no evidence of macular edema or proliferative retinopathy[45].
Age is a significant predictor of retinopathy in T1DM but not young-onset T2DM, with a 5% increased risk of developing retinopathy for every 1-year increase in age[47]. Studies have identified more marked subclinical structural and functional retinal abnormalities in adolescents with young-onset T2DM compared to T1DM, despite a shorter overall duration of T2DM[48].
The data regarding the prevalence of retinopathy in early- vs late-onset T2DM of same duration is conflicting. One study reported a higher prevalence of retinopathy in individuals with young- vs usual-onset DM after ≥ 10 years’ disease duration[49]. In this study, longer duration of diabetes was a significant predictor of disease, while age of onset was not. However, in another study on Pima Indian people, retinopathy was relatively lower in patients with young- rather than later-onset T2DM[50]. In a recent meta-analysis, the most significant increase in the prevalence of T2DM was observed at more than 5 years after diagnosis[51]. Overall, available studies suggest that having younger onset T2DM increases the risk of retinopathy more than having T1DM of same duration, but the differences in risk between young-onset and usual onset T2DM remain unclear.
Akin to adults, children and youth with T2DM should be screened for retinopathy at diagnosis and then periodically thereafter, with more frequent monitoring in those with inadequate glycemic control. Fundoscopy has been found to be less sensitive in detecting retinopathy than stereoscopic fundus photography in children.
Neuropathy: Studies on neuropathy in young-onset DM are relatively few. In the SEARCH study, the prevalence of diabetic neuropathy calculated by the Michigan Neuropathy Screening Instrument (MNSI) was 26% in young-onset T2DM. However, in the MNSI, small-fiber dysfunction was not assessed. The prevalence was 21% in an Australian study using thermal and vibration threshold testing for small and large fiber neuropathy respectively[47,52]. Additionally, more than 50% had autonomic neuropathy tested using pupillary reactivity[47].
In the SEARCH study, those with young-onset T2DM had almost double the age-adjusted prevalence of peripheral neuropathy than those with T1DM (17.7% vs 8.5%)[36]. However, there were no differences in the prevalence of cardiac autonomic neuropathy between the two groups. In another study on young people aged < 18 years, although no differences were noted in the prevalence of peripheral and autonomic neuropathy, the mean duration of disease in those with T2DM was only 1.3 years compared to 6.8 years for those with T1DM[47]. There have been no systematic studies comparing the risk of neuropathy in young- to late-onset T2DM. One Australian study showed that people with T2DM diagnosed between 15 years and 30 years of age have a greater severity of neuropathy compared to those diagnosed between 40 years and 50 years of age and with a similar duration of DM[29].
The prevalence of neuropathy in young-onset T2DM is at least as frequent as in adults. Thus, diagnosing neuropathy using appropriate screening tests should be at least as frequent in youth as in adulthood - at diagnosis and annually. The neuropathy screening tests should be predominantly aimed at the detection of at-risk foot for diabetic foot ulcer and can be conducted easily using clinical parameters such as foot inspection, assessment of pedal pulses and testing for pinprick sensation, 10-g monofilament and vibration using a 128-Hz tuning fork, and ankle jerks, all of which involve no or inexpensive equipment. Novel risk factors that have been identified for the development of peripheral neuropathy and cardiac autonomic neuropathy in children with DM include central obesity, smoking, puberty, and eating disorders. Therefore, young T2DM with any of these represent a priority group for neuropathy screening[53]. In an Indian study of 4555 individuals with childhood and adolescent-onset diabetes, 19.5% had T2DM while 71.4% had T1DM. Age-adjusted incidence of retinopathy was 52.9/1000 person-years in T1DM, which was slightly higher than that in T2DM (49.8/1000 person-years). However, the rates of nephropathy and neuropathy were higher in youth with T2DM compared to those with T1DM (6.2 vs 13.8 and 8.8 vs 24 in T1DM vs T2DM, respectively)[54].
A study on first nation Canadian adolescents with T2DM found a striking mortality rate of 9% over a follow-up period of 9 years[55]. In an Australian study comparing standardized mortality rate (SMR) in patients with T2DM diagnosed between 15 years and 30 years of age with that of those diagnosed between 40 years and 50 years, the investigators found an inverse relationship between age of diabetes onset and SMR, which was the highest for T2DM diagnosed between 15 years and 30 years of age (SMR 3.4 [95% confidence interval [CI]: 2.7-4.2]), with the highest SMR of more than 6 in early midlife. Notably, SMR for the older-onset group was similar to the non-diabetic background population[3]. The excess risk of death progressively declined until it reached values similar to those of the background population in those who were diagnosed with T2DM beyond 69 years of age. A Swedish study showed up to two- to three-fold increased risk of all-cause mortality in people with diabetes who were aged less than 55 years compared to those who were older. When adjusted for glycemia and albuminuria, excess mortality persisted in those who were younger than 55 years even when the hemoglobin A1c (HbA1c) was < 7% and in the absence of albuminuria[56].
In a large registry from Australia, those with young-onset T2DM had higher all-cause mortality, CV mortality, and stroke but slightly lower cancer-related mortality[57]. Earlier age of diagnosis of diabetes by 10 years, was associated with about 30% higher risk for all-cause mortality and a 60% elevated risk of CV mortality. A Danish study reported that younger age at diagnosis of T2DM increased mortality, but this association was markedly weaker in women than in men[58]. The decline in mortality over several decades with improved healthcare was also evident in men with diabetes but not women. The life expectancy for young-onset diabetes diagnosed between 20 years and 40 years of age is reduced by 14 years in men and 16 years in women compared with those without T2DM, and this reduction in life expectancy is greater than that of T1DM, which is about 12 years[57].
Hypertension and dyslipidemia: In a United Kingdom study comparing young-onset T2DM to T1DM, the former cohort showed an increased prevalence of obesity, hypertension, as well as dyslipidemia, although they also had an older age of onset (P < 0.0005) despite having similar glycemic control in both groups. In another hospital-based study, the hypertension prevalence among T2DM patients was 59.5%, with the most common being stage 1 hypertension (30.95%). The risk of hypertension was higher among those aged between 50 years and 60 years, those with longer duration of T2DM, having BMI ≥ 25 kg/m2, those with poor glycemic control, and smokers[59]. In a recent meta-analysis of 60 studies involving 3463 participants, hypertension prevalence was 25.33%, with higher risk among male participants than females[42].
Investigators of the TODAY study group found that at the end of follow-up of 13.3 ± 1.8 years, the incidence of hypertension was 67.5% and that of dyslipidemia was 51.6%. There was development of at least one macrovascular or microvascular complication in 60.1% of the participants, and at least two complications in 28.4% of the participants and the independent risk factors for the development of complications included hyperglycemia, hypertension, and dyslipidemia[60].
Studies in individuals with familial hypercholesterolemia have demonstrated that statins reduce CIMT with similar efficacy in youth as in adults[61]. However, a multicenter study in T1DM failed to show any effect of statins on CIMT, although low-density lipoprotein cholesterol was lowered[62]. There is not enough longitudinal data on statin use in youth-onset T2DM. However, the fact that dyslipidemia in youth will track into adulthood is well known, and the presence of diabetes with dyslipidemia could potentially increase the CV risk associated with the latter by several-fold. Therefore, a reasonable approach to managing dyslipidemia should be aligned with the general recommendations for dyslipidemia in diabetes.
Obstructive sleep apnea: Obstructive sleep apnea (OSA) is associated with obesity and insulin resistance in adults and children and is established as a risk factor for future CVD[63-65]. OSA can affect glycemic control in individuals with diabetes, and treatment of OSA might lead to improved glycemic profile, HbA1c, and insulin sensitivity as well as inflammatory markers, as has been seen in studies in adult T2DM[66,67]. The implications in young-onset T2DM remain to be explored. A detailed sleep study is expensive but evaluation for OSA using some general questions about sleep quality, snoring, apneic spells, early morning headaches, and excessive daytime sleepiness can be easily done. A pediatric Epworth sleepiness scale has been validated with a score of 10 or more indicative of excessive daytime sleepiness in children[68].
Polycystic ovarian syndrome: Overweight and obese adolescent girls have higher prevalence of polycystic ovarian syndrome (PCOS) compared with normal weight adolescent girls[69]. Adolescent girls with PCOS and obesity have a high prevalence of impaired glucose tolerance (30%) and T2DM (3.7%)[70]. Up to 21% of adolescent girls in the TODAY cohort had oligomenorrhea, and these girls had higher androgen levels but lower sex hormone-binding globulin and estradiol levels[71].
In girls with T2DM and PCOS, metformin in conjunction with lifestyle modification can improve not only metabolic dysfunction but also improve menstrual regularity as well as reduce hyperandrogenism[70]. However, in the TODAY study, all of the girls were on metformin and differences between effects of the three treatment groups (metformin alone, metformin along with lifestyle changes, and metformin with rosiglitazone) on menses or sex steroids could not be demonstrated[71]. Although some of the hormonal combined oral contraceptive pills (COCPs) have been associated with adverse metabolic status and CV risk, COCPs still form the cornerstone of therapy for hyperandrogenism and anovulation in PCOS and they have no contraindications in young women with T2DM. While pelvic ultrasound-detected polycystic morphology is not indicative of a diagnosis of PCOS in adolescents, it can be diagnosed based on the presence of oligo- or amenorrhea with clinical or biochemical evidence of hyperandrogenism.
Non-alcoholic fatty liver disease: Non-alcoholic fatty liver disease (NAFLD) can be seen in up to 50% of children with T2DM[72]. Longitudinal studies revealed that after 20 years, 6% of non-diabetic adolescents with NAFLD died or required a liver transplant[73]. The figures might be even worse for those with young-onset T2DM and concomitant NAFLD but data are lacking in this regard.
In a multicenter study on youth with NAFLD, 23.4% had prediabetes and 6.5% had T2DM[74]. Furthermore, greater NAFLD histologic severity has been seen in young-onset T2DM compared to adults, with a higher risk of progression to hepatic fibrosis, cirrhosis, and liver failure[74,75]. While transaminase levels have good sensitivity for the detection of advanced stages of hepatitis or fibrosis, they have poor specificity for NAFLD and it is necessary to rule out non-NAFLD causes of chronic liver disease. It is recommended to evaluate young T2DM for the presence of possible NAFLD by measuring alanine transaminase and aspartate transaminase at diagnosis and annually thereafter. Liver ultrasound can detect liver fat > 30% but its sensitivity is poor with lesser degrees of fat infiltration and in morbidly obese patients. Liver biopsy is still considered the gold standard, and magnetic resonance spectroscopy is the non-invasive test with highest sensitivity for detecting liver fat[76]. It should be noted here that the non-invasive scores for fibrosis assessment such as fibrosis-4 and tools like transient elastography are not validated in children or adolescents and should not be used.
The rate of loss of β-cell function is more accelerated in young-onset T2DM with about β-cell function loss per year being 25%-30% in young people compared to 7% per year for those having adult-onset T2DM[22]. This accelerated rate of β-cell loss persists beyond youth and a rapid progression to glycemic failure can be seen up to the age of 40 years[77]. The loss is particularly steep during adolescence due to the effects of growth hormone surges as well as gonadal steroids leading to a decrease in insulin sensitivity. In the TODAY study, participants who experienced treatment failure had significantly reduced β-cell function. These findings were similar to those observed in the RISE study and large population-level studies in Europe and China. However, poor glycemic durability could not be ameliorated by early insulin treatment, indicating it was not related to poor glycemic control alone[22,23,77].
In one study, adults with early-onset T2DM had an 80% higher likelihood of requiring insulin therapy compared to those with usual-onset T2DM, although they had a similar average time to initiation of insulin (about 2.2 years)[41].
Mental health problems including depression and anxiety are commonly seen in people living with diabetes and the risk might be particularly higher in the younger people. Notably, in another registry-based study, about 40% of hospitalizations among young individuals with T2DM were attributable to mental health issues[78]. In a study on Swedish people with young-onset T2DM, the risk of bipolar disorder, anxiety, depression or stress-related events was higher by up to 4-fold[79]. Among youth with T2DM, younger age at diagnosis was found to be an independent predictor for depression[80]. It is important to assess psychiatric problems such as depression, diabetes-related distress, body image disorders, and eating disorders in all children and adolescents with T2DM. While there is a validated questionnaire for assessing diabetes distress in children such as the Problem Areas in Diabetes-Child score, they have mostly been used in children with T1DM who have other factors like fear of needles and a high risk for life-threatening complications[81]. Fear of hypoglycemia should be assessed. A lot of the issues can be handled with timely identification and appropriate counseling. Socioeconomic factors adding to their distress such as food security, housing stability, child labor, school drop-outs, and exam-related stress should be kept in mind when devising a treatment plan after detailed discussion with the children and should ideally include the family.
Evidence suggests a higher frequency of diabetes-related cancers in those with young-onset T2DM compared to adult-onset T2DM[82]. In the Nurses’ Health study, early-onset T2DM was associated with increased risk of early-onset cancers by 1.47-times, of obesity-related cancers by a 1.75-times, and of diabetes-related cancers by a 2.11-times, all of which were higher than the rates observed in usual onset T2DM across all ages[82,83].
In the TODAY study reporting the outcomes of 260 pregnancies among 141 women with young-onset T2DM, 65% of the women reported complications during their pregnancy. About one-fourth (25.3%) suffered pregnancy loss while preterm birth was seen in 32.6% of pregnancies. One-third of pregnant women had HbA1c higher than 8%. Congenital abnormalities were seen in up to 10% of pregnancies, of which 3.7% resulted in stillbirths, which was three times higher than the national rates for the United States during that time. Of the offspring, 7.8% were small for gestational age, 26.8% were born large for gestational age while 17.9% of infants were in the macrosomic range[84]. In the National Pregnancy data from the United Kingdom, pregnant women with T2DM had increased perinatal deaths than women with T1DM in all HbA1c categories[85]. The risk is particularly high for adolescent mothers with T2DM in whom as high as 38% of women experienced pregnancy loss in a Canadian study[55]. Much of the risk can be eliminated or reduced with appropriate pre-conception counseling, meticulous glycemic control, and folic acid supplementation (5 mg/d) combined with close monitoring of the mother and fetus. Unfortunately, studies have shown that compared to women with T1DM, a significantly higher proportion of women with young-onset T2DM receive potentially teratogenic medications, and < 50% had knowledge or used adequate contraception[86].
Currently, there is dearth of guidelines by societies that focus on the management issues of young-onset T2DM. A position statement to this regard was released by American Diabetes Association (ADA) in 2018 and another consensus guideline from International Society of Pediatric and Adolescent Diabetes (ISPAD) in 2022, the salient points of those are summarized in Table 1[87,88]. Overall, in addition to glycemic status assessment the investigations that should be done at diagnosis and annual review should include lipid profile, urine for albuminuria, serum creatinine levels, retinopathy screening, neuropathy screening with foot check-ups as well as liver function tests. Preferably, one should also assess for OSA in all patients, for possibility of PCOS in adolescent and young girls along with a psychological assessment for emotional well-being and quality of life. Although most guidelines recommend that complication screening in T1DM should start after 5 years of diagnosis or at puberty, whichever is earlier in T1DM, the same conditions may not be appropriate in T2DM given that there would be an unknown duration of hyperglycemia before T2DM is diagnosed.
| Complication | American diabetes association 2018[87] | International society for paediatric and adolescent diabetes 2022[91] |
| Cardiovascular disease | Intensive lifestyle interventions focusing on weight loss, dyslipidemia, hypertension, and dysglycemia are important to prevent overt macrovascular disease in early adulthood. Routine ECG, echocardiography, stress tests or other screening tests for cardiovascular disease are not warranted in the absence of cardiac symptoms | |
| Retinopathy | Screening for retinopathy should be performed by dilated fundoscopy or retinal photography as soon as possible after diagnosis and then annually. Less frequent examination (every 2 yr) may be considered for those with adequate glycemic control and a normal eye examination | Screening of youth with T2DM at the time of initial diagnosis and annually by an ophthalmologist or optometrist by a comprehensive eye examination with dilated pupils or retinal photograph. More frequent examinations are required if retinopathy is of higher grade, progressing and if there is suboptimal glycemic control |
| Neuropathy | Screening for the presence of neuropathy by foot examination at diagnosis and then annually, including inspection, assessment of foot pulses, pinprick and 10-g monofilament tests, testing of vibration perception using a 128-Hz tuning fork, and ankle reflexes | Foot examination (including sensation, vibration sense, light touch and ankle reflexes) at diagnosis and then annually is recommended to detect at risk feet |
| Nephropathy | UACR to be obtained at diagnosis and then annually thereafter. Elevated UACR (> 30 mg/g creatinine) should be confirmed on two out of three samples. eGFR should be determined at the time of diagnosis and annually thereafter. Those with nephropathy should undergo continued monitoring with yearly UACR, eGFR, and serum potassium. Referral to nephrology is recommended if etiology is uncertain or if there is worsening UACR, or decline in eGFR | Albuminuria screening should occur at diagnosis and annually thereafter using three first morning urine collections. If UACR is > 30 mg/g (3 mg/mmol) and BP is elevated or UACR is > 300 mg/g (30 mg/mmol) irrespective of BP, ACEi, or ARB should be started and BP normalized. If albuminuria is present, serum potassium and renal function should be evaluated annually. Renal function to be evaluated using calculated eGFR from validated formulas cystatin C measurement is currently not recommended as it shows high variability and is affected by age, sex, BMI, and diabetes control. A repeat UACR may be helpful 6 mo after the start of ACEi or ARB to ensure albuminuria is normalized. Non-diabetes-related causes of renal disease should be considered and consultation with a nephrologist obtained if severely increased albuminuria (UACR > 300 mg/g or 30 mg/mmol) or if hypertension is present |
| Hypertension | BP should be measured at every visit and optimized if necessary. If BP is > 95th percentile for age, sex, and height, increased emphasis should be placed on lifestyle management. Antihypertensive therapy should be initiated if BP is not optimized by 6 mo. ACEi or ARB) should be initial therapeutic agents. Other BP–lowering agents may be added if needed to optimize BP | BP should be measured at diabetes diagnosis and at every subsequent visit, in the seated position. ABPM can be considered if there is suspicion of white coat hypertension or to confirm hypertension. Echocardiographic evaluation is recommended in youth with confirmed hypertension to assess for left ventricular target organ injury |
| Dyslipidemia | Lipid testing after initial glycemic control has been achieved and repeated annually thereafter. Optimal goals are: LDL-C < 100 mg/dL (2.6 mmol/L), HDL-C > 35 mg/dL (0.905 mmol/L), triglycerides < 150 mg/dL (1.7 mmol/L). Dietary counseling using the AHA Step 2 diet. If LDL-C remains above goal after 6 mo of dietary intervention, to initiate statins with goal of LDL-C < 100 mg/dL. If triglycerides are > 400 mg/dL (4.7 mmol/L) fasting or > 1000 mg/dL (11.6 mmol/L) non-fasting, to optimize glycemia and begin fibrate, with a goal of < 400 mg/dL (4.7 mmol/L) fasting to reduce risk of pancreatitis | Testing for dyslipidemia should occur once glycemic control has been achieved or after 3 mo of initiation of medication, and annually thereafter unless abnormal |
| NAFLD | Evaluation for NAFLD (by measuring ALT and AST) should be done at diagnosis and annually thereafter. Referral to gastroenterology should be considered for persistently elevated or worsening transaminases | Liver enzymes (ALT, AST, and GGT) should be evaluated at T2DM diagnosis and annually thereafter, preferably sooner if abnormal |
| Psychosocial | Assessment of social factors, including food and housing stability, financial constraints. Appropriate patient-appropriate validated tools to assess mental health issues including diabetes distress, depression and disordered eating behaviours, and referral to specialty care if needed. At every visit, to check medication adherence. Effects of medication on body weight must be considered. Screening for smoking and alcohol use at diagnosis and at regular intervals | Youth with T2DM should be screened for psychological co-morbidities including depression, diabetes distress, and disordered eating at diagnosis and at regular follow-up intervals. Providers should specifically consider household food security, housing stability, and family financial resources when devising a treatment plan with the youth and family |
| OSA | OSA screening should be done at each visit, and referral to a pediatric sleep specialist for evaluation and a polysomnogram, if indicated, is recommended. OSA should be treated when documented | Youth with T2DM should be screened for symptoms of OSA at diagnosis and annually thereafter unless there is excessive weight gain, which requires an earlier review of OSA symptoms. OSA can be initially evaluated using general questions about snoring, sleep quality, apnea, morning headaches, daytime sleepiness, nocturia, and enuresis. If symptoms are suggestive of OSA, the diagnosis of OSA is confirmed by a sleep study and referral to a sleep specialist. Nocturnal pulse oximetry can be an initial useful evaluation if there is limited access to a sleep study |
| PCOS | Adolescents with T2DM should be evaluated for PCOS including laboratory studies when indicated. Metformin in addition to lifestyle modification can be used to improve the menstrual cyclicity and hyperandrogenism in girls with T2DM. Weight loss and metformin may improve the menstrual disorder. If hormonal contraception is commenced, effects on metabolic risk should be considered in agent-selection. Oral contraceptives, if required for treatment of PCOS, are not contraindicated for girls with T2DM | PCOS screening should occur at diagnosis in pubertal girls and yearly after that with an evaluation of menstrual history and evidence of hyperandrogenism (hirsutism and/or moderate to severe acne and/or total testosterone measurement). PCOS is diagnosed based on the presence of oligo- or amenorrhea with clinical or biochemical evidence of hyperandrogenism (total testosterone) after exclusion of other possible causes. Pelvic ultrasound is not recommended for the diagnosis of PCOS within 8 yr post-menarche |
| Pregnancy | Pre-conception counseling should be incorporated into routine diabetes clinic visits for all females of child-bearing potential, starting from puberty |
Given the fact that youth-onset T2DM has a more aggressive presentation and earlier appearance of complications, and the rising prevalence of young-onset T2DM, it is expected to portend a significant clinical and socioeconomic burden with high financial burden. Complications would result in both increased direct costs of hospitalization and indirect medical costs due to work absenteeism, reduced productivity while working, reduced productivity at work place, loss of jobs and disability-adjusted life years (DALYs) due to disease-related disability and also premature deaths. Data on the socioeconomic burden of diabetes in youth-onset T2DM are lacking but extrapolating the data from adults, it can be hypothesized that there is a significant increase in health-care costs due to delayed diagnosis of complications and comorbidities in young-onset T2DM, thus making it necessary for timely and periodic screening tests for complications[89]. Instead of focusing on costly investigations, complication screening should start with inexpensive clinic-based tests such as anthropometry, clinical neuropathy assessment, fundoscopy for retinopathy, history and examination for oligomenorrhoea, and hyperandrogenism for PCOS and validated questionnaire for diabetes-distress and excessive daytime sleepiness in children. Laboratory investigations may be limited to those with abnormalities detected on screening.
Using a similar logic, children and young adults who have complications such as atherosclerotic CVD, cardiomyopathy, peripheral arterial disease, kidney disease, or neuropathy must undergo screening for prediabetes or diabetes as a first-line investigation, irrespective of their age. While these are rare, comorbidities such as obesity, dyslipidemia, and PCOS are commonly encountered in adolescents. Available guidelines recommend screening for prediabetes or diabetes in overweight or obese children or adolescents with additional risk factors such as hypertension, dyslipidemia, and PCOS. Screening for diabetes preconceptionally and gestational DM screening in adolescent pregnancy should proceed in the same lines as during adulthood.
Guidelines from the ADA, ISPAD, and American Heart Association advocate a lifestyle modification along with pharmacotherapy in youth-onset T2DM[90-92]. Dietary modification targeting a daily caloric deficit and enhanced nutrient intake is an integral part of the management of youth with T2DM[93]. Diet modification should be advised for the entire family and emphasizing healthy parenting practices related to diet should be the goal, while avoiding excessively restricted food intake[91]. The ADA recommends adding 4-5 sessions involving 30-60 min vigorous physical activity weekly to regular school physical education sessions for children and adolescents with T2DM and obesity[87]. Nevertheless, weight reduction remains a challenge in the management of T2DM and obesity in youth. Barriers to achieving recommended weight loss in are multifactorial. Intrinsic biological factors are there that hinder weight loss, while the lack of engagement is related to family education, motivation, accessibility of social support, and diverse socioeconomic barriers[94]. The rate of attendance in lifestyle sessions was only 60% of the planned sessions in the TODAY study[95]. However, participants who attended more than 75% of the program duration achieved higher body weight loss and improvement in body composition. Despite the encouraging results in adults, the TODAY trial demonstrated that intensive lifestyle interventions plus metformin did not improve weight-related outcomes compared to the group that received metformin only[96]. In the TODAY trial, only 53.6% of participants met the physical activity target, and this failure might have contributed to the suboptimal response in the metformin plus lifestyle intervention group. The efficacy of lifestyle modification in T2DM youth may be less than expected and inability to improve of fitness, HbA1c levels or weight may have a physiologic basis[97]. Although diet and exercise can reduce BMI and alleviate the risk of diabetes related complications, these lifestyle interventions should always be used in conjunction with pharmacotherapy for T2DM in children and adolescents because of the aggressive nature of the disease.
The treatment of young individuals with T2DM remains a challenge. Pharmacological treatment options approved by the regulatory bodies were limited to metformin and insulin until 2019, when liraglutide once daily, a glucagon-like peptide 1 receptor agonist (GLP-1 RA) gained indication for children aged 10 years or above based on an acceptable safety data and a -1.3% placebo-corrected reduction in HbA1c after 52 wk of treatment[98]. In the subsequent 3 years, the once-weekly GLP-1 RAs exenatide[99] and dulaglutide[100] were approved by the United States Food and Drug Administration (FDA) for use in children and adolescents who have T2DM. In Europe, exenatide has received approval in 2023 and dulaglutide is expected to gain approval in 2024. The indication of the (SGLT-2) inhibitor dapagliflozin was extended to children aged 10 years or more subsequent to a small study of young people, aged 10-24 years in Europe[101]. The placebo-corrected reduction in HbA1c was -0.85% for exenatide, -1.4% for dulaglutide, and -0.75% for dapagliflozin. In a recent study of metformin and/or insulin treated youth (aged 10–17 years) with T2DM having mean baseline HbA1c about 8%, dipeptidyl peptidase (DPP-4) inhibitor linagliptin did not provide a clinically significant HbA1c reduction when added to metformin or insulin therapy, and SGLT-2 inhibitor empagliflozin only showed a modest effect on HbA1c in a randomized clinical trial (DINAMO study)[102]. Similar safety profiles of empagliflozin and linagliptin were observed as found in adults with T2DM. Diabetic ketoacidosis episodes were not reported. Of note, empagliflozin has recently been approved to manage T2DM in children 10 years or older based on results from DINAMO trial. The findings with empagliflozin and linagliptin are similar to those from earlier studies in youth-onset T2DM with dapagliflozin (SGLT-2 inhibitor) and sitagliptin (DPP-4 inhibitor)[101,103]. Use of sulfonylureas, DPP-4 inhibitors, or thiazolinidiones is not approved for the treatment of T2DM in children and adolescents.
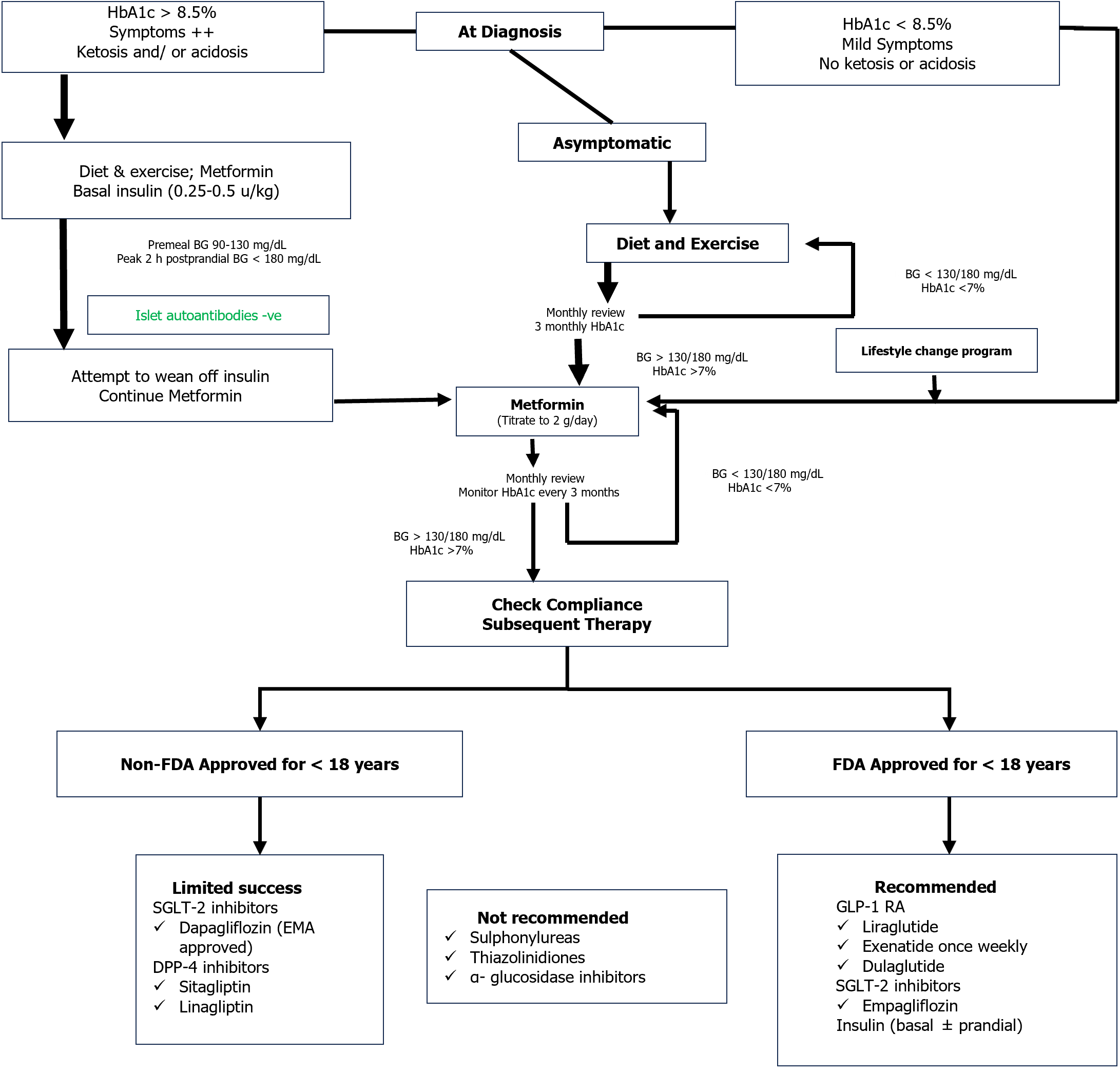
Initial and subsequent therapy: Metformin is the initial treatment of choice for T2DM in children and adolescents together with healthy lifestyle changes[90,91]. If HbA1c < 8.5% in absence of ketosis or hyperglycemic symptoms may be initiated on metformin at 500 mg a day with weekly slow titration to 1000 mg twice daily. Insulin replacement is indicated in combination with metformin in youth with symptoms or HbA1c ≥ 8.5%. Once daily intermediate- or long-acting basal insulin (starting dose 0.25-0.5 units/kg) is necessary in the face of ketosis or acidosis[91]. In general, transition to metformin only can be obtained within the following 2-6 wk. If the goal of initial treatment (HbA1c < 7.0%) is not attained with metformin monotherapy in adolescents, addition of basal insulin or GLP-1 RA may be considered. With higher HbA1c values (> 9%), the preferred option is basal insulin. If the glycemic target is not achieved on a combination of metformin and basal insulin (up to 1.5 units/kg), initiation of mealtime insulin should be considered[91]. An algorithm on the drug treatment of T2DM in children and adolescents is provided in Figure 2[104].
In contrast to the several therapies available to adults with T2DM, metformin and liraglutide remain the only non-insulin treatments formally approved in the United States for use in children and adolescents with T2DM. Metformin monotherapy fails to achieve glycemic control in about half of adolescents with T2DM, especially among adolescents with severe metabolic abnormalities at diagnosis[24,105]. The RISE Consortium data demonstrated that pediatric patients with recent-onset T2DM had continued deterioration of β-cell function regardless of metformin therapy[10]. Data are limited with regard to the use of liraglutide in children and adolescents with T2DM. Despite the FDA approval of liraglutide in 2019 for pediatric T2D, the uptake of GLP-1RAs in this population has been slow due to injectable way of administration and cost. Furthermore, contrary to findings from adult studies, the pivotal GLP-1RA trials in pediatric T2DM do not demonstrate significant weight loss. In addition, the long-term safety and CV and kidney impact of GLP1 RAs have not been investigated in youth-onset T2DM. Although numerous formulations of insulin are commonly used in children and adolescents with T2DM, they have not been studied for the particular indication of pediatric T2DM. Dapagliflozin was the first oral glucose-lowering medication since metformin to be approved in Europe for children (≥ 10-years-old) and young adults with T2DM. Given the adverse cardio- and microvascular profile[41,106] of many young individuals with T2DM, the non-glycemic benefits of dapagliflozin may become increasingly important in the management of youth-onset T2DM. However, due to the lack of published data, SGLT-2 inhibitors are not yet widely used for the treatment of T2DM in children and adolescents. Obesity is the key pathophysiological driver of T2DM and development of CV risk factors in most children and adolescents. Therefore, studies testing semaglutide and tirzepatide, which have shown impressive weight loss together with robust effects on glycemic control in adults with T2DM, are now needed in young people with T2DM to get these drugs approved as future treatments. Overall, the therapies currently available are limited and often inadequate. Although promising results have been shown by some treatments in short-term, it is imperative to ensure that long-term benefits of these therapeutic options are evidently showed in youth with T2DM. Future research should aim to surmount the challenges seen in clinical trials in this population in order to address the unmet need for the best possible management of T2DM in children and adolescents.
As per current international guidelines, metabolic bariatric surgery (MBS) can be recommended in adolescent (10-19 years of age) T2DM patients with a BMI of more than 35 kg/m2 or more than 120% of the 95th percentile of age and sex matched population[107]. Among the adolescent populations, vertical sleeve gastrectomy (VSG), laparoscopic adjustable gastric band and the roux-en-Y gastric bypass (RYGB) are the commonly performed surgeries[108]. In a meta-analysis of 29 cohort studies, a total of 4970 adolescents with at least 5 years follow up after bariatric surgery were included. A remarkable remission rate of 90% (95%CI: 83.2–95.6) for T2DM following MBS was reported in this study[109]. When the metabolic effects of MBS were compared between adolescent and adult populations, the chance of remission of T2DM was found to be significantly higher in adolescents than adults (86% vs 53%; risk ratio: 1.27; 95%CI: 1.03 to 1.57) even with similar degree of weight loss[110]. In the 3 years follow up of Teen- Longitudinal Assessment of Bariatric Surgery (LABS) study, 76% remission was found even among pre-diabetes adolescents who underwent MBS[111]. In the adolescent population, both RYGB as well as VSG showed comparable T2DM remission rates[112,113]. Thus, MBS can be used as a tool for early intervention for prevention as well as better control of dysglycemia in adolescents. On the other hand, patients undergoing MBS at early age are at higher risk for both micro as well as macronutrient deficiencies[114]. Moreover, as maximum bone accrual occurs at this age, bone health can also be affected due to the risk of nutrient deficiency associated with MBS[115]. Further studies evaluating the long-term safety data of MBS performed in adolescents are needed in the future[116]. Reoperation can also be necessary either due to complications (hernia, leak, band slip) or failure (inadequate weight loss or resolution of co-morbidities) of primary surgery[117]. Limited expertise and availability, cost of therapy, lack of insurance coverage, stigma, and lack of awareness and family support are the common barriers to performing MBS in adolescents[118,119]. Medically correctable obesity, substance abuse, current or planned pregnancy within 12 to 18 mo, eating disorders, the chance of non-adherence to post-operative recommendations, and poor family support are considered relative contraindications to MBS[108]. Overall, as the chance of remission of T2DM is significantly high with MBS in adolescents, it should be considered in carefully selected morbidly obese adolescents with T2DM. Among the minimally invasive newer procedures, intragastric balloon treatment and aspiration therapy are now being tried in adolescent obesity[120,121].
As per ADA recommendations, HbA1c should be measured every 3 mo and the HbA1c target for children or adolescents with T2DM should be < 7%[87]. However, ADA also recommends a more stringent target of < 6.5% if that can be achieved without causing any significant adverse effects including hypoglycemia[87]. The lower HbA1c target is preferred in young T2DM patients because of the following reasons. Firstly, the risk of complications due to diabetes is higher in younger patients because of cumulatively longer duration of exposure to dysglycemia[45,122]. Second, young T2DM have a greater degree of β-cell dysfunction[123]. Moreover, they are prone to rapid deterioration of beta cell function. In TODAY study, HbA1c cut-off as low as prediabetes range (> 6.3%) was associated with a higher risk of rapid loss of glycemic control within 48 mo of follow-up[124]. Thus, more stringent control can help to preserve β-cell function. Thirdly, with the advent of newer molecules like SGLT-2i as well as incretin-based therapies, the risk of hypoglycemia is much less when compared to intensive treatment with traditional sulphonylureas and insulin[125]. Moreover, the risk of hypoglycemia is reported to be very low in adolescents even when insulin therapy has been used[126]. Thus, in young T2DM patients, stringent glycemic control is possible without increasing the risk of hypoglycemia significantly. To summarize, the HbA1c target in adolescents with T2DM should be on the lower side. However, the target should be individualized depending on the risk of hypoglycemia. The frequency and targets for self-monitoring of blood glucose monitoring should also be individualized[87].
As per the international expert group consensus report, ‘remission’ of T2DM is considered if HbA1c remains < 6.5% for at least 3 mo without any glucose lowering pharmacotherapy[127]. The HbA1c testing should be done at least 3 mo after any surgical or pharmacological intervention or after at least 6 mo from the initiation lifestyle-based intervention[127]. The studies which evaluated the chance of T2DM remission in adolescent patients are mostly limited to lifestyle and surgical interventions. In a small study by Willi et al[128], ketogenic very low calorie diet (VLCD; 680-800 Kcal/d) had been reported to be effective in compliant children with T2DM allowing withdrawal of exogenous insulin and oral antidiabetic medications. However, in TODAY study done in T2DM adolescents, lifestyle interventions (VLCD 1200-1500 Kcal/d with 200-300 min of weekly moderate exercise) failed to show any additional benefit when combined with metformin[24]. Moreover, adherence rate, long term durability and adverse effects of VLCD in children and adolescents with T2DM are not well studied. On the other hand, the effect of MBS on induction of remission of T2DM in adolescents is encouraging. In the Teen-LABS study, among 29 adolescents who underwent MBS, 95% reported remission from diabetes at 3 years[111]. In the same study, 76% remission rate had been reported in adolescents with pre-diabetes. In the meta-analysis by Wu et al[109], 90% diabetes remission rate was reported after at least 5 years of undergoing MBS. The MBS are found to be more effective if inducing remission if done earlier than later in life. Moreover, remission occurs very rapidly following MBS even before significant weight loss suggesting additional mechanisms of diabetes remission beyond sustained weight loss[129].
One of the chief drivers as well as a major modifiable risk factor for young-onset T2DM is excess body weight starting from childhood. The risk starts in utero with the nutritional status of the mother along with the fetal birth weight having important implications on the metabolic health of the child. Thus, interventions that reverse or reduce obesity in children and adolescents at the community level are essential in preventing young-onset T2DM. In this regard, school-based health interventions are gaining importance in addressing and mitigating environmental and behavioral factors that increase the risk for the development of obesity and T2DM.
A wide range of prevalence data on childhood obesity (BMI > 95th centile) in T2DM has been reported- from 64.5% in Asian populations to 89.9% in European populations[130]. Important evidence regarding the impact of lifestyle interventions for youth with T2DM comes from the TODAY study. While the goal was to achieve a 7%-10% decrease in weight with lifestyle changes, the addition of metformin monotherapy to lifestyle intervention was not associated with any increase in the duration of metabolic control beyond that with metformin[96]. Youth with T2DM receiving metformin plus lifestyle intervention demonstrated short-term weight loss and body composition improvement but the changes were not sustained over a long period. Irrespective of treatment, sustained weight losses above 7% led to improvements in HbA1c, high-density lipoprotein cholesterol, and C-peptide levels. Family-based behavioral weight management programs have been found to have some positive impact on weight and metabolic risk factors of school-going children without diabetes but were not very effective in adolescents and children who had greater degrees of obesity[131]. A comprehensive pediatric lifestyle intervention should involve the family and employ evidence-based behavioral strategies that have been demonstrated to develop sustainable changes in nutrition and physical activity. Therefore, young-onset T2DM who are overweight/obese should receive appropriate comprehensive lifestyle programs, involving their family members, targeting to achieve a 7%–10% reduction in body weight. Nutrition advice should include healthy eating patterns with increased consumption of nutrient-dense and decreased consumption of calorie-dense, nutrient-poor foods, like sugar-sweetened beverages. Physical activity must include at least 30-60 min of moderate to vigorous physical activity at least 5 d every wk along with strength-training activities for a minimum of 3 d per wk[87]. A meta-analysis found the short-term efficacy of very-low calorie in reducing weight in obese people aged below 18 years, but no long-term follow-up has been undertaken[132]. Younger people have shown better uptake of behavioral interventions in the digital format[133]. Data regarding the efficacy and safety of pharmacotherapy for weight loss in the youth are limited. Most anti-obesity medications except orlistat and liraglutide are not yet approved for use in children. Overall, there is limited evidence base to support diabetes screening in all adolescents or young adults with obesity which would not be a very cost-effective approach[134]. Some guidelines recommend screening for dysglycemia in obese youth who have additional co-morbidities like hypertension[91]. Several risk scores to identify individuals at risk for and needing screening for diabetes have been developed and validated in different countries, including clinical and polygenic factors[135]. The trajectories from normoglycemia to T2DM might differ in young people, thus narrowing the window of opportunity to screen at later ages. Another target group for preventive strategies especially includes women with a history of gestational diabetes. A study showed that the adjusted hazards in women with gestational diabetes for progression to T2DM were 71.9 per 1000 person-years[136]. Such women should receive timely and appropriate advice regarding lifestyle, nutrition, and monitoring.
The development of best practices for managing youth-onset T2DM is limited by gaps in the understanding of glucose metabolism abnormalities during adolescence and development of complications and the long-term outcomes of young-onset T2DM. Even the definitions of prediabetes and T2DM in children and adolescents are not evidence-based, as they have only been extrapolated from glycemic indices predicting microvascular complications in adults. Hyperglycemia appears to be temporary in some youth, and rapid loss of glycemic control on oral monotherapy is seen in others requiring insulin treatment[137]. Therefore, a better understanding of disease heterogeneity is needed to envisage disease trajectory that would allow customized approaches to treatment. The long-term outcomes for early-onset dyslipidemia, hypertension, kidney disease, NAFLD, and CV dysfunction are largely not known[138]. Research is required to improve understanding of what diabetes-related complications may require pediatric-specific approaches.
Recent studies suggest that pediatric T2DM differs from adult-onset T2D in a variety of ways and adolescents with T2DM display more insulin resistance and glycemic failure compared with adults[139]. Consequently, there is an urgent need for an expanded set of treatment options. However, the social and environmental complexities surrounding youth with T2DM hinder recruitment into and completion of clinical trials[138]. As a consequence, trial recruitment for youth with T2DM has had limited success as a relatively new endeavor. Other barriers to study implementation include a relatively small number of available study participants (compared with adults) with an increasing number of trials competing for that restricted pool of available patients, restrictive eligibility criteria, the difficulty of participants/caregivers repeatedly taking time off from school or work, and the small number of research sites with resources dedicated to pediatric T2DM trials[140-142].
Knowing the realities of the epidemiology, disease heterogeneity, and socioeconomic challenges of youth-onset T2DM, as well as the clinical research experience to date, it is imperative to find solutions to this situation in order to progress in understanding the pathophysiology of T2DM in children and adolescents and successful completion of research programs for this population. Research recommendations and possible solutions to barriers are summarized in Table 2.
| Knowledge gaps in youth-onset T2DM |
| 1 Future studies are needed with larger and more diverse samples to understand the unique aspects of T2DM in children and adolescent |
| 2 What are the physiological barriers to exercise seen in obese youth and youth with T2DM? Are lifestyle interventions successful, durable, and sufficient? |
| 3 What are effective ways to increase compliance with lifestyle interventions and medication in adolescents with T2DM? |
| 4 What medications (alone or in combination) achieve durable glycemic control in youth-onset T2DM? Should disease-modifying therapies (SGLT2 inhibitors and GLP-1 RAs) be deployed earlier in the treatment algorithms? |
| 5 What is the optimal approach to management of comorbidities and complications in youth-onset T2DM? |
| Possible solutions to barriers |
| 1 Prioritization of clinical and translational research addressing the gaps in knowledge regarding the unique physiological features of youth-onset T2DM |
| 2 Increased explorations of the psychological and socioeconomic aspects of youth-onset T2DM |
| 3 Collaboration among academic leaders, government and charitable sponsors, industry, and regulatory agencies to delineate research strategies |
| 4 Increasing research and infrastructure capacity for youth-onset T2DM through the development of research centers of excellence those are uniquely staffed and maintained |
| 5 Increasing the proportion of youth with T2DM who participate in clinical drug trials through trial designs appropriate for the typical youth with T2DM and creative strategies to overcome barriers to care and research |
T2DM in children and adolescents is an emergent public health concern globally that presents with distinctive characteristics, demographics, and disease progression in comparison to adult-onset T2DM. It shows lower rates of response to oral pharmacotherapy than in adults. Given the relatively recent emergence of T2DM in children and adolescents, evidence base about prevention, optimal treatment approaches, and monitoring of this population is lagging behind the steady rise in cases. The available treatment options to manage youth-onset T2DM and prevent the development of complications are limited. However, two more GLP-1RAs have already been approved. The trials of oral semaglutide (Pioneer Teens) and tirzepatide (SURPASS-PEDS) in type 2 diabetic children and adolescents are ongoing. We hope that additional therapies will not only help in the optimization of glycemic control but also slow the progression of disease and decrease long-term co-morbidities and complications. T2DM prevention in youth is another priority. Detection of children and adolescents who are at the highest risk of developing T2DM will aid the development of more efficient interventions to prevent or delay the disease onset. A focus on improving knowledge regarding the pathophysiology as well as biology and environment of youth-onset T2DM, finding the optimal approach to working with this population, and utilizing more realistic and effective study designs and interventions suitable to this population is needed to help improve the care of children and adolescents with T2DM across the world.
| 1. | Fagot-Campagna A, Pettitt DJ, Engelgau MM, Burrows NR, Geiss LS, Valdez R, Beckles GL, Saaddine J, Gregg EW, Williamson DF, Narayan KM. Type 2 diabetes among North American children and adolescents: an epidemiologic review and a public health perspective. J Pediatr. 2000;136:664-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 736] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 2. | TODAY Study Group; Zeitler P, Hirst K, Pyle L, Linder B, Copeland K, Arslanian S, Cuttler L, Nathan DM, Tollefsen S, Wilfley D, Kaufman F. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. 2012;366:2247-2256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 662] [Cited by in RCA: 726] [Article Influence: 55.8] [Reference Citation Analysis (2)] |
| 3. | Al-Saeed AH, Constantino MI, Molyneaux L, D'Souza M, Limacher-Gisler F, Luo C, Wu T, Twigg SM, Yue DK, Wong J. An Inverse Relationship Between Age of Type 2 Diabetes Onset and Complication Risk and Mortality: The Impact of Youth-Onset Type 2 Diabetes. Diabetes Care. 2016;39:823-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 180] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 4. | Zeitler P, Chou HS, Copeland KC, Geffner M. Clinical trials in youth-onset type 2 diabetes: needs, barriers, and options. Curr Diab Rep. 2015;15:28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Lawrence JM, Divers J, Isom S, Saydah S, Imperatore G, Pihoker C, Marcovina SM, Mayer-Davis EJ, Hamman RF, Dolan L, Dabelea D, Pettitt DJ, Liese AD; SEARCH for Diabetes in Youth Study Group. Trends in Prevalence of Type 1 and Type 2 Diabetes in Children and Adolescents in the US, 2001-2017. JAMA. 2021;326:717-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 368] [Article Influence: 92.0] [Reference Citation Analysis (0)] |
| 6. | Wagenknecht LE, Lawrence JM, Isom S, Jensen ET, Dabelea D, Liese AD, Dolan LM, Shah AS, Bellatorre A, Sauder K, Marcovina S, Reynolds K, Pihoker C, Imperatore G, Divers J; SEARCH for Diabetes in Youth study. Trends in incidence of youth-onset type 1 and type 2 diabetes in the USA, 2002-18: results from the population-based SEARCH for Diabetes in Youth study. Lancet Diabetes Endocrinol. 2023;11:242-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 113] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 7. | Lynch JL, Barrientos-Pérez M, Hafez M, Jalaludin MY, Kovarenko M, Rao PV, Weghuber D. Country-Specific Prevalence and Incidence of Youth-Onset Type 2 Diabetes: A Narrative Literature Review. Ann Nutr Metab. 2020;76:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Wu H, Patterson CC, Zhang X, Ghani RBA, Magliano DJ, Boyko EJ, Ogle GD, Luk AOY. Worldwide estimates of incidence of type 2 diabetes in children and adolescents in 2021. Diabetes Res Clin Pract. 2022;185:109785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 73] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 9. | Shield JP, Lynn R, Wan KC, Haines L, Barrett TG. Management and 1 year outcome for UK children with type 2 diabetes. Arch Dis Child. 2009;94:206-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | RISE Consortium. Metabolic Contrasts Between Youth and Adults With Impaired Glucose Tolerance or Recently Diagnosed Type 2 Diabetes: II. Observations Using the Oral Glucose Tolerance Test. Diabetes Care. 2018;41:1707-1716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 11. | Bjerregaard LG, Jensen BW, Ängquist L, Osler M, Sørensen TIA, Baker JL. Change in Overweight from Childhood to Early Adulthood and Risk of Type 2 Diabetes. N Engl J Med. 2018;378:1302-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 258] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 12. | Writing Group for the SEARCH for Diabetes in Youth Study Group; Dabelea D, Bell RA, D'Agostino RB Jr, Imperatore G, Johansen JM, Linder B, Liu LL, Loots B, Marcovina S, Mayer-Davis EJ, Pettitt DJ, Waitzfelder B. Incidence of diabetes in youth in the United States. JAMA. 2007;297:2716-2724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 688] [Cited by in RCA: 686] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 13. | Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3033] [Cited by in RCA: 4762] [Article Influence: 1587.3] [Reference Citation Analysis (36)] |
| 14. | Xie J, Wang M, Long Z, Ning H, Li J, Cao Y, Liao Y, Liu G, Wang F, Pan A. Global burden of type 2 diabetes in adolescents and young adults, 1990-2019: systematic analysis of the Global Burden of Disease Study 2019. BMJ. 2022;379:e072385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 115] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 15. | Dabelea D, Mayer-Davis EJ, Lamichhane AP, D'Agostino RB Jr, Liese AD, Vehik KS, Narayan KM, Zeitler P, Hamman RF. Association of intrauterine exposure to maternal diabetes and obesity with type 2 diabetes in youth: the SEARCH Case-Control Study. Diabetes Care. 2008;31:1422-1426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 316] [Cited by in RCA: 308] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 16. | Shah RD, Chernausek SD, El Ghormli L, Geffner ME, Keady J, Kelsey MM, Farrell R, Tesfaldet B, Tryggestad JB, Van Name M, Isganaitis E. Maternal Diabetes in Youth-Onset Type 2 Diabetes Is Associated With Progressive Dysglycemia and Risk of Complications. J Clin Endocrinol Metab. 2023;108:1120-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Mayer-Davis EJ, Dabelea D, Lamichhane AP, D'Agostino RB Jr, Liese AD, Thomas J, McKeown RE, Hamman RF. Breast-feeding and type 2 diabetes in the youth of three ethnic groups: the SEARCh for diabetes in youth case-control study. Diabetes Care. 2008;31:470-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Kahn LG, Philippat C, Nakayama SF, Slama R, Trasande L. Endocrine-disrupting chemicals: implications for human health. Lancet Diabetes Endocrinol. 2020;8:703-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 440] [Article Influence: 88.0] [Reference Citation Analysis (0)] |
| 19. | Donnelly LA, Zhou K, Doney ASF, Jennison C, Franks PW, Pearson ER. Rates of glycaemic deterioration in a real-world population with type 2 diabetes. Diabetologia. 2018;61:607-615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 20. | Dabelea D, Mayer-Davis EJ, Andrews JS, Dolan LM, Pihoker C, Hamman RF, Greenbaum C, Marcovina S, Fujimoto W, Linder B, Imperatore G, D'Agostino R Jr. Clinical evolution of beta cell function in youth with diabetes: the SEARCH for Diabetes in Youth study. Diabetologia. 2012;55:3359-3368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 21. | Arslanian SA, El Ghormli L, Kim JY, Tjaden AH, Barengolts E, Caprio S, Hannon TS, Mather KJ, Nadeau KJ, Utzschneider KM, Kahn SE; RISE Consortium. OGTT Glucose Response Curves, Insulin Sensitivity, and β-Cell Function in RISE: Comparison Between Youth and Adults at Randomization and in Response to Interventions to Preserve β-Cell Function. Diabetes Care. 2021;44:817-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 22. | Sam S, Edelstein SL, Arslanian SA, Barengolts E, Buchanan TA, Caprio S, Ehrmann DA, Hannon TS, Tjaden AH, Kahn SE, Mather KJ, Tripputi M, Utzschneider KM, Xiang AH, Nadeau KJ; RISE Consortium; RISE Consortium Investigators. Baseline Predictors of Glycemic Worsening in Youth and Adults With Impaired Glucose Tolerance or Recently Diagnosed Type 2 Diabetes in the Restoring Insulin Secretion (RISE) Study. Diabetes Care. 2021;44:1938-1947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 23. | Galderisi A, Giannini C, Weiss R, Kim G, Shabanova V, Santoro N, Pierpont B, Savoye M, Caprio S. Trajectories of changes in glucose tolerance in a multiethnic cohort of obese youths: an observational prospective analysis. Lancet Child Adolesc Health. 2018;2:726-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 24. | TODAY Study Group. Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and β-cell function in TODAY. Diabetes Care. 2013;36:1749-1757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 243] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 25. | Arslanian S, Kim JY, Nasr A, Bacha F, Tfayli H, Lee S, Toledo FGS. Insulin sensitivity across the lifespan from obese adolescents to obese adults with impaired glucose tolerance: Who is worse off? Pediatr Diabetes. 2018;19:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 26. | Almgren P, Lehtovirta M, Isomaa B, Sarelin L, Taskinen MR, Lyssenko V, Tuomi T, Groop L; Botnia Study Group. Heritability and familiality of type 2 diabetes and related quantitative traits in the Botnia Study. Diabetologia. 2011;54:2811-2819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 197] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 27. | Srinivasan S, Chen L, Todd J, Divers J, Gidding S, Chernausek S, Gubitosi-Klug RA, Kelsey MM, Shah R, Black MH, Wagenknecht LE, Manning A, Flannick J, Imperatore G, Mercader JM, Dabelea D, Florez JC; ProDiGY Consortium. The First Genome-Wide Association Study for Type 2 Diabetes in Youth: The Progress in Diabetes Genetics in Youth (ProDiGY) Consortium. Diabetes. 2021;70:996-1005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 28. | Misra S, Ke C, Srinivasan S, Goyal A, Nyriyenda MJ, Florez JC, Khunti K, Magliano DJ, Luk A. Current insights and emerging trends in early-onset type 2 diabetes. Lancet Diabetes Endocrinol. 2023;11:768-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 88] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 29. | Constantino MI, Molyneaux L, Limacher-Gisler F, Al-Saeed A, Luo C, Wu T, Twigg SM, Yue DK, Wong J. Long-term complications and mortality in young-onset diabetes: type 2 diabetes is more hazardous and lethal than type 1 diabetes. Diabetes Care. 2013;36:3863-3869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 273] [Cited by in RCA: 310] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 30. | Gregg EW, Hora I, Benoit SR. Resurgence in Diabetes-Related Complications. JAMA. 2019;321:1867-1868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 197] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 31. | TODAY Study Group; Bjornstad P, Drews KL, Caprio S, Gubitosi-Klug R, Nathan DM, Tesfaldet B, Tryggestad J, White NH, Zeitler P. Long-Term Complications in Youth-Onset Type 2 Diabetes. N Engl J Med. 2021;385:416-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 318] [Article Influence: 79.5] [Reference Citation Analysis (0)] |
| 32. | Chan JC, Lau ES, Luk AO, Cheung KK, Kong AP, Yu LW, Choi KC, Chow FC, Ozaki R, Brown N, Yang X, Bennett PH, Ma RC, So WY. Premature mortality and comorbidities in young-onset diabetes: a 7-year prospective analysis. Am J Med. 2014;127:616-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 33. | Wadwa RP, Urbina EM, Anderson AM, Hamman RF, Dolan LM, Rodriguez BL, Daniels SR, Dabelea D; SEARCH Study Group. Measures of arterial stiffness in youth with type 1 and type 2 diabetes: the SEARCH for diabetes in youth study. Diabetes Care. 2010;33:881-886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 34. | Bacha F, Edmundowicz D, Sutton-Tyrell K, Lee S, Tfayli H, Arslanian SA. Coronary artery calcification in obese youth: what are the phenotypic and metabolic determinants? Diabetes Care. 2014;37:2632-2639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Levitt Katz L, Gidding SS, Bacha F, Hirst K, McKay S, Pyle L, Lima JA; TODAY Study Group. Alterations in left ventricular, left atrial, and right ventricular structure and function to cardiovascular risk factors in adolescents with type 2 diabetes participating in the TODAY clinical trial. Pediatr Diabetes. 2015;16:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 36. | Dabelea D, Stafford JM, Mayer-Davis EJ, D'Agostino R Jr, Dolan L, Imperatore G, Linder B, Lawrence JM, Marcovina SM, Mottl AK, Black MH, Pop-Busui R, Saydah S, Hamman RF, Pihoker C; SEARCH for Diabetes in Youth Research Group. Association of Type 1 Diabetes vs Type 2 Diabetes Diagnosed During Childhood and Adolescence With Complications During Teenage Years and Young Adulthood. JAMA. 2017;317:825-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 476] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 37. | Yoo EG, Choi IK, Kim DH. Prevalence of microalbuminuria in young patients with type 1 and type 2 diabetes mellitus. J Pediatr Endocrinol Metab. 2004;17:1423-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 38. | Pinhas-Hamiel O, Zeitler P. Acute and chronic complications of type 2 diabetes mellitus in children and adolescents. Lancet. 2007;369:1823-1831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 249] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 39. | Yokoyama H, Okudaira M, Otani T, Sato A, Miura J, Takaike H, Yamada H, Muto K, Uchigata Y, Ohashi Y, Iwamoto Y. Higher incidence of diabetic nephropathy in type 2 than in type 1 diabetes in early-onset diabetes in Japan. Kidney Int. 2000;58:302-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 136] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 40. | Dart AB, Sellers EA, Martens PJ, Rigatto C, Brownell MD, Dean HJ. High burden of kidney disease in youth-onset type 2 diabetes. Diabetes Care. 2012;35:1265-1271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 41. | Hillier TA, Pedula KL. Complications in young adults with early-onset type 2 diabetes: losing the relative protection of youth. Diabetes Care. 2003;26:2999-3005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 285] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 42. | Cioana M, Deng J, Hou M, Nadarajah A, Qiu Y, Chen SSJ, Rivas A, Banfield L, Chanchlani R, Dart A, Wicklow B, Alfaraidi H, Alotaibi A, Thabane L, Samaan MC. Prevalence of Hypertension and Albuminuria in Pediatric Type 2 Diabetes: A Systematic Review and Meta-analysis. JAMA Netw Open. 2021;4:e216069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 43. | Burgert TS, Dziura J, Yeckel C, Taksali SE, Weiss R, Tamborlane W, Caprio S. Microalbuminuria in pediatric obesity: prevalence and relation to other cardiovascular risk factors. Int J Obes (Lond). 2006;30:273-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 44. | Bartz SK, Caldas MC, Tomsa A, Krishnamurthy R, Bacha F. Urine Albumin-to-Creatinine Ratio: A Marker of Early Endothelial Dysfunction in Youth. J Clin Endocrinol Metab. 2015;100:3393-3399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 45. | TODAY Study Group. Retinopathy in youth with type 2 diabetes participating in the TODAY clinical trial. Diabetes Care. 2013;36:1772-1774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 46. | Yokoyama H, Okudaira M, Otani T, Takaike H, Miura J, Saeki A, Uchigata Y, Omori Y. Existence of early-onset NIDDM Japanese demonstrating severe diabetic complications. Diabetes Care. 1997;20:844-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 70] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 47. | Eppens MC, Craig ME, Cusumano J, Hing S, Chan AK, Howard NJ, Silink M, Donaghue KC. Prevalence of diabetes complications in adolescents with type 2 compared with type 1 diabetes. Diabetes Care. 2006;29:1300-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 329] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 48. | Bronson-Castain KW, Bearse MA Jr, Neuville J, Jonasdottir S, King-Hooper B, Barez S, Schneck ME, Adams AJ. Early neural and vascular changes in the adolescent type 1 and type 2 diabetic retina. Retina. 2012;32:92-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 49. | Song SH, Gray TA. Early-onset type 2 diabetes: high risk for premature diabetic retinopathy. Diabetes Res Clin Pract. 2011;94:207-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 50. | Krakoff J, Lindsay RS, Looker HC, Nelson RG, Hanson RL, Knowler WC. Incidence of retinopathy and nephropathy in youth-onset compared with adult-onset type 2 diabetes. Diabetes Care. 2003;26:76-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 99] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 51. | Cioana M, Deng J, Nadarajah A, Hou M, Qiu Y, Chen SSJ, Rivas A, Toor PP, Banfield L, Thabane L, Chaudhary V, Samaan MC. Global Prevalence of Diabetic Retinopathy in Pediatric Type 2 Diabetes: A Systematic Review and Meta-analysis. JAMA Netw Open. 2023;6:e231887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 52. | Jaiswal M, Lauer A, Martin CL, Bell RA, Divers J, Dabelea D, Pettitt DJ, Saydah S, Pihoker C, Standiford DA, Rodriguez BL, Pop-Busui R, Feldman EL; SEARCH for Diabetes in Youth Study Group. Peripheral neuropathy in adolescents and young adults with type 1 and type 2 diabetes from the SEARCH for Diabetes in Youth follow-up cohort: a pilot study. Diabetes Care. 2013;36:3903-3908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 53. | Akinci G, Savelieff MG, Gallagher G, Callaghan BC, Feldman EL. Diabetic neuropathy in children and youth: New and emerging risk factors. Pediatr Diabetes. 2021;22:132-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 54. | Amutha A, Ranjit U, Anjana RM, Shanthi R CS, Rajalakshmi R, Venkatesan U, Muthukumar S, Philips R, Kayalvizhi S, Gupta PK, Sastry NG, Mohan V. Clinical profile and incidence of microvascular complications of childhood and adolescent onset type 1 and type 2 diabetes seen at a tertiary diabetes center in India. Pediatr Diabetes. 2021;22:67-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 55. | Dean H, Flett B. Natural history of type 2 diabetes diagnosed in childhood: long term follow-up in young adult years. Diabetes. 2002;51 (Suppl. 2):A24. |
| 56. | Sattar N, Rawshani A, Franzén S, Svensson AM, Rosengren A, McGuire DK, Eliasson B, Gudbjörnsdottir S. Age at Diagnosis of Type 2 Diabetes Mellitus and Associations With Cardiovascular and Mortality Risks. Circulation. 2019;139:2228-2237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 347] [Article Influence: 69.4] [Reference Citation Analysis (0)] |
| 57. | Huo L, Magliano DJ, Rancière F, Harding JL, Nanayakkara N, Shaw JE, Carstensen B. Impact of age at diagnosis and duration of type 2 diabetes on mortality in Australia 1997-2011. Diabetologia. 2018;61:1055-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 119] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 58. | Færch K, Carstensen B, Almdal TP, Jørgensen ME. Improved survival among patients with complicated type 2 diabetes in Denmark: a prospective study (2002-2010). J Clin Endocrinol Metab. 2014;99:E642-E646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 59. | Akalu Y, Belsti Y. Hypertension and Its Associated Factors Among Type 2 Diabetes Mellitus Patients at Debre Tabor General Hospital, Northwest Ethiopia. Diabetes Metab Syndr Obes. 2020;13:1621-1631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 60. | Song SH. Complication characteristics between young-onset type 2 versus type 1 diabetes in a UK population. BMJ Open Diabetes Res Care. 2015;3:e000044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 61. | Vuorio A, Kuoppala J, Kovanen PT, Humphries SE, Tonstad S, Wiegman A, Drogari E, Ramaswami U. Statins for children with familial hypercholesterolemia. Cochrane Database Syst Rev. 2019;2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 62. | Marcovecchio ML, Chiesa ST, Bond S, Daneman D, Dawson S, Donaghue KC, Jones TW, Mahmud FH, Marshall SM, Neil HAW, Dalton RN, Deanfield J, Dunger DB; AdDIT Study Group. ACE Inhibitors and Statins in Adolescents with Type 1 Diabetes. N Engl J Med. 2017;377:1733-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 63. | Cappuccio FP, Taggart FM, Kandala NB, Currie A, Peile E, Stranges S, Miller MA. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31:619-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1358] [Cited by in RCA: 1458] [Article Influence: 85.8] [Reference Citation Analysis (0)] |
| 64. | Jean-Louis G, Zizi F, Clark LT, Brown CD, McFarlane SI. Obstructive sleep apnea and cardiovascular disease: role of the metabolic syndrome and its components. J Clin Sleep Med. 2008;4:261-272. [PubMed] |
| 65. | Nedeltcheva AV, Kessler L, Imperial J, Penev PD. Exposure to recurrent sleep restriction in the setting of high caloric intake and physical inactivity results in increased insulin resistance and reduced glucose tolerance. J Clin Endocrinol Metab. 2009;94:3242-3250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 231] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 66. | Zychowski KE, Sanchez B, Pedrosa RP, Lorenzi-Filho G, Drager LF, Polotsky VY, Campen MJ. Serum from obstructive sleep apnea patients induces inflammatory responses in coronary artery endothelial cells. Atherosclerosis. 2016;254:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 67. | Mokhlesi B, Grimaldi D, Beccuti G, Abraham V, Whitmore H, Delebecque F, Van Cauter E. Effect of One Week of 8-Hour Nightly Continuous Positive Airway Pressure Treatment of Obstructive Sleep Apnea on Glycemic Control in Type 2 Diabetes: A Proof-of-Concept Study. Am J Respir Crit Care Med. 2016;194:516-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 68. | Janssen KC, Phillipson S, O'Connor J, Johns MW. Validation of the Epworth Sleepiness Scale for Children and Adolescents using Rasch analysis. Sleep Med. 2017;33:30-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 150] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 69. | Christensen SB, Black MH, Smith N, Martinez MM, Jacobsen SJ, Porter AH, Koebnick C. Prevalence of polycystic ovary syndrome in adolescents. Fertil Steril. 2013;100:470-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 70. | Palmert MR, Gordon CM, Kartashov AI, Legro RS, Emans SJ, Dunaif A. Screening for abnormal glucose tolerance in adolescents with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87:1017-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 180] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 71. | Kelsey MM, Braffett BH, Geffner ME, Levitsky LL, Caprio S, McKay SV, Shah R, Sprague JE, Arslanian SA; TODAY Study Group. Menstrual Dysfunction in Girls From the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) Study. J Clin Endocrinol Metab. 2018;103:2309-2318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 72. | Bloomgarden ZT. Nonalcoholic fatty liver disease and insulin resistance in youth. Diabetes Care. 2007;30:1663-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 73. | Feldstein AE, Charatcharoenwitthaya P, Treeprasertsuk S, Benson JT, Enders FB, Angulo P. The natural history of non-alcoholic fatty liver disease in children: a follow-up study for up to 20 years. Gut. 2009;58:1538-1544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 572] [Cited by in RCA: 554] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 74. | Newton KP, Hou J, Crimmins NA, Lavine JE, Barlow SE, Xanthakos SA, Africa J, Behling C, Donithan M, Clark JM, Schwimmer JB; Nonalcoholic Steatohepatitis Clinical Research Network. Prevalence of Prediabetes and Type 2 Diabetes in Children With Nonalcoholic Fatty Liver Disease. JAMA Pediatr. 2016;170:e161971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 186] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 75. | Xanthakos SA, Jenkins TM, Kleiner DE, Boyce TW, Mourya R, Karns R, Brandt ML, Harmon CM, Helmrath MA, Michalsky MP, Courcoulas AP, Zeller MH, Inge TH; Teen-LABS Consortium. High Prevalence of Nonalcoholic Fatty Liver Disease in Adolescents Undergoing Bariatric Surgery. Gastroenterology. 2015;149:623-34.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 106] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 76. | Tsai E, Lee TP. Diagnosis and Evaluation of Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis, Including Noninvasive Biomarkers and Transient Elastography. Clin Liver Dis. 2018;22:73-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 77. | Dennis JM, Shields BM, Henley WE, Jones AG, Hattersley AT. Disease progression and treatment response in data-driven subgroups of type 2 diabetes compared with models based on simple clinical features: an analysis using clinical trial data. Lancet Diabetes Endocrinol. 2019;7:442-451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 273] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 78. | Ke C, Lau E, Shah BR, Stukel TA, Ma RC, So WY, Kong AP, Chow E, Clarke P, Goggins W, Chan JCN, Luk A. Excess Burden of Mental Illness and Hospitalization in Young-Onset Type 2 Diabetes: A Population-Based Cohort Study. Ann Intern Med. 2019;170:145-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 79. | Liu S, Leone M, Ludvigsson JF, Lichtenstein P, Gudbjörnsdottir S, Landén M, Bergen SE, Taylor MJ, Larsson H, Kuja-Halkola R, Butwicka A. Early-Onset Type 2 Diabetes and Mood, Anxiety, and Stress-Related Disorders: A Genetically Informative Register-Based Cohort Study. Diabetes Care. 2022;45:2950-2956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 80. | Barker MM, Davies MJ, Zaccardi F, Brady EM, Hall AP, Henson JJ, Khunti K, Lake A, Redman EL, Rowlands AV, Speight J, Yates T, Sargeant JA, Hadjiconstantinou M. Age at Diagnosis of Type 2 Diabetes and Depressive Symptoms, Diabetes-Specific Distress, and Self-Compassion. Diabetes Care. 2023;46:579-586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 81. | Patton SR, Kahhan N, Pierce JS, Benson M, Fox LA, Clements MA. Parental diabetes distress is a stronger predictor of child HbA1c than diabetes device use in school-age children with type 1 diabetes. BMJ Open Diabetes Res Care. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 82. | Zhang Y, Song M, Cao Y, Eliassen AH, Wolpin BM, Stampfer MJ, Willett WC, Wu K, Ng K, Hu FB, Giovannucci EL. Incident Early- and Later-Onset Type 2 Diabetes and Risk of Early- and Later-Onset Cancer: Prospective Cohort Study. Diabetes Care. 2023;46:120-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 83. | Noto H, Tsujimoto T, Sasazuki T, Noda M. Significantly increased risk of cancer in patients with diabetes mellitus: a systematic review and meta-analysis. Endocr Pract. 2011;17:616-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 121] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 84. | TODAY Study Group. Pregnancy Outcomes in Young Women With Youth-Onset Type 2 Diabetes Followed in the TODAY Study. Diabetes Care. 2021;45:1038-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 85. | Murphy HR, Howgate C, O'Keefe J, Myers J, Morgan M, Coleman MA, Jolly M, Valabhji J, Scott EM, Knighton P, Young B, Lewis-Barned N; National Pregnancy in Diabetes (NPID) advisory group. Characteristics and outcomes of pregnant women with type 1 or type 2 diabetes: a 5-year national population-based cohort study. Lancet Diabetes Endocrinol. 2021;9:153-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 207] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 86. | Makda SI, Davies MJ, Wilmot E, Bankart J, Yates T, Varghese EM, Fisher H, Anwar A, Khunti K. Prescribing in pregnancy for women with diabetes: use of potential teratogenic drugs and contraception. Diabet Med. 2013;30:457-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 87. | Arslanian S, Bacha F, Grey M, Marcus MD, White NH, Zeitler P. Evaluation and Management of Youth-Onset Type 2 Diabetes: A Position Statement by the American Diabetes Association. Diabetes Care. 2018;41:2648-2668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 232] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 88. | Wong J, Ross GP, Zoungas S, Craig ME, Davis EA, Donaghue KC, Maple-Brown LJ, McGill MJ, Shaw JE, Speight J, Wischer N, Stranks S. Management of type 2 diabetes in young adults aged 18-30 years: ADS/ADEA/APEG consensus statement. Med J Aust. 2022;216:422-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 89. | Rodriquez IM, O'Sullivan KL. Youth-Onset Type 2 Diabetes: Burden of Complications and Socioeconomic Cost. Curr Diab Rep. 2023;23:59-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 90. | ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, Kahan S, Khunti K, Leon J, Lyons SK, Perry ML, Prahalad P, Pratley RE, Seley JJ, Stanton RC, Gabbay RA; on behalf of the American Diabetes Association. 14. Children and Adolescents: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46:S230-S253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 113] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 91. | Shah AS, Zeitler PS, Wong J, Pena AS, Wicklow B, Arslanian S, Chang N, Fu J, Dabadghao P, Pinhas-Hamiel O, Urakami T, Craig ME. ISPAD Clinical Practice Consensus Guidelines 2022: Type 2 diabetes in children and adolescents. Pediatr Diabetes. 2022;23:872-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 82] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 92. | Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, Michos ED, Miedema MD, Muñoz D, Smith SC Jr, Virani SS, Williams KA Sr, Yeboah J, Ziaeian B. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596-e646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1000] [Cited by in RCA: 1762] [Article Influence: 293.7] [Reference Citation Analysis (0)] |
| 93. | McGavock J, Dart A, Wicklow B. Lifestyle therapy for the treatment of youth with type 2 diabetes. Curr Diab Rep. 2015;15:568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 94. | Salama M, Biggs BK, Creo A, Prissel R, Al Nofal A, Kumar S. Adolescents with Type 2 Diabetes: Overcoming Barriers to Effective Weight Management. Diabetes Metab Syndr Obes. 2023;16:693-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 95. | Berkowitz RI, Marcus MD, Anderson BJ, Delahanty L, Grover N, Kriska A, Laffel L, Syme A, Venditti E, Van Buren DJ, Wilfley DE, Yasuda P, Hirst K; TODAY Study Group. Adherence to a lifestyle program for youth with type 2 diabetes and its association with treatment outcome in the TODAY clinical trial. Pediatr Diabetes. 2018;19:191-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 96. | Marcus MD, Wilfley DE, El Ghormli L, Zeitler P, Linder B, Hirst K, Ievers-Landis CE, van Buren DJ, Walders-Abramson N; TODAY Study Group. Weight change in the management of youth-onset type 2 diabetes: the TODAY clinical trial experience. Pediatr Obes. 2017;12:337-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 97. | Burns N, Finucane FM, Hatunic M, Gilman M, Murphy M, Gasparro D, Mari A, Gastaldelli A, Nolan JJ. Early-onset type 2 diabetes in obese white subjects is characterised by a marked defect in beta cell insulin secretion, severe insulin resistance and a lack of response to aerobic exercise training. Diabetologia. 2007;50:1500-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 98. | Tamborlane WV, Barrientos-Pérez M, Fainberg U, Frimer-Larsen H, Hafez M, Hale PM, Jalaludin MY, Kovarenko M, Libman I, Lynch JL, Rao P, Shehadeh N, Turan S, Weghuber D, Barrett T; Ellipse Trial Investigators. Liraglutide in Children and Adolescents with Type 2 Diabetes. N Engl J Med. 2019;381:637-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 230] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 99. | Tamborlane WV, Bishai R, Geller D, Shehadeh N, Al-Abdulrazzaq D, Vazquez EM, Karoly E, Troja T, Doehring O, Carter D, Monyak J, Sjöström CD. Once-Weekly Exenatide in Youth With Type 2 Diabetes. Diabetes Care. 2022;45:1833-1840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 56] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 100. | Arslanian SA, Hannon T, Zeitler P, Chao LC, Boucher-Berry C, Barrientos-Pérez M, Bismuth E, Dib S, Cho JI, Cox D; AWARD-PEDS Investigators. Once-Weekly Dulaglutide for the Treatment of Youths with Type 2 Diabetes. N Engl J Med. 2022;387:433-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 84] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 101. | Tamborlane WV, Laffel LM, Shehadeh N, Isganaitis E, Van Name M, Ratnayake J, Karlsson C, Norjavaara E. Efficacy and safety of dapagliflozin in children and young adults with type 2 diabetes: a prospective, multicentre, randomised, parallel group, phase 3 study. Lancet Diabetes Endocrinol. 2022;10:341-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 52] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 102. | Laffel LM, Danne T, Klingensmith GJ, Tamborlane WV, Willi S, Zeitler P, Neubacher D, Marquard J; DINAMO Study Group. Efficacy and safety of the SGLT2 inhibitor empagliflozin versus placebo and the DPP-4 inhibitor linagliptin versus placebo in young people with type 2 diabetes (DINAMO): a multicentre, randomised, double-blind, parallel group, phase 3 trial. Lancet Diabetes Endocrinol. 2023;11:169-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 30.5] [Reference Citation Analysis (1)] |
| 103. | Jalaludin MY, Deeb A, Zeitler P, Garcia R, Newfield RS, Samoilova Y, Rosario CA, Shehadeh N, Saha CK, Zhang Y, Zilli M, Scherer LW, Lam RLH, Golm GT, Engel SS, Kaufman KD, Shankar RR. Efficacy and safety of the addition of sitagliptin to treatment of youth with type 2 diabetes and inadequate glycemic control on metformin without or with insulin. Pediatr Diabetes. 2022;23:183-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 104. | Karavanaki K, Paschou SA, Tentolouris N, Karachaliou F, Soldatou A. Type 2 diabetes in children and adolescents: distinct characteristics and evidence-based management. Endocrine. 2022;78:280-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 105. | Bacha F, Cheng P, Gal RL, Kollman C, Tamborlane WV, Klingensmith GJ, Manseau K, Wood J, Beck RW; for the Pediatric Diabetes Consortium. Initial Presentation of Type 2 Diabetes in Adolescents Predicts Durability of Successful Treatment with Metformin Monotherapy: Insights from the Pediatric Diabetes Consortium T2D Registry. Horm Res Paediatr. 2018;89:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 106. | Zuckerman Levin N, Cohen M, Phillip M, Tenenbaum A, Koren I, Tenenbaum-Rakover Y, Admoni O, Hershkovitz E, Haim A, Mazor Aronovitch K, Zangen D, Strich D, Brener A, Yeshayahu Y, Schon Y, Rachmiel M, Ben-Ari T, Levy-Khademi F, Tibi R, Weiss R, Lebenthal Y, Pinhas-Hamiel O, Shehadeh N. Youth-onset type 2 diabetes in Israel: A national cohort. Pediatr Diabetes. 2022;23:649-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 107. | Pratt JSA, Browne A, Browne NT, Bruzoni M, Cohen M, Desai A, Inge T, Linden BC, Mattar SG, Michalsky M, Podkameni D, Reichard KW, Stanford FC, Zeller MH, Zitsman J. ASMBS pediatric metabolic and bariatric surgery guidelines, 2018. Surg Obes Relat Dis. 2018;14:882-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 354] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 108. | Armstrong SC, Bolling CF, Michalsky MP, Reichard KW; SECTION ON OBESITY, SECTION ON SURGERY. Pediatric Metabolic and Bariatric Surgery: Evidence, Barriers, and Best Practices. Pediatrics. 2019;144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 165] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 109. | Wu Z, Gao Z, Qiao Y, Chen F, Guan B, Wu L, Cheng L, Huang S, Yang J. Long-Term Results of Bariatric Surgery in Adolescents with at Least 5 Years of Follow-up: a Systematic Review and Meta-Analysis. Obes Surg. 2023;33:1730-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 110. | Inge TH, Courcoulas AP, Jenkins TM, Michalsky MP, Brandt ML, Xanthakos SA, Dixon JB, Harmon CM, Chen MK, Xie C, Evans ME, Helmrath MA; Teen–LABS Consortium. Five-Year Outcomes of Gastric Bypass in Adolescents as Compared with Adults. N Engl J Med. 2019;380:2136-2145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 242] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 111. | Inge TH, Courcoulas AP, Jenkins TM, Michalsky MP, Helmrath MA, Brandt ML, Harmon CM, Zeller MH, Chen MK, Xanthakos SA, Horlick M, Buncher CR; Teen-LABS Consortium. Weight Loss and Health Status 3 Years after Bariatric Surgery in Adolescents. N Engl J Med. 2016;374:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 509] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 112. | Maffazioli GD, Stanford FC, Campoverde Reyes KJ, Stanley TL, Singhal V, Corey KE, Pratt JS, Bredella MA, Misra M. Comparing Outcomes of Two Types of Bariatric Surgery in an Adolescent Obese Population: Roux-en-Y Gastric Bypass vs. Sleeve Gastrectomy. Front Pediatr. 2016;4:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 113. | Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273:219-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1241] [Cited by in RCA: 1262] [Article Influence: 105.2] [Reference Citation Analysis (0)] |
| 114. | Xanthakos SA, Inge TH. Nutritional consequences of bariatric surgery. Curr Opin Clin Nutr Metab Care. 2006;9:489-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 115. | Harkness LS, Bonny AE. Calcium and vitamin D status in the adolescent: key roles for bone, body weight, glucose tolerance, and estrogen biosynthesis. J Pediatr Adolesc Gynecol. 2005;18:305-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 116. | Inge TH, Jenkins TM, Xanthakos SA, Dixon JB, Daniels SR, Zeller MH, Helmrath MA. Long-term outcomes of bariatric surgery in adolescents with severe obesity (FABS-5+): a prospective follow-up analysis. Lancet Diabetes Endocrinol. 2017;5:165-173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 210] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 117. | Gill RS, Birch DW, Shi X, Sharma AM, Karmali S. Sleeve gastrectomy and type 2 diabetes mellitus: a systematic review. Surg Obes Relat Dis. 2010;6:707-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 179] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 118. | Campbell EG, Alasmar A, Lawrence R, Kurpius-Brock M, DeCamp M, Kovar A, Schoen J, Inge T, Kelsey MM, Boles R, Engel S. Barriers to metabolic bariatric surgery in adolescents: results of a qualitative study. Surg Obes Relat Dis. 2022;18:794-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 119. | Nunez Lopez O, Jupiter DC, Bohanon FJ, Radhakrishnan RS, Bowen-Jallow KA. Health Disparities in Adolescent Bariatric Surgery: Nationwide Outcomes and Utilization. J Adolesc Health. 2017;61:649-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 120. | Fittipaldi-Fernandez RJ, Guedes MR, Galvao Neto MP, Klein MRST, Diestel CF. Efficacy of Intragastric Balloon Treatment for Adolescent Obesity. Obes Surg. 2017;27:2546-2551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 121. | Swei EC, Sullivan SA. Aspiration Therapy. Tech Vasc Interv Radiol. 2020;23:100659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 122. | TODAY Study Group. Lipid and inflammatory cardiovascular risk worsens over 3 years in youth with type 2 diabetes: the TODAY clinical trial. Diabetes Care. 2013;36:1758-1764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 123. | Arslanian S, El Ghormli L, Bacha F, Caprio S, Goland R, Haymond MW, Levitsky L, Nadeau KJ, White NH, Willi SM; TODAY Study Group. Adiponectin, Insulin Sensitivity, β-Cell Function, and Racial/Ethnic Disparity in Treatment Failure Rates in TODAY. Diabetes Care. 2017;40:85-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 124. | Zeitler P, Hirst K, Copeland KC, El Ghormli L, Levitt Katz L, Levitsky LL, Linder B, McGuigan P, White NH, Wilfley D; TODAY Study Group. HbA1c After a Short Period of Monotherapy With Metformin Identifies Durable Glycemic Control Among Adolescents With Type 2 Diabetes. Diabetes Care. 2015;38:2285-2292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 125. | Skyler JS, Bergenstal R, Bonow RO, Buse J, Deedwania P, Gale EA, Howard BV, Kirkman MS, Kosiborod M, Reaven P, Sherwin RS; American Diabetes Association; American College of Cardiology Foundation; American Heart Association. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care. 2009;32:187-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 490] [Cited by in RCA: 437] [Article Influence: 27.3] [Reference Citation Analysis (1)] |
| 126. | TODAY Study Group. Safety and tolerability of the treatment of youth-onset type 2 diabetes: the TODAY experience. Diabetes Care. 2013;36:1765-1771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 127. | Riddle MC, Cefalu WT, Evans PH, Gerstein HC, Nauck MA, Oh WK, Rothberg AE, le Roux CW, Rubino F, Schauer P, Taylor R, Twenefour D. Consensus Report: Definition and Interpretation of Remission in Type 2 Diabetes. Diabetes Care. 2021;44:2438-2444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 242] [Article Influence: 60.5] [Reference Citation Analysis (0)] |
| 128. | Willi SM, Martin K, Datko FM, Brant BP. Treatment of type 2 diabetes in childhood using a very-low-calorie diet. Diabetes Care. 2004;27:348-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 129. | Ricci M, Mancebo-Sevilla JJ, Cobos Palacios L, Sanz-Cánovas J, López-Sampalo A, Hernández-Negrin H, Pérez-Velasco MA, Pérez-Belmonte LM, Bernal-López MR, Gómez-Huelgas R. Remission of type 2 diabetes: A critical appraisal. Front Endocrinol (Lausanne). 2023;14:1125961. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 130. | Cioana M, Deng J, Nadarajah A, Hou M, Qiu Y, Chen SSJ, Rivas A, Banfield L, Toor PP, Zhou F, Guven A, Alfaraidi H, Alotaibi A, Thabane L, Samaan MC. The Prevalence of Obesity Among Children With Type 2 Diabetes: A Systematic Review and Meta-analysis. JAMA Netw Open. 2022;5:e2247186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 131. | Savoye M, Shaw M, Dziura J, Tamborlane WV, Rose P, Guandalini C, Goldberg-Gell R, Burgert TS, Cali AM, Weiss R, Caprio S. Effects of a weight management program on body composition and metabolic parameters in overweight children: a randomized controlled trial. JAMA. 2007;297:2697-2704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 359] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 132. | Gow ML, Pham-Short A, Jebeile H, Varley BJ, Craig ME. Current Perspectives on the Role of Very-Low-Energy Diets in the Treatment of Obesity and Type 2 Diabetes in Youth. Diabetes Metab Syndr Obes. 2021;14:215-225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 133. | Ross JAD, Barron E, McGough B, Valabhji J, Daff K, Irwin J, Henley WE, Murray E. Uptake and impact of the English National Health Service digital diabetes prevention programme: observational study. BMJ Open Diabetes Res Care. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 134. | US Preventive Services Task Force, Mangione CM, Barry MJ, Nicholson WK, Cabana M, Chelmow D, Coker TR, Davidson KW, Davis EM, Donahue KE, Jaén CR, Kubik M, Li L, Ogedegbe G, Pbert L, Ruiz JM, Stevermer J, Tseng CW, Wong JB. Screening for Prediabetes and Type 2 Diabetes in Children and Adolescents: US Preventive Services Task Force Recommendation Statement. JAMA. 2022;328:963-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 135. | Mohan V, Anbalagan VP. Expanding role of the Madras Diabetes Research Foundation - Indian Diabetes Risk Score in clinical practice. Indian J Endocrinol Metab. 2013;17:31-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 136. | Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373:1773-1779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2141] [Cited by in RCA: 2332] [Article Influence: 145.8] [Reference Citation Analysis (1)] |
| 137. | Bacha F, Pyle L, Nadeau K, Cuttler L, Goland R, Haymond M, Levitsky L, Lynch J, Weinstock RS, White NH, Caprio S, Arslanian S; TODAY Study Group. Determinants of glycemic control in youth with type 2 diabetes at randomization in the TODAY study. Pediatr Diabetes. 2012;13:376-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 138. | Nadeau KJ, Anderson BJ, Berg EG, Chiang JL, Chou H, Copeland KC, Hannon TS, Huang TT, Lynch JL, Powell J, Sellers E, Tamborlane WV, Zeitler P. Youth-Onset Type 2 Diabetes Consensus Report: Current Status, Challenges, and Priorities. Diabetes Care. 2016;39:1635-1642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 280] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 139. | Shah AS, Nadeau KJ, Dabelea D, Redondo MJ. Spectrum of Phenotypes and Causes of Type 2 Diabetes in Children. Annu Rev Med. 2022;73:501-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 140. | Karres J, Pratt V, Guettier JM, Temeck J, Tamborlane WV, Dunger D, Bejnariu C, De Beaufort C, Tomasi P. Joining forces: a call for greater collaboration to study new medicines in children and adolescents with type 2 diabetes. Diabetes Care. 2014;37:2665-2667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 141. | Karres J, Tomasi P. New medicines for type 2 diabetes in adolescents: many products, few patients. Expert Rev Clin Pharmacol. 2013;6:227-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 142. | Van Name M, Klingensmith G, Nelson B, Wintergerst K, Mitchell J, Norris K, Tamborlane WV; Pediatric Diabetes Consortium. Transforming Performance of Clinical Trials in Pediatric Type 2 Diabetes: A Consortium Model. Diabetes Technol Ther. 2020;22:330-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |