Published online Jun 9, 2023. doi: 10.5409/wjcp.v12.i3.77
Peer-review started: January 28, 2023
First decision: April 2, 2023
Revised: April 26, 2023
Accepted: May 8, 2023
Article in press: May 8, 2023
Published online: June 9, 2023
Processing time: 130 Days and 15.2 Hours
Comprehensive guidelines on seropositive autoimmune hepatitis have been published for both adults and children, although these guidelines comprise only limited knowledge about seronegative autoimmune hepatitis. Autoimmune hepatitis presents as an acute or chronic progressive disease and poor outcomes are inevitable if left untreated. The absence of autoantibody positivity, hypergammaglobulinemia and lack of comprehensive algorithms makes seronegative autoimmune hepatitis a mysterious disease. In general, seronegative autoimmune hepatitis often presents with acute hepatitis, and its treatment and prognosis similar to seropositive autoimmune hepatitis. The present review focuses on the known characteristics of seronegative autoimmune hepatitis in childhood, and those of which current knowledge is vague.
Core Tip: Seronegative autoimmune hepatitis is a diagnostic dilemma. The absence of autoantibodies and hypergammaglobulinemia which is not uncommon in seronegative autoimmune hepatitis complicates the diagnosis. Seronegative autoimmune hepatitis often presents with acute hepatitis, and its treatment and prognosis similar to seropositive autoimmune hepatitis. This review discusses whether seronegativity is only a matter of laboratory issue or if it implies an entity with different pathogenesis, clinical features, treatment algorithms, and prognosis.
- Citation: Islek A, Tumgor G. Seronegative autoimmune hepatitis in childhood. World J Clin Pediatr 2023; 12(3): 77-85
- URL: https://www.wjgnet.com/2219-2808/full/v12/i3/77.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v12.i3.77
The diagnosis of autoimmune hepatitis is based on the ruling out of other causes of liver damage with the presence of non-organ specific autoantibodies and specific histological findings. The value of autoantibodies cannot be denied in the diagnosis and subtyping of autoimmune hepatitis and in the follow-up of disease activity. Failure to diagnose may increase mortality in children presenting for the first time with acute hepatic failure or cirrhosis will be inevitable in children due to progressive disease course if not treated. Autoimmune hepatitis is a progressive inflammatory disease that responds well to immunosuppressive therapy and is diagnosed with histologically interface hepatitis, serologically presence of non-organ-specific autoantibodies, and biochemically elevated transaminase and immunoglobulin G (IgG) levels[1,2]. The term seronegativity refers to the condition in which no autoantibodies are detected, but other features partially or completely support the diagnosis of autoimmune hepatitis, although the term seronegative autoimmune hepatitis has not been widely accepted. It is not known whether seronegativity is only a matter of laboratory issue or if it implies an entity with different pathogenesis, clinical features, treatment algorithms, and prognosis. We present here a discussion of the aspects of seronegative cases that are already known or those that require further elucidation.
Autoimmune hepatitis can present in childhood or adulthood. Different incidence and prevalence rates have been reported for autoimmune hepatitis from different countries, with reported incidences in the range of 0.23-0.4 cases/100.000 people and prevalence in the range of 2.4-9 cases/100.000 people. Differences in the incidence and prevalence in the same ethnic origins but in different geographic regions suggest that factors other than genetic predisposition play a role in disease development. Autoimmune hepatitis is more commonly observed in females than in males[3]. There is a lack of detailed information about the prevalence of seronegative autoimmune hepatitis in which conventional autoantibodies are not detected. Seronegative autoimmune hepatitis account for 5%-20% of all autoimmune hepatitis, and on top of the uncertainties regarding which patients should be considered seronegative-it is no secret that some seronegative cases are classified as cryptogenic hepatitis-there is also a possibility of false-negative results in autoantibodies[4]. In our series of 54 patients, 27.7% (15/54) were identified as seronegative autoimmune hepatitis at the time of diagnosis, and a significant proportion (60%) of the cases were female, as is the case with type-1 autoimmune hepatitis. The average age at presentation was 5.69 years in those with seronegative autoimmune hepatitis. The mean age at presentation was 11 years in patients with type-1 autoimmune hepatitis and 5.1 years in those with type-2 autoimmune hepatitis[5].
The liver cells become damaged in cases of autoimmune hepatitis as a result of the joint effects of cellular and humoral immunity. It is suggested that damage develops in the target organ with the loss of immunity control that is triggered by a virus in individuals with a genetic predisposition. The loss of immune tolerance to hepatocyte antigens (these antigens are considered to be asialoglycoprotein receptors, liver cytosolic antigens, and soluble liver antigens) due to the compromised functioning of regulatory T cells (Tregs) results in the activation of the CD4 and CD8 T-cells, which release such proinflammatory cytokines such as interferon-γ and tumor necrosis factor-α[6]. A recent study suggested a decrease in Tregs in the peripheral blood of patients with untreated seropositive autoimmune hepatitis, together with increased Treg/T-cell and Treg/B-cell ratios identified on liver biopsy, and this finding was associated with the migration of these cells to the center of inflammation[7]. Proinflammatory cytokines initiate hepatocyte damage and result in the immune activation and proliferation of macrophages, B and T-cells, and natural killer cells. B-cells subsequently transform into plasma cells, which produce the antibodies that serve as the diagnostic instruments in autoimmune hepatitis[6]. The antibodies and target antigens described in seropositive autoimmune hepatitis are listed in Table 1.
| Antibody | Target antigen |
| ANA | Single-stranded/double-stranded DNA, ribonucleoproteins |
| ASMA | Filamentous actin, vimentin, desmin |
| LKM | Cytochrome P450 2D6 (CYP2D6) |
| anti-SLA | UGA serine transfer RNA associated protein |
| LC-1 | Formiminotransferase cyclo-deaminase |
| pANCA | Nuclear lamina proteins |
| ASGP-R | Asialoglycoprotein receptor |
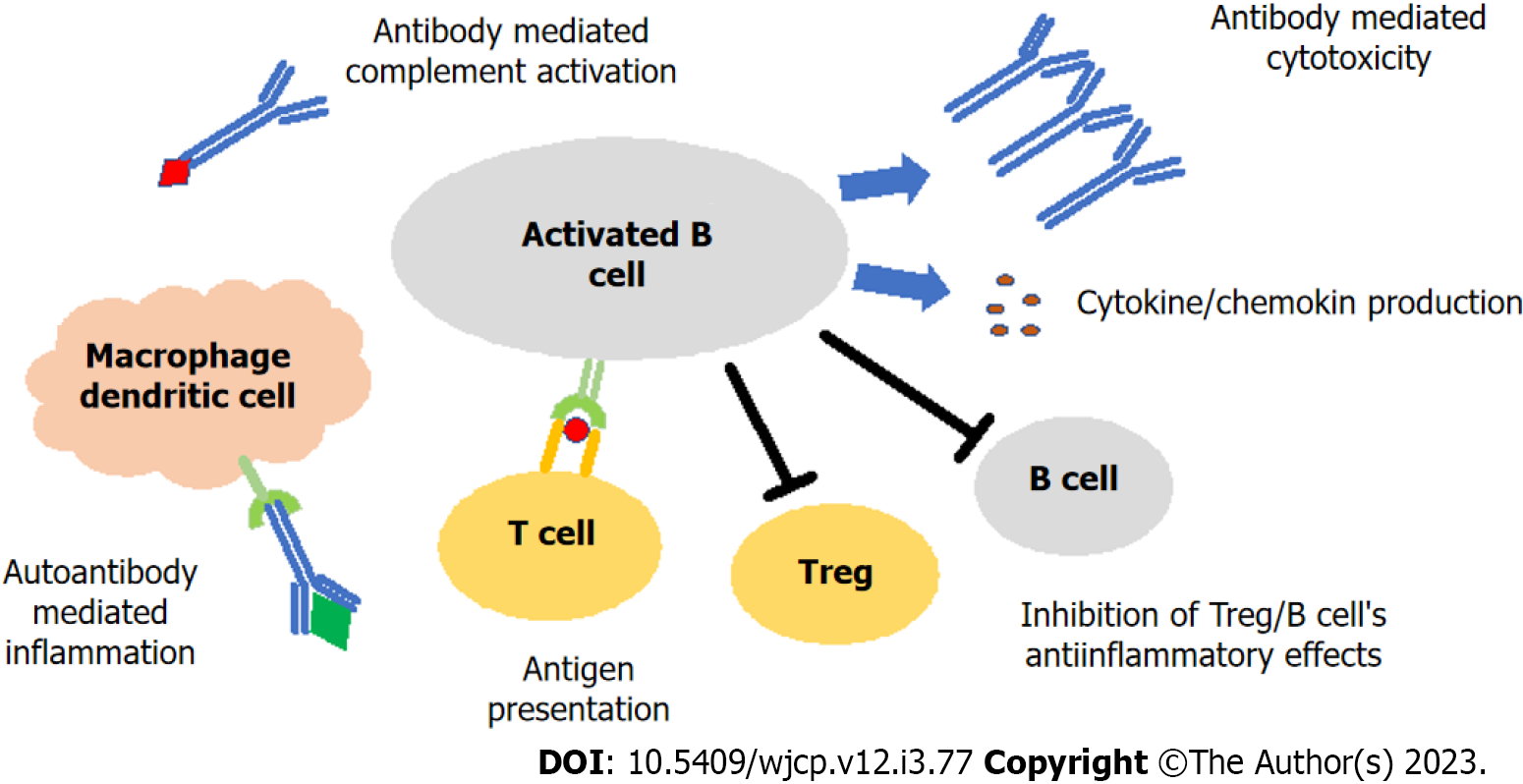
T-cells serve as the trigger in the initiation of autoimmune hepatitis and mediate the activation of humoral immunity[8]. Autoantibodies are currently used in the diagnosis of autoimmune hepatitis, and for the determination of the subtype and the assessment of remission. Anti-nuclear antibody (ANA) and/or anti-smooth muscle antibody (ASMA) are detected in type-1 autoimmune hepatitis, and anti-liver-kidney microsomal antibody (LKM) and/or liver cytosol type-1 antibody (LC-1) in type-2 autoimmune hepatitis. ANA and ASMA are positive in approximately half of the patients with autoimmune hepatitis, while ANA alone is positive in 10%–15% and ASMA alone in 30%–35% of patients. Both anti-LKM and anti-LC-1 are positive in approximately half of the patients with type-2 autoimmune hepatitis. A positive anti-LC-1 alone is reported in 10% of patients with type-2 autoimmune hepatitis. A positive anti-soluble liver antigen/liver–pancreas antigen (anti-SLA) reported in both types of autoimmune hepatitis, being positive in approximately one-fifth of those with type-1 and half of those with type-2 autoimmune hepatitis. A positive anti-SLA alone is reported in less than 10% of all patients[9]. Serum concentrations of anti-LKM, anti-LC-1, and ASMA have been identified as indicators of disease severity. In other words, hepatocyte necrosis can be severe, and the disease may progress rapidly to cirrhosis[2,9]. This raises the question: “Is the pathogenesis of seronegative autoimmune hepatitis different, and does it have a milder disease course?” Under normal circumstances, T cells initiate the immune cascade in seropositive autoimmune hepatitis, while the inflammatory events continue with B cells (Figure 1). Activated B cells, which are switched to plasma cells with the stimulation of T cells, release autoantibodies and cytokines/chemokines, cause autoantibody-mediated activation of complement and macrophage/dendritic cells and continue to contribute to inflammation with antigen presentation to T cells, while on the other hand suppress Treg and B cells' anti-inflammatory effects[10-12]. There is a lack of comprehensive data on the contribution of autoantibodies to cell damage in seronegative autoimmune hepatitis. The presence of plasma cells in the liver biopsies of patients with seronegative autoimmune hepatitis suggests that similar physiopathological mechanisms are involved in the pathogenesis of seronegative autoimmune hepatitis. Perhaps a defect in differentiation and maturation of B cells could impair antibody production, but its effects on T cell regulation were preserved. There is, however, a lack of evidence supporting this theory in seronegative patients.
There may also be a case of pseudo-seronegativity. This may be attributed to several reasons for this. Firstly, the autoantibody tests may not have been performed as recommended. The International Autoimmune Hepatitis Group has determined a threshold of ≥ 1:40 for autoantibodies to be detected by an indirect immunofluorescence method by diluting the serum in adults[13]. The recommended autoantibody threshold levels in children are lower than those determined for adults, with a titer of 1:20 for ANA and ASMA and a titer of 1:10 for anti-LKM being considered significant[14]. In adults, thresholds for all antibodies are ≥ 1:40. False-negative results may be obtained if standard high dilutions are used in the laboratory designated for adult patients. This problem can be overcome by communicating with the laboratory personnel for testing serum in low dilutions or it may be beneficial to test such autoantibodies using an enzyme-linked immunosorbent assay (ELISA), even if its validity has not been fully demonstrated. Secondly, previous studies have suggested that the commonly used immunofluorescence tests may initially produce false-negative results due to an intense antigen-antibody reaction in patients presenting with acute hepatic failure and acute hepatitis[8,15]. This theory is supported by the observation of seroconversion during the disease course in some patients who were initially seronegative. Furthermore, immunosuppressive agents such as corticosteroids and azathioprine may lead to conversion from seronegative to seropositive with the elimination of the effect of unknown factors. It is currently unknown when, and in what titers will the autoantibodies be positive due to limited data. In an earlier study by the present authors, the majority of patients with seronegative autoimmune hepatitis had an acute presentation (13 out of 15 patients), and the autoantibody tests turned positive in half of the patients (7 of the 15 patients) during follow-up, with a median time to seroconversion of 28 d (18–51 d)[5]. Thirdly, antibodies other than ANA, ASMA, and anti-LKM such as anti-SLA and anti-LC-1, tested by ELISA or immunoblotting methods and cannot be studied in many centers, can be checked[16]. The investigation of these autoantibodies and novel antibodies that are yet to be discovered can reduce the number of cases labeled as seronegative autoimmune hepatitis when conventional antibodies are negative.
A liver biopsy is essential for the establishment of a diagnosis of autoimmune hepatitis. The anticipated primary histological finding is lymphoplasmacytic infiltration in portal areas extending to the lobule center, in other words, interface hepatitis. That said, interface hepatitis can also be observed in other liver diseases, such as acute and chronic viral hepatitis, drug-induced liver disease, and Wilson’s disease. Other histological findings include portal lymphoplasmacytic infiltration, rosette formation, emperipolesis, central perivenulitis, and portal-to-portal or portal-to-central fibrosis. Centrilobular necrosis and multilobular collapse can be observed in acute presentations. Interface hepatitis may not be obvious in cirrhotic patients and those receiving immunosuppressive therapy. It has been stated that the presence of central perivenulitis and hyaline droplets in Kupffer cells may be an important diagnostic finding in the absence of interface hepatitis[1,6].
Studies of adult patients have suggested that a histopathological finding specific to seronegative autoimmune hepatitis is lacking, although there have been contradictory statements on whether the severity of histopathological findings differs from that observed in seropositive autoimmune hepatitis. Previous studies have reported that advanced histological-stage cirrhosis is more common in seronegative patients than in seropositive patients[17]. Despite the limited data on pediatric patients, we commonly encounter centrilobular necrosis in patients with seronegative autoimmune hepatitis in our practice due to the frequency of acute presentation.
In one study, CD8 T-cell-dominant infiltration was identified in the liver biopsies of a small number of pediatric patients with seronegative autoimmune hepatitis and aplastic anemia[18]. In a study of 33 patients in whom the authors described the underlying disease as “acute hepatic failure with an unknown cause”, including eight patients who had hepatitis-associated aplastic anemia (HAAA), CD8 T-cell infiltration was identified in the livers of the majority of cases. The patients diagnosed with “acute hepatic failure with an unknown cause” actually fully meet the definition of seronegative autoimmune hepatitis. The authors state, however, that CD8 T-cell infiltration presented to various degrees in a small number of patients with autoimmune hepatitis, although further research is needed in this regard[19].
Seropositive autoimmune hepatitis can be discovered incidentally with asymptomatic transaminase elevation but can also be diagnosed after presentation with a severe clinical course. The presentation may vary between different regions but is generally as follows: Approximately one-third of patients present with fatigue, nausea, abdominal pain, and jaundice, as in acute viral hepatitis (acute presentation), while the other third presents with such symptoms of chronic hepatitis as lack of appetite, weight loss, amenorrhea, and intermittent jaundice. Less than one-fifth presents with the symptoms of cirrhosis and its complications, such as ascites, abdominal distention, splenomegaly, and variceal hemorrhage. Type-1 autoimmune hepatitis is often observed in adolescence, while type-2 autoimmune hepatitis is mostly encountered in young children. Patients with type-2 autoimmune hepatitis commonly present with acute hepatitis or acute hepatic failure[1]. The data derived from adult patients suggests that acute presentations are uncommon in seronegative autoimmune hepatitis[20]. There are no detailed clinical findings related seronegative autoimmune hepatitis in children. In a study of 38 pediatric patients with seronegative autoimmune hepatitis, three-quarters presented with acute symptoms[16]. In our previous study, 13 of 15 patients who were considered to have seronegative autoimmune hepatitis presented with acute hepatitis[5]. There is another terminology called HAAA in the literature[21]. Aplastic anemia develops within a couple of weeks or months after the onset of hepatitis, while it rarely develops after one year. Patients can be recognized at the acute hepatitis stage with symptoms such as vomiting and jaundice but can also be diagnosed after the development of aplastic anemia. HAAA, is thought to develop as a result of autoimmunity, triggered by an unknown factor, although a virus is suspected. Although HAAA can be diagnosed at any age, including adulthood, reports in literature focus mostly on pediatric cases, and most are case reports or small case series[22]. The prevalence of HAAA was found to be 5% in a multicenter European study of 3916 adult patients with aplastic anemia[23]. While previous studies have described the histopathological findings of the liver as lobular inflammations as in viral hepatitis[24], some have described features of autoimmune hepatitis and termed it as seronegative autoimmune hepatitis with the absence of autoantibodies[25]. The confusing situation for the diagnosis of autoimmune hepatitis is the spontaneous normalization of transaminases without any treatment. Our clinical experience suggests that transaminase elevation is no longer observed once aplastic anemia has developed. Autoimmune hepatitis in fact has a chronic disease course that is characterized by fluctuations. In our previous study, aplastic anemia developed in two patients with seronegative autoimmune hepatitis who presented with cholestatic hepatitis, despite dramatic improvement in hypertransaminasemia and direct bilirubinemia after corticosteroid treatment[5]. Furthermore, hepatitis did not recur within two years of the discontinuation of immunosuppressive therapy.
As is the case with seropositive autoimmune hepatitis, there is no pathognomonic finding of seronegative autoimmune hepatitis, so it is important to rule out other conditions such as viral hepatitis, alpha-1 antitrypsin deficiency, metabolic conditions such as Wilson’s disease, and drug-induced liver injury. Hypergammaglobulinemia is one of the vital criteria for a diagnosis of seropositive autoimmune hepatitis[4]. Alongside the absence of autoantibodies, hypergammaglobulinemia which is not uncommon in seronegative autoimmune hepatitis, further complicates diagnosis. While serum IgG levels may be within normal ranges for age in seronegative cases, a decrease from baseline may be observed with treatment[12,25]. Patients diagnosed in the hepatitis stage who subsequently develop aplastic anemia are often found to have lymphocytopenia before the development of aplastic anemia. It has been found that the CD4 T-cell count is decreased in these patients[18]. If a patient is found to have isolated persistent lymphocytopenia without stigmas of a viral infection, the patient will most likely develop aplastic anemia in the future. Finally, it is essential to identify any histopathological liver features specific to autoimmune hepatitis to confirm a diagnosis of seronegative autoimmune hepatitis, although some histopathological findings can be observed also in other liver diseases.
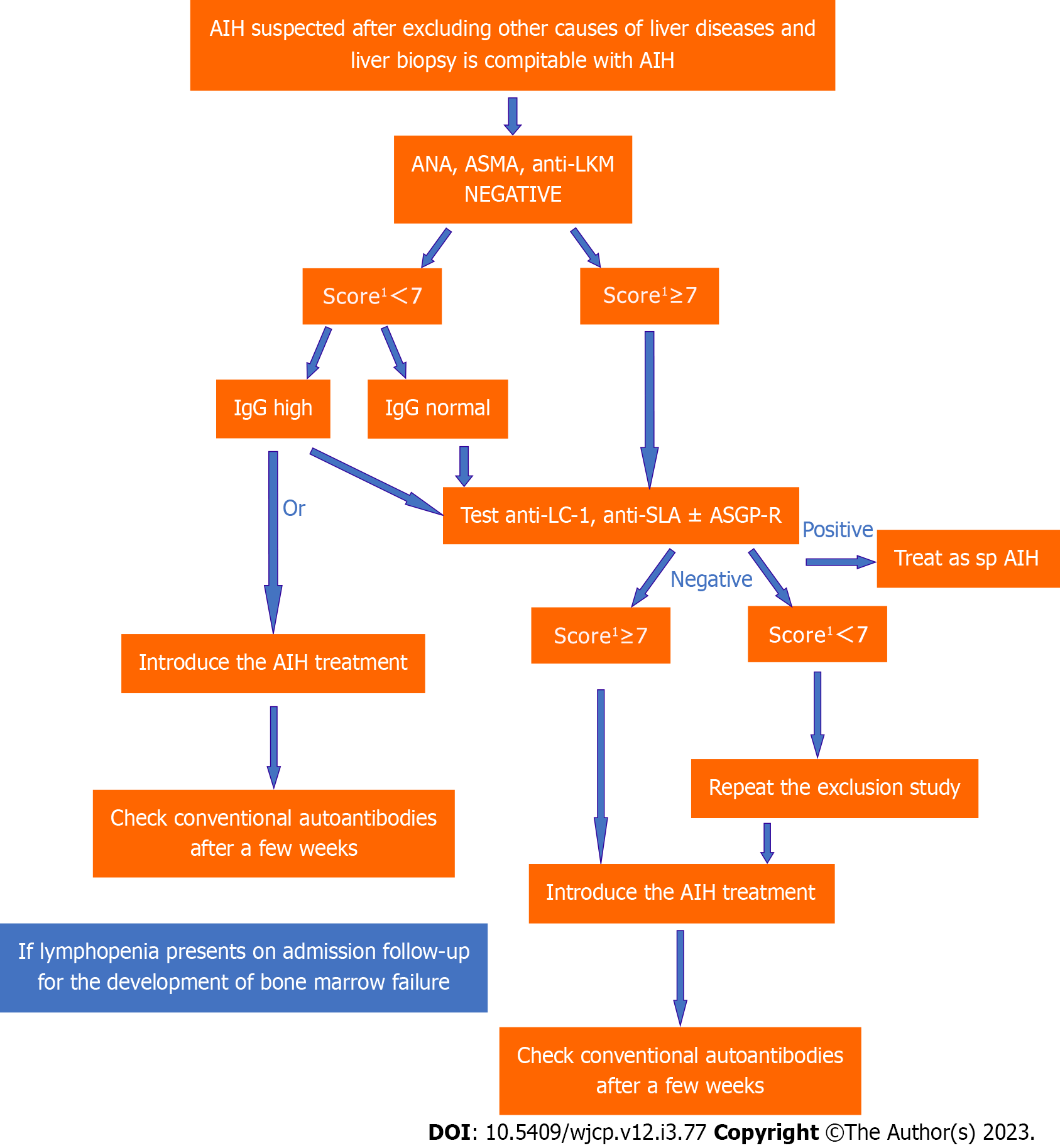
Simplified diagnostic criteria is used for research purposes in autoimmune hepatitis but may also be used in cases that pose diagnostic challenges[4]. This scoring system has been found to have a sensitivity of 77% and a false-negative rate of 17%, and these false-negative cases are indeed sero
Delays in treatment can lead to disease progression in patients with autoimmune hepatitis, while timely treatment can prevent such severe complications as cirrhosis. The treatment of seronegative auto
The benefits of corticosteroid therapy are arguable, even in patients with seropositive autoimmune hepatitis who present with acute hepatic failure. Survival without transplantation is possible in patients treated with corticosteroid, although an increased risk of sepsis and mortality has been reported[1]. The appropriate approach to patients presenting with acute hepatic failure and unresponsive to corticosteroid therapy-if steroid therapy has been attempted—and in those with decompensated cirrhosis is liver transplantation.
Despite the limited data related to pediatric patients, prognosis is generally good in patients with seronegative autoimmune hepatitis. In a study of 38 seronegative patients, Maggiore et al[25] reported that all were still alive after 4-17 years. Among the study participants, one presented with acute hepatic failure underwent liver transplantation, while four patients with aplastic anemia underwent bone marrow transplantations. The median time to the cessation of immunosuppressive therapy in 20 out of the 38 cases was 4 years (1-6 years), and nine in whom the immunosuppressive therapy was stopped had aplastic anemia[25]. One adult study reported a high risk of developing hepatitis in the transplanted livers of patients with seronegative autoimmune hepatitis[20], although there is a lack of long-term data that would allow us to speculate on the situation in children. The differences between seropositive and seronegative autoimmune hepatitis are summarized in Table 2.
| Seropositive individuals | Seronegative individuals |
| High IgG | Normal IgG (may be high) |
| Presents with an acute or chronic course of disease | Generally, presents with acute manifestations |
| Does not show bone marrow abnormality | Lymphopenia may accompany (generally initially) and bone marrow failure may develop |
| Autoantibodies are detectabl on admission | Autoantibody positivity may develop after immunosuppressive therapy |
| Disease onset is usually in the second decade for type 1 AIH and at any age in the first decade for type 2 | Disease onset is similar to type 2 AIH (may be at any age) |
| Treatment response is generally good | Treatment response is generally good |
| Immunosuppressant withdrawal possible (Recurrence rate is higher in type 2 AIH than type 1) | Immunosuppressant withdrawal possible (Recurrence rate is unknown) |
While seronegative autoimmune hepatitis is not a rare entity, little is known about its diagnosis and treatment. However, based on this limited literature and our experience, we can summarize seronegative autoimmune hepatitis as follows. Since autoantibody positivity at low dilutions in immunofluorescence is sufficient for a diagnosis of autoimmune hepatitis in children, it should be ensured that the laboratory conditions are arranged for opportunity to test autoantibodies in low dilutions, or other non-conventional autoantibodies should be studied. The presence of lymphopenia points to the development of bone marrow failure in the future. Treatment should not be delayed after other causes have been ruled out in patients with liver biopsy features consistent with autoimmune hepatitis to prevent the development of complications. The treatment of seronegative autoimmune hepatitis is similar to seropositive autoimmune hepatitis and the authors believe that the prognosis is good in seronegative autoimmune hepatitis.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ng HY, China; Wu X, China; Yu F, China S-Editor: Fan JR L-Editor: A P-Editor: Ju JL
| 1. | Kerkar N, Mack CL. Autoimmune hepatitis in children. In: Suchy FJ, Sokol RJ, Balistreri WF, Bezerra JA, Mack CL, Shneider BL. Liver Disease in Children, 5th ed. Cambridge, UK: Cambridge University Press, 2021: 321-332. |
| 2. | Sebode M, Weiler-Normann C, Liwinski T, Schramm C. Autoantibodies in Autoimmune Liver Disease-Clinical and Diagnostic Relevance. Front Immunol. 2018;9:609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 3. | Palle SK, Naik KB, McCracken CE, Kolachala VL, Romero R, Gupta NA. Racial disparities in presentation and outcomes of paediatric autoimmune hepatitis. Liver Int. 2019;39:976-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Mieli-Vergani G, Vergani D, Czaja AJ, Manns MP, Krawitt EL, Vierling JM, Lohse AW, Montano-Loza AJ. Autoimmune hepatitis. Nat Rev Dis Primers. 2018;4:18017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 275] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 5. | Islek A, Keskin H. Seronegative autoimmune hepatitis in children: a single-center experience. Acta Gastroenterol Belg. 2021;84:305-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Terziroli Beretta-Piccoli B, Mieli-Vergani G, Vergani D. Autoimmmune hepatitis. Cell Mol Immunol. 2022;19:158-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 93] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 7. | Diestelhorst J, Junge N, Schlue J, Falk CS, Manns MP, Baumann U, Jaeckel E, Taubert R. Pediatric autoimmune hepatitis shows a disproportionate decline of regulatory T cells in the liver and of IL-2 in the blood of patients undergoing therapy. PLoS One. 2017;12:e0181107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Assis DN. Immunopathogenesis of Autoimmune Hepatitis. Clin Liver Dis (Hoboken). 2020;15:129-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 9. | Terziroli Beretta-Piccoli B, Mieli-Vergani G, Vergani D. Serology in autoimmune hepatitis: A clinical-practice approach. Eur J Intern Med. 2018;48:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Mieli-Vergani G, Vergani D, Baumann U, Czubkowski P, Debray D, Dezsofi A, Fischler B, Gupte G, Hierro L, Indolfi G, Jahnel J, Smets F, Verkade HJ, Hadžić N. Diagnosis and Management of Pediatric Autoimmune Liver Disease: ESPGHAN Hepatology Committee Position Statement. J Pediatr Gastroenterol Nutr. 2018;66:345-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 212] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 11. | Narkewicz MR, Horslen S, Belle SH, Rudnick DA, Ng VL, Rosenthal P, Romero R, Loomes KM, Zhang S, Hardison RM, Squires RH; Pediatric Acute Liver Failure Study Group. Prevalence and Significance of Autoantibodies in Children With Acute Liver Failure. J Pediatr Gastroenterol Nutr. 2017;64:210-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Taylor SA, Assis DN, Mack CL. The Contribution of B Cells in Autoimmune Liver Diseases. Semin Liver Dis. 2019;39:422-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 13. | Hennes EM, Zeniya M, Czaja AJ, Parés A, Dalekos GN, Krawitt EL, Bittencourt PL, Porta G, Boberg KM, Hofer H, Bianchi FB, Shibata M, Schramm C, Eisenmann de Torres B, Galle PR, McFarlane I, Dienes HP, Lohse AW; International Autoimmune Hepatitis Group. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1205] [Cited by in RCA: 1253] [Article Influence: 73.7] [Reference Citation Analysis (0)] |
| 14. | Khedr MA, Salem TA, Boghdadi GM, Elharoun AS, El-Shahaway AA, Atallah HR, Sira MM. Seronegative autoimmune hepatitis in children : A real diagnostic challenge. Wien Klin Wochenschr. 2022;134:195-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Bhumi SA, Wu GY. Seronegative Autoimmune Hepatitis. J Clin Transl Hepatol. 2023;11:459-465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 16. | Czaja AJ. Autoantibody-negative autoimmune hepatitis. Dig Dis Sci. 2012;57:610-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Kumari N, Kathuria R, Srivastav A, Krishnani N, Poddar U, Yachha SK. Significance of histopathological features in differentiating autoimmune liver disease from nonautoimmune chronic liver disease in children. Eur J Gastroenterol Hepatol. 2013;25:333-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Kemme S, Stahl M, Brigham D, Lovell MA, Nakano T, Feldman AG, Mack C. Outcomes of Severe Seronegative Hepatitis-associated Aplastic Anemia: A Pediatric Case Series. J Pediatr Gastroenterol Nutr. 2021;72:194-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Chapin CA, Burn T, Meijome T, Loomes KM, Melin-Aldana H, Kreiger PA, Whitington PF, Behrens EM, Alonso EM. Indeterminate pediatric acute liver failure is uniquely characterized by a CD103(+) CD8(+) T-cell infiltrate. Hepatology. 2018;68:1087-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 20. | Donaghy L, Barry FJ, Hunter JG, Stableforth W, Murray IA, Palmer J, Bendall RP, Elsharkawy AM, Dalton HR. Clinical and laboratory features and natural history of seronegative hepatitis in a nontransplant centre. Eur J Gastroenterol Hepatol. 2013;25:1159-1164. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Patel KR, Bertuch A, Sasa GS, Himes RW, Wu H. Features of Hepatitis in Hepatitis-associated Aplastic Anemia: Clinical and Histopathologic Study. J Pediatr Gastroenterol Nutr. 2017;64:e7-e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Gonnot M, Neumann F, Huet F, Maudinas R, Leblanc T, Lacaille F. Hepatitis-associated Aplastic Anemia. J Pediatr Gastroenterol Nutr. 2022;75:553-555. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 23. | Locasciulli A, Bacigalupo A, Bruno B, Montante B, Marsh J, Tichelli A, Socié G, Passweg J; Severe Aplastic Anemia Working Party of the European Blood and Marrow Transplant Group (SAA-WP, EBMT). Hepatitis-associated aplastic anaemia: epidemiology and treatment results obtained in Europe. A report of The EBMT aplastic anaemia working party. Br J Haematol. 2010;149:890-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Gonzalez-Casas R, Garcia-Buey L, Jones EA, Gisbert JP, Moreno-Otero R. Systematic review: hepatitis-associated aplastic anaemia--a syndrome associated with abnormal immunological function. Aliment Pharmacol Ther. 2009;30:436-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Maggiore G, Socie G, Sciveres M, Roque-Afonso AM, Nastasio S, Johanet C, Gottrand F, Fournier-Favre S, Jacquemin E, Bernard O. Seronegative autoimmune hepatitis in children: Spectrum of disorders. Dig Liver Dis. 2016;48:785-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 26. | Arcos-Machancoses JV, Molera Busoms C, Julio Tatis E, Bovo MV, Martín de Carpi J. Accuracy of the Simplified Criteria for Autoimmune Hepatitis in Children: Systematic Review and Decision Analysis. J Clin Exp Hepatol. 2019;9:147-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |