Published online Jun 9, 2023. doi: 10.5409/wjcp.v12.i3.115
Peer-review started: December 3, 2022
First decision: February 21, 2023
Revised: March 8, 2023
Accepted: March 30, 2023
Article in press: March 30, 2023
Published online: June 9, 2023
Processing time: 186 Days and 19.3 Hours
Mycoplasma pneumoniae (MP) is a prevalent pathogen that causes respiratory infections in children and adolescents.
To assess the differences in the clinical features of MP-associated community-acquired pneumonia (CAP) in children who presented with mild or severe myco
This work is a retrospective study. We identified children between 2 mo and 16 years of age with clinical and radiological findings consistent with CAP. We admitted patients to the inpatient department of the Second Hospital of Jilin University, Changchun, China, from January 2019 to December 2019.
A total of 409 hospitalized patients were diagnosed with MPP. Among them were 214 (52.3%) males and 195 (47.7%) females. The duration of fever and cough was the longest in severe MPP cases. Similarly, plasma levels of highly sensitive C-reactive protein (t = -2.834, P < 0.05), alanine transaminase (t = -2.511, P < 0.05), aspartate aminotransferase (t = -2.939, P < 0.05), and lactate dehydrogenase (LDH) (t = -2.939, P < 0.05) were all elevated in severe MPP cases compared with mild MPP cases, and these elevations were statistically significant (P < 0.05). Conversely, the neutrophil percentage was significantly lower in severe MPP cases than in mild MPP cases. The incidence of myocardial damage was significantly higher in severe MPP cases than in mild MPP cases (χ2 = 157.078, P < 0.05).
Mycoplasma pneumoniae is the main cause of CAP. The incidence of myocardial damage was higher and statistically significant in severe MPP cases than in mild MPP cases.
Core Tip: Our study highlighted which clinical parameters should be focused on to differentiate between mild and severe mycoplasma pneumoniae pneumonia (MPP), which is crucial for pediatricians as it would enable us to make a quick diagnosis and consequently prompt treatment in case of severe MPP. We found that the duration of fever and cough was longer in the severe MPP group than in the mild MPP group. Similarly, the high sensitivity C-reactive protein levels, procalcitonin, alanine transaminase, aspartate aminotransferase, and lactate dehydrogenase were significantly higher in the severe MPP cohort than in the mild MPP group. Paradoxically, the neutrophil count was significantly higher in the mild MPP group than in the severe MPP group. More importantly, the incidence of myocardial damage was significantly higher in the severe MPP group than in mild MPP cases. However, it is unknown whether there is a causal link between severe MPP and myocardial damage; therefore, to ascertain this hypothesis, future research is recommended.
- Citation: Yusuf SO, Chen P. Clinical characteristics of community-acquired pneumonia in children caused by mycoplasma pneumoniae with or without myocardial damage: A single-center retrospective study. World J Clin Pediatr 2023; 12(3): 115-124
- URL: https://www.wjgnet.com/2219-2808/full/v12/i3/115.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v12.i3.115
Community-acquired pneumonia (CAP) is a pulmonary infection (parenchyma or pleura) acquired outside the hospital[1]. CAP is a significant cause of inpatient hospitalization and mortality in children. The annual incidence of CAP requiring hospitalization is over 20 million[2]. In 2015, data from Asia showed that 15% of all fatalities in children under five years of age were caused by pneumonia, with an estimated 922000 children in this age group dying[3]. Similarly, global mortality is estimated at 14%, ranging from 2% of those treated as outpatients to 37% of those admitted to intensive care units (ICUs)[4].
In China, the incidence density of pneumonia in children under five years of age is 0.06 to 0.27% per year. According to a systematic review of data from the China Mortality Surveillance System from 2001 to 2015, the mortality rate for children under the age of five was 153.2 per 100000 live births[5]. Multiple microorganisms drive the pathophysiology of CAP. Classical typical pneumonia is caused by bacteria, while atypical pneumonia is caused by atypical pathogens, such as Mycoplasma pneumoniae (MP), Legionella pneumoniae, and Chlamydia pneumoniae. These three pathogens combined are responsible for 21% to 28% of adult CAP worldwide[6]. MP is one of the most common pathogens causing respiratory illness in adolescents and children, accounting for up to 40% of CAP in children above five years of age[7].
Mycoplasma is a small cell wall-deficient prokaryote. Microbes are cell-free and malleable organisms that can grow and proliferate in a cell-free environment[8].
According to the mycoplasma pneumoniae pneumonia (MPP) diagnostic criteria of CAP in children, MP patients are classified as mild mycoplasma pneumoniae pneumonia (MMPP) and severe myco
SMPP is defined as MPP with protracted fevers, worsening clinical symptoms, and persistent radiological features following a week-long routine of macrolide antibiotic therapy[9]. Similarly, SMPP is defined as a fever (> 38.5°C), persistent cough for more than two weeks, CRP > 40 mg/L, radiological features showing consolidation in two or more pulmonary lobes, and extrapulmonary complications were the criteria to diagnose SMPP as per the algorithm of community-acquired pneumonia in children[10,11].
Consensus on the definition of SMPP is lacking because it can affect any part of the body, including the musculoskeletal system, neurological system, hematological system, and skin[12]. However, MP infection most severely affects the respiratory system; hence, respiratory and metabolic acid-base disturbances may indirectly indicate severe disease. Therefore, prompt and effective treatment is recommended[13]. Moreover, immune evasion by specific pathogens via the transmission of host-derived lipid membranes can lead to uninhibited proliferation, resulting in overt clinical symptoms and a worsening disease course[14].
MP is contagious and can be transmitted through aerosols from coughing and sneezing, causing acute upper and lower respiratory tract inflammation[15]. These respiratory pathogens are ubiquitous on environmental surfaces, and mucous membrane contact with these contaminated surfaces aids in disease transmission. The propensity for children to play with toys and have poor hand hygiene make children a high-risk and susceptible group in daycare and school settings[16]. MP infection also causes nonrespiratory symptoms, including myocarditis, arthritis, and thrombosis, in newborns. If left untreated, multiple organ failures may ensue[17]. Acute myocardial injury in people hospitalized with community-acquired pneumonia (CAP) is caused by many different factors. These factors include type-2 myocardial infarction with or without prior coronary artery disease (CAD) due to an imbalance between demand and supply and non-CAD myocardial damage caused by toxins, direct myocardial infection, inflammatory mediators, and stress-induced cardiomyopathy[18]. MP-induced myocarditis is usually confirmed via an electrocardiogram (ECG), which shows conduction arrhythmias and myocardial atrioventricular block. Chest pain can be a sign of myocarditis or pericarditis and has been linked to anti-cardiolipin antibodies[19].
Although uncommon, the prevalence of myocarditis in children with MP ranges from 1% to 8%, and the prevalence rate is slightly higher in adults than in children[20]. Mycoplasma-associated carditis (myo- or pericarditis) is a rare condition that has affected 1%-5% of patients since Pönkä's study in 1979. However, individuals with mycoplasma carditis seem to be older on average. This study supports Pönkä's conclusion that the mean age was 32. This recurring finding is not fully understood. However, it may be related to the increased rates of mycoplasma infection in older persons appearing as pneumonia, which is more common in patients with carditis[21].
This study aims to assess the differences in the clinical characteristics of children diagnosed with CAP caused by MP and to further identify the cohort of patients who developed MP-induced myocardial damage and those without heart failure.
This study was a single-center retrospective study. We identified children between 2 mo and 16 years of age with clinical and radiological findings consistent with CAP admitted to the inpatient department of the Second Hospital of Jilin University, Changchun, China, from January 2019 to December 2019. The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of The Second Hospital of Jilin University (In 2022, research review No. 073).
(1) Age between 2 mo and 16 years old; (2) Children admitted to the inpatient pediatric department of the Second Hospital of Jilin University in 2019 with diagnosed MPP; (3) Radiological findings, such as interstitial infiltration, linear opacities, patchy infiltration, segmental or lobar consolidation, reticulonodular infiltrate, or pleural effusion; and (4) Diagnosed with MP by serology with an immunoglobulin M (IgM) titer of > 1.1 considered positive. MP-IgM was identified by enzyme-linked immunosorbent assay (ELISA) as serum MP antibodies.
(1) Children aged less than two months and greater than 16 years old; (2) Children with chronic respiratory tract infection or other pulmonary illness; (3) Community-acquired pneumonia caused by other species, such as chlamydia pneumonia; and (4) Concomitant other pathogenic infections
Clinical data were collected uniformly from the Second Hospital of Jilin University pediatrics inpatient department database. Based on the following symptoms, all patients were diagnosed with CAP: Cough, tachypnea, chest retractions, fever, wheezing, crackles or reduced breath sounds, and radiological abnormalities with pulmonary fever, wheezing, or infiltrates. IgM titer (> 1.1), age, gender, white blood cell (WBC) count, lymphocyte percentage, neutrophil percentage, C-reactive protein (CRP), high-sensitivity C-reactive protein, procalcitonin, alanine transaminase (ALT), aspartate aminotransferase (AST), creatine phosphokinase and lactate dehydrogenase (LDH) were obtained from the patient chart. Fever (> 38.5°C), persistent cough for more than two weeks, CRP > 40 mg/L, and intra- and extrapulmonary complications were the criteria to diagnose SMPP per the algorithm of community-acquired pneumonia in children. White blood cell (WBC) count > 15000 cells/mL or < 5500 cells/mL for children < 5 years old and WBC count > 11000 cells/mL or < 3000 cells/mL for children ≥ 5 years old were considered abnormal. Fever (> 38.5°C), persistent cough for more than two weeks, CRP > 40 mg/L, and intra- and extrapulmonary complications were the criteria to diagnose SMPP per the algorithm of community-acquired pneumonia in children. According to myocardial damage, an ECG was performed to check for different heart conditions. We also performed blood tests to check for proteins associated with heart damage, such as troponin.
The data were populated in Microsoft Excel 2016. Categorical data and comparisons among these two groups between mild and severe MPP were analyzed using the chi-squared or Fisher's exact tests. An independent t test was used to compare the differences between continuous variables. Statistical analyses were performed using IBM SPSS statistics 26, and statistical significance was set at a P value of < 0.05. The results are presented as the mean ± SD for numerical variables.
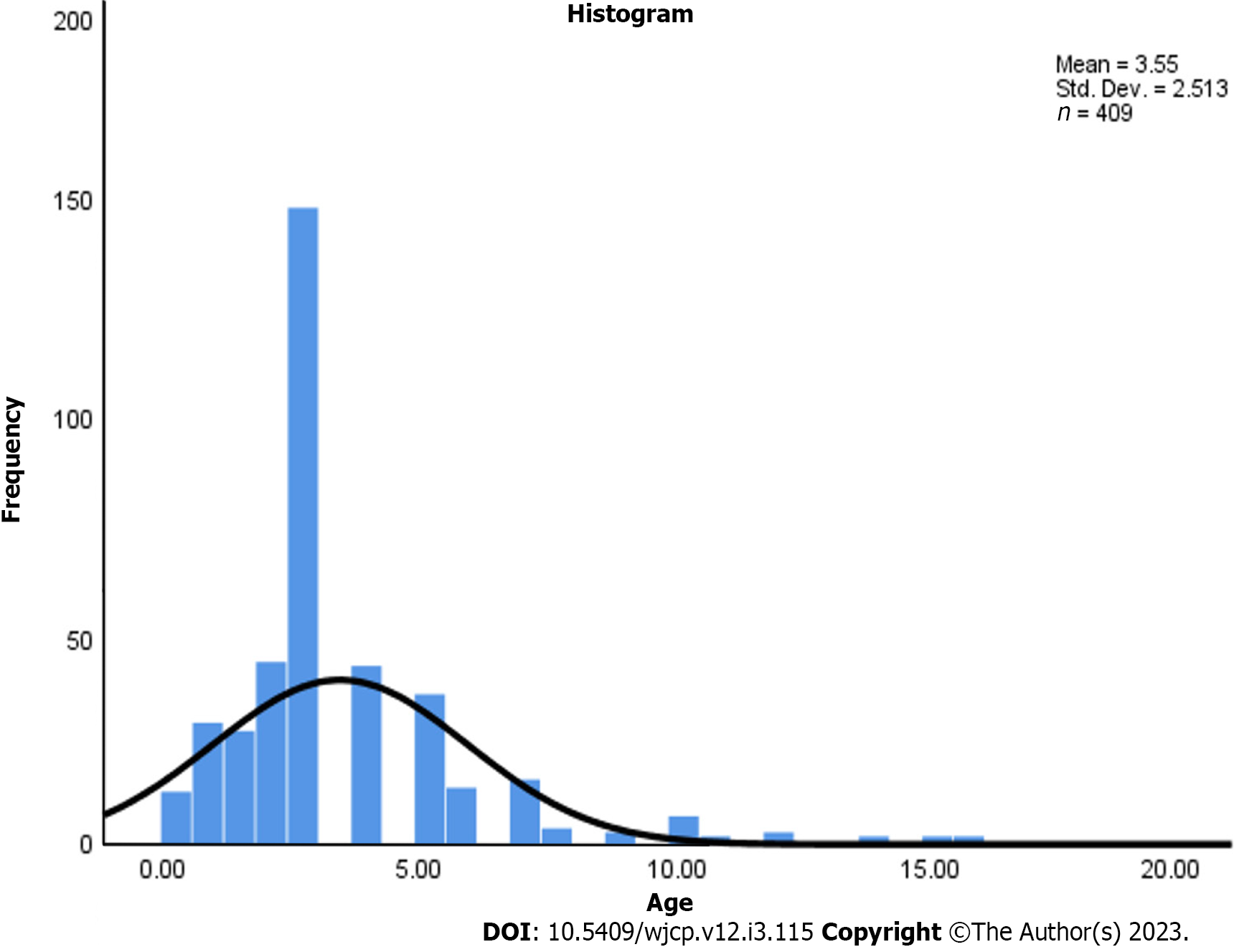
In our study, 409 children with CAP were included, among whom 214 (52.3%) were males and 195 (47.7%) were females. MP infection was more prevalent in males than females (Table 1). Among the mild MPP cases, 232 patients were < 5 years old, accounting for 77.6% of patients: 67 patients were ≥ 5 years old, accounting for 22.4% of patients. In severe cases, 85 patients were < 5 years old, accounting for 77.3% old, while 25 patients were ≥ 5 years, accounting for 22.7% of patients, as shown (Table 2 and Figure 1).
| Gender | Frequency | Percentage |
| Female | 195 | 47.68 |
| Male | 214 | 52.32 |
| Total | 409 | 100.00 |
| Age group | Total | Mild MPP | Severe MPP |
| < 5 yr | 317 | 232 (77.6) | 85 (77.3) |
| ≥ 5 yr | 92 | 67 (22.4) | 25 (22.7) |
| Total | 409 | 299 | 110 |
The seasonal distribution of MP infection: The seasonal distribution of hospitalized children with mild MPP (102/299, 34.10%) and severe MPP indicated that 41/110 children (37.30%) were diagnosed during winter. In comparison, mild MPP (80/299, 26.80%) and severe MPP (28/110, 25.50%) patients were diagnosed during the spring. Moreover, 28/299 (9.40%) patients with mild MPP and 28/110 (10.00%) patients with severe MPP were diagnosed during summer, whereas 89/299 (29.80%) patients with mild MPP and 30/110 (27.3%) patients with severe MPP were diagnosed during autumn. These results indicated that the prevalence rate of M pneumonia in hospitalized children was highest in winter (Table 3).
| Season | Mild MPP | Severe MPP |
| Winter | 102 (34.10) | 41 (37.30) |
| Spring | 80 (26.80) | 28 (25.50) |
| Summer | 28 (9.40) | 11 (10.00) |
| Autumn | 89 (29.80) | 30 (27.30) |
According to Pearson's chi-square test, a significant difference was not detected between seasons according to severe MPP cases and mild MPP cases (χ2 = 0.487, P > 0.05).
Clinical symptoms of children in both groups were compared head-to-head. In our study, the incidences of mild and severe MPP cases did not differ by sex or age (t = 0.54, P > 0.05, t = 0.69, P > 0.05, respectively) (Table 4). Common clinical symptoms included phlegm (31/409, 7.6%), hoarseness (22/409, 5.3%), rhinorrhea (12/409, 3%), diarrhea (9/409, 2.2%), vomiting (7/409, 1.7%), and rash (7/409, 1.7%). These symptoms, signs, or physical findings did not significantly differ between mild and severe MPP.
| Parameters | Mild MPP (n = 299) | Severe MPP (n = 110) | Z | P value |
| Gender | 1.48 ± 0.501 | 1.45 ± 0.500 | 0.54 | 0.58 |
| Age (yr) | 3.59 ± 2.63 | 3.40 ± 2.16 | 0.69 | 0.49 |
| Fever (℃) | 38.73 ± 0.86 | 38.71 ± 0.83 | 0.163 | 0.87 |
| Duration of fever | 4.27 ± 3.37 | 5.76 ± 5.39 | -2.72 | 0.007 |
| Duration of cough | 5.98 ± 6.9 | 11.4 ± 10.33 | -5.103 | < 0.001 |
| WBC (109/L) | 8.98 ± 4.77 | 9.34 ± 4.23 | -0.691 | 0.49 |
| Lymphocyte (%) | 35.87 ± 14.04 | 38.93 ± 18.29 | -1.59 | 0.114 |
| Neutrophil (%) | 54.48 ± 15.46 | 49.98 ± 20.28 | 2.113 | 0.036 |
| Neutrophil to lymphocyte ratio | 2.06 ± 1.702 | 2.046 ± 2.20 | 0.078 | 0.938 |
| CRP (mg/L) | 12.59 ± 19.24 | 10.48 ± 17.82 | 1 | 0.318 |
| hsCRP (mg/L) | 6.1 ± 5.84 | 8.35 ± 7.5 | -2.834 | 0.005 |
| PCT (ng/mL) | 0.42 ± 0.93 | 0.76 ± 3.15 | -1.097 | 0.27 |
| Alanine transaminase (U/L) | 15.95 ± 8.42 | 24.97 ± 37.32 | -2.511 | 0.013 |
| Aspartate aminotransferase (U/L) | 32.25 ± 20.31 | 41.37 ± 30.11 | -2.939 | 0.004 |
| Creatine kinase (U/L) | 97.3 ± 110.38 | 101.4 ± 83 | -0.354 | 0.724 |
| Lactate dehydrogenase (U/L) | 286.55 ± 69.12 | 317.55 ± 93.77 | -3.633 | < 0.001 |
However, auscultated respiratory sounds significantly differed between severe and mild MPP cases in our study (χ2 = 11.915, P < 0.05) (Table 5).
| Parameters | Mild MPP | Severe MPP | χ2 | P value | |
| 1 Auscultation | Crackles | 37 (12.40) | 23 (20.90) | 11.915 | 0.008 |
| Normal | 141 (47.20) | 35 (31.80) | |||
| Wet rales | 7 (2.30) | 7 (6.40) | |||
| Wheezing | 114 (38.10) | 45 (40.90) | |||
| 2 Myocardial damage | Yes | 0 (0.0) | 52 (47.30) | 157.078 | < 0.001 |
| No | 299 (100) | 58 (52.70) |
Similarly, myocardial damage significantly differed between mild and severe MPP cases (χ2 = 157.078, P < 0.05). Specifically, the incidence of myocardial damage was higher in severe MPP cases than in mild MPP cases (Table 5).
Independent t test samples showed that the duration of fever was significantly different between severe and mild MPP (t = -2.72, P < 0.05). The duration of cough was also significantly longer in severe MPP cases than in mild MPP cases (t = -5.103, P < 0.05). Conversely, the neutrophil percentage was significantly higher in mild MPP cases than in severe MPP cases (t = 2.113, P < 0.05) (Table 4).
High sensitivity C-reactive protein was significantly higher in severe MPP cases than in mild MPP cases (t = -2.834, P < 0.05). Both ALT and AST were significantly higher in severe MPP cases than in mild MPP cases (t = -2.511, P < 0.05 and t = -2.939, P < 0.05, respectively). Lactate dehydrogenase (LDH) was also significantly higher in severe MPP cases than in mild MPP cases (t = -2.939, P < 0.05). The remaining variables did not significantly differ (P > 0.05) between severe and mild MPP (Table 4).
This work is the first study to discuss the relationship between Mycoplasma pneumoniae pneumonia and myocardial damage in children. We investigated the clinical and laboratory characteristics of children with CAP caused by MP and compared them with those with mild MPP and severe MPP. Our study showed that MP was the leading cause of CAP in hospitalized children in Changchun, China, in 2019.
CAP is a nonhospital-acquired illness of the lower respiratory tract[22]. MP is one of the most common infections in children with CAP. MPP is usually self-limiting and is adequately treated with macrolides. Conversely, severe MPP is common and may result in problems such as pleural effusion, atelectasis, and lung consolidation[23]. In recent years, an upsurge in the number of refractory, severe, and even deadly MPP cases has been reported[24]. The pathogen MP, discovered in the 1940s, causes a wide range of clinical symptoms with a unique seasonal pattern; it is most active in the fall/early winter, with favorable peak rates of 3% to 4% between September and January[25]. MP is a benign, self-limiting disease; however, missed early detection opportunities, clinical misdiagnosis, and drug resistance often lead to poor outcomes[26].
Following the outbreak of the coronavirus disease 2019 (COVID-19) pandemic, comprehensive testing and positivity rates of MP plummeted compared to previous years[27]. A national multicenter prospective survey of all-age patients (52.2% were aged 18 years) with acute respiratory tract infections in China between 2009 and 2019 revealed a peak in the positivity rate of MP in 2011 and a gradual upward trend in the positivity rate of MP from 2015 to 2019 (the majority being pediatric patients)[28]. MP is contagious and can be transmitted through aerosols from coughing and sneezing, causing acute upper and lower respiratory tract inflammation. The positivity rate for MP was low during 2020, which coincided with the COVID-19 era, suggesting that the implementation of nationwide countermeasures, such as strict face mask-wearing and population quarantine measures, may have also effectively prevented the concurrent spread of MP[29]. Our data showed that the rate of MP in hospitalized children was higher in winter than in autumn, which corroborated previously published data[30]. Conversely, a study from Serbia reported that the highest number of MP infections was recorded in the fall (33.3%), and this rate was higher than that in winter (29.2%)[31]. Similar studies on the seasonality of MP infection from Shanghai, China, showed a peak in spring that declined precipitously until the following summer[29]. However, some studies from Italy and Tunisia found no seasonal correlation in MP infection[31]. In our research, we found that children < 5 years of age were likely to have more severe MPP than mild MPP, accounting for 77.3% of cases. This finding was similar to data from a study conducted in Luzhou, China, which reported an MP positivity rate of 75% in children between the ages of 5 and 1 year[32]. However, some studies also report a higher risk of MP infection in children above > 5 years of age compared to those < 5 years of age[31]. Severe Mycoplasma infections are prevalent not only in children older than five years but also in those aged 2 to 4 years, and 10% of MP-infected patients admitted to the ICU were less than two years old. In addition, prior research identified MP as a significant cause of CAP in babies younger than one year[33]. Our findings are not easily compared to those of other studies because of the wide range of epidemiological conditions, varying populations, and different diagnostic modalities.
We also compared mild and severe MPP and found that the duration of fever and cough was longer in the severe MPP group than in the mild MPP group. Similarly, the high-sensitivity C-reactive protein, alanine transaminase, aspartate aminotransferase, and lactate dehydrogenase levels were significantly higher in the severe MPP cohort than in the mild MPP group. Paradoxically, the neutrophil count was significantly higher in the mild MPP group than in the severe MPP group, and we found that the neutrophil-to-lymphocyte ratio (NLR) did not significantly between mild MPP and severe MPP cases in children (P > 0.05). In another study of adults, the NLR was shown to have good prognostic value for short- and long-term mortality, ICU admission, and rehospitalization. This ratio is distinguished by high death and morbidity rates, particularly among older individuals. As a result, the NLR was linked with post-CAP mortality better than standard pneumonia ratings (Patient-specific instrumentation; and Confusion, Urea, Respiratory rate, and Blood pressure, aged 65 and older, CURB-65)[34].
As a result, novel, simple, specific, and low-cost biomarkers to diagnose and monitor CAP are still needed. Neutrophilia and lymphocytopenia are innate immune system physiological responses to systemic inflammation. Lymphocytopenia is characterized by rapid apoptosis and the margination of lymphocytes in the reticuloendothelial system, liver, and splanchnic lymphatic system as well as lymphocyte redistribution throughout the lymphatic system. Neutrophilia is the opposite phenomenon and occurs during systemic inflammation due to neutrophil emargination and growth factor stimulation of stem cells (granulocyte-colony stimulating factor)[34]. In previously published research, WBC count and CRP were also demonstrated to be possible markers of pneumonia; however, they did not play a significant role in determining the causative pathogen of pneumonia, similar to our findings[35]. More importantly, the incidence of myocardial damage was significantly higher in the severe MPP group than in the mild MPP group. The remaining parameters, did not significantly differ between the two cohorts (P > 0.05).
Cardiac complications caused by MP are infrequent, with a prevalence rate of 1.0-8.5% and a slightly higher prevalence rate in adults than in children. Almost half of MP patients showed symptoms or evidence of cardiac abnormalities at a mean of 16 mo following infection. Constrictive pericarditis caused by MP infection has also been documented[20].
In some instances, MP-related extrapulmonary illnesses may be this infection's most obvious clinical issue, particularly in young people and children. MP infection has also been connected to several extrarespiratory symptoms. Numerous disorders affecting the skin, musculoskeletal, neurological, hematological, digestive, and renal systems have been described in pediatric populations[36]. CD4- T cells, B cells, and plasma cells invade the lungs, causing additional immunological amplification, including lymphocyte proliferation, immunoglobulin synthesis, and the release of proinflammatory cytokines. Total immunoglobulin, immunoglobulin A (IgA), IgM, and IgG levels in serum have previously been shown to rise throughout the convalescent phase of the illness; furthermore, IgE specific to MP is produced during infection[37]. The concentrations of serum cytokines, such as Interleukins (IL) IL-1, IL-4, and IL-6, also increase. The intensity of inflammation is determined by its degree. The production of acute-phase proteins, such as C-reactive protein, the amount of leukocytosis, the level of fibrinogen, and the rate of erythrocyte sedimentation are all pathogenetically relevant in pneumonia, determining the severity of the illness and increased mortality[38].
Our study highlighted the clinical parameters that should be focused on to differentiate mild and severe MPP cases. These paratmeters are crucial for pediatricians because they allow for rapid diagnosis and prompt treatment in cases of severe MPP.
This study's limitations include its retrospective nature and small sample size. Some data were omitted because they were missing and could not be extrapolated to represent hospitalized children with CAP throughout the year. Moreover, only cases were analyzed and this study lacked a control group.
Mycoplasma pneumoniae is the leading cause of community-acquired pneumonia. We found a significantly higher incidence of myocardial damage in children with severe MPP than in those with mild MPP. However, a causal link between severe MPP and myocardial damage has not yet been identified; therefore, future research is recommended to confirm this hypothesis.
Community-acquired pneumonia (CAP) is a significant cause of inpatient hospitalization and mortality in children.
This is crucial for pediatricians as it would enable us to make a quick diagnosis and consequently prompt treatment in case of severe mycoplasma pneumoniae pneumonia (MPP).
Our study highlighted which clinical parameters should be focused on to differentiate between mild and severe MPP.
We identified children between 2 mo and 16 years of age with clinical and radiological findings consistent with CAP.
We found that the duration of fever and cough was longer in the severe MPP group than in the mild MPP group. Similarly, the high sensitivity C-reactive protein levels, procalcitonin, alanine transaminase, aspartate aminotransferase, and lactate dehydrogenase were significantly higher in the severe MPP cohort than in the mild MPP group. Paradoxically, the neutrophil count was significantly higher in the mild MPP group than in the severe MPP group.
The incidence of myocardial damage was significantly higher in the severe MPP group than in mild MPP cases.
It is unknown whether there is a causal link between severe MPP and myocardial damage; therefore, to ascertain this hypothesis, future research is recommended.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Moshref RH, Saudi Arabia S-Editor: Liu JH L-Editor: A P-Editor: Zhang XD
| 1. | Leung AKC, Wong AHC, Hon KL. Community-Acquired Pneumonia in Children. Recent Pat Inflamm Allergy Drug Discov. 2018;12:136-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 2. | Otheo E, Rodríguez M, Moraleda C, Domínguez-Rodríguez S, Martín MD, Herreros ML, Vázquez C, Folgueira MD, Pérez-Rivilla A, Jensen J, López A, Berzosa A, Sanz de Santaeufemia FJ, Jiménez AB, Sainz T, Llorente M, Santos M, Garrote E, Muñoz C, Sánchez P, Illán M, Coca A, Barrios A, Pacheco M, Arquero C, Gutiérrez L, Epalza C, Rojo P, Serna-Pascual M, Mota I, Moreno S, Galán JC, Tagarro A; VALS-DANCE and PCAPE Working Groups. Viruses and Mycoplasma pneumoniae are the main etiological agents of community-acquired pneumonia in hospitalized pediatric patients in Spain. Pediatr Pulmonol. 2022;57:253-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Chi H, Huang YC, Liu CC, Chang KY, Lin HC, Chang LY, Ho YH, Tsao KC, Mu JJ, Huang LM, Hsieh YC; Taiwan Pediatric Infectious Disease Alliance. Characteristics and etiology of hospitalized pediatric community-acquired pneumonia in Taiwan. J Formos Med Assoc. 2020;119:1490-1499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Rejas J, Sicras-Mainar A, Sicras-Navarro A, Lwoff N, Méndez C. All-cause community acquired pneumonia cost by age and risk in real-world conditions of care in Spain. Expert Rev Pharmacoecon Outcomes Res. 2022;22:853-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 5. | Ning G, Wang X, Wu D, Yin Z, Li Y, Wang H, Yang W. The etiology of community-acquired pneumonia among children under 5 years of age in mainland China, 2001-2015: A systematic review. Hum Vaccin Immunother. 2017;13:2742-2750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 6. | Liu L, Maharjan S, Sun JL, Li YC, Cheng HJ. Prevalence and clinical characteristics of septicemia in children with Mycoplasma pneumoniae pneumonia. J Int Med Res. 2021;49:3000605211021733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 7. | Zhang Y, Huang Y, Ai T, Luo J, Liu H. Effect of COVID-19 on childhood Mycoplasma pneumoniae infection in Chengdu, China. BMC Pediatr. 2021;21:202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Naghib M, Hatam-Jahromi M, Niktab M, Ahmadi R, Kariminik A. Mycoplasma pneumoniae and toll-like receptors: A mutual avenue. Allergol Immunopathol (Madr). 2018;46:508-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Gong H, Sun B, Chen Y, Chen H. The risk factors of children acquiring refractory mycoplasma pneumoniae pneumonia: A meta-analysis. Medicine (Baltimore). 2021;100:e24894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 10. | Yan C, Xue G, Zhao H, Feng Y, Li S, Cui J, Ni S, Sun H. Molecular and clinical characteristics of severe Mycoplasma pneumoniae pneumonia in children. Pediatr Pulmonol. 2019;54:1012-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 11. | Fan L, Li D, Zhang L, Hao C, Sun H, Shao X, Xu J, Chen Z. Pediatric clinical features of Mycoplasma pneumoniae infection are associated with bacterial P1 genotype. Exp Ther Med. 2017;14:1892-1898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Zhou H, Chen X, Li J. Effect of Methylprednisolone Plus Azithromycin on Fractional Exhaled Nitric Oxide and Peripheral Blood Eosinophils in Children with Refractory Mycoplasma Pneumoniae Pneumonia. J Coll Physicians Surg Pak. 2022;32:33-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Waites KB, Xiao L, Liu Y, Balish MF, Atkinson TP. Mycoplasma pneumoniae from the Respiratory Tract and Beyond. Clin Microbiol Rev. 2017;30:747-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 488] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 14. | Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39:529-539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1895] [Cited by in RCA: 1790] [Article Influence: 223.8] [Reference Citation Analysis (0)] |
| 15. | Jiang Z, Zhou R, Leung PHM, Deng Z, Li S. An attenuated multiple genetic mutant of Mycoplasma pneumoniae imparts good immuno-protection against M. pneumoniae pneumonia in BALB/c mice. Microb Pathog. 2022;165:105463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 16. | Shah SS. Mycoplasma pneumoniae as a Cause of Community-Acquired Pneumonia in Children. Clin Infect Dis. 2019;68:13-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Jiang Y, Wang W, Zhang Z, Ma X, Sang Y, Wang J, Xu G, Feng Q, Zhao S. Serum amyloid a, C-reactive protein, and procalcitonin levels in children with Mycoplasma pneumoniae infection. J Clin Lab Anal. 2022;36:e24265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 18. | Khatib M, Elbaz-Greener G, Nitzan O, Soboh S, Peretz A, Hazanov E, Kinany W, Halahla Y, Grosman-Rimon L, Houle H, Amir O, Carasso S. Unmasking Myocardial Dysfunction in Patients Hospitalized for Community-Acquired Pneumonia Using a 4-Chamber 3-Dimensional Volume/Strain Analysis. J Digit Imaging. 2022;35:1654-1661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 19. | Nagashima M, Higaki T, Satoh H, Nakano T. Cardiac thrombus associated with Mycoplasma pneumoniae infection. Interact Cardiovasc Thorac Surg. 2010;11:849-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Li CM, Gu L, Yin SJ, Yang R, Xie Y, Guo XZ, Fu YX, Cheng D. Age-specific Mycoplasma pneumoniae pneumonia-associated myocardial damage in children. J Int Med Res. 2013;41:1716-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Paz A, Potasman I. Mycoplasma-associated carditis. Case reports and review. Cardiology. 2002;97:83-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, Cooley LA, Dean NC, Fine MJ, Flanders SA, Griffin MR, Metersky ML, Musher DM, Restrepo MI, Whitney CG. Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45-e67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1279] [Cited by in RCA: 2165] [Article Influence: 433.0] [Reference Citation Analysis (36)] |
| 23. | Yang M, Meng F, Gao M, Cheng G, Wang X. Cytokine signatures associate with disease severity in children with Mycoplasma pneumoniae pneumonia. Sci Rep. 2019;9:17853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Liu J, Zhao F, Lu J, Xu H, Liu H, Tang X, Yang H, Zhang J, Zhao S. High Mycoplasma pneumoniae loads and persistent long-term Mycoplasma pneumoniae DNA in lower airway associated with severity of pediatric Mycoplasma pneumoniae pneumonia. BMC Infect Dis. 2019;19:1045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Kim CH, Lee J. Mycoplasma pneumoniae Pleural Effusion in Adults. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 26. | Zhou Y, Wang J, Chen W, Shen N, Tao Y, Zhao R, Luo L, Li B, Cao Q. Impact of viral coinfection and macrolide-resistant mycoplasma infection in children with refractory Mycoplasma pneumoniae pneumonia. BMC Infect Dis. 2020;20:633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 27. | Rothstein TE, Cunningham SA, Rieke RA, Mainella JM, Mutchler MM, Patel R. Macrolide Resistance in Mycoplasma pneumoniae, Midwestern United States, 2014 to 2021. Antimicrob Agents Chemother. 2022;66:e0243221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Li ZJ, Zhang HY, Ren LL, Lu QB, Ren X, Zhang CH, Wang YF, Lin SH, Zhang XA, Li J, Zhao SW, Yi ZG, Chen X, Yang ZS, Meng L, Wang XH, Liu YL, Wang X, Cui AL, Lai SJ, Jiang T, Yuan Y, Shi LS, Liu MY, Zhu YL, Zhang AR, Zhang ZJ, Yang Y, Ward MP, Feng LZ, Jing HQ, Huang LY, Xu WB, Chen Y, Wu JG, Yuan ZH, Li MF, Wang Y, Wang LP, Fang LQ, Liu W, Hay SI, Gao GF, Yang WZ; Chinese Centers for Disease Control and Prevention (CDC) Etiology of Respiratory Infection Surveillance Study Team. Etiological and epidemiological features of acute respiratory infections in China. Nat Commun. 2021;12:5026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 233] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 29. | Chen J, Zhang J, Lu Z, Chen Y, Huang S, Li H, Lin S, Yu J, Zeng X, Ji C, Zheng Y, Dai F, Dong W, Xu H, Chen W, Jin X, Cui Z, Qiao J, Qin W, Chen H, Jiang W, Zhang X, Song J, Shao J, Su W, Wang C, Liu F, Zhao Y, Zou Y, Guo R, Zhang L, Wu J, Yuan S, Tang M, Wu Y, Lin J, Chen X, Sun X, Yin Y. Mycoplasma pneumoniae among Chinese Outpatient Children with Mild Respiratory Tract Infections during the Coronavirus Disease 2019 Pandemic. Microbiol Spectr. 2022;10:e0155021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 30. | Su M, Wang Q, Li D, Wang LL, Wang CY, Wang JL, Zhang Q, Du LY, Liu JY, Xie GC. Prevalence and clinical characteristics of hospitalized children with community-acquired Mycoplasma pneumoniae pneumonia during 2017/2018, Chengde, China. Medicine (Baltimore). 2021;100:e23786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Medjo B, Atanaskovic-Markovic M, Radic S, Nikolic D, Lukac M, Djukic S. Mycoplasma pneumoniae as a causative agent of community-acquired pneumonia in children: clinical features and laboratory diagnosis. Ital J Pediatr. 2014;40:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 32. | Chen A, Song L, Chen Z, Luo X, Jiang Q, Yang Z, Hu L, He J, Zhou L, Yu H. Immunoglobulin M profile of viral and atypical pathogens among children with community acquired lower respiratory tract infections in Luzhou, China. BMC Pediatr. 2019;19:280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Lee KL, Lee CM, Yang TL, Yen TY, Chang LY, Chen JM, Lee PI, Huang LM, Lu CY. Severe Mycoplasma pneumoniae pneumonia requiring intensive care in children, 2010-2019. J Formos Med Assoc. 2021;120:281-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 34. | Cataudella E, Giraffa CM, Di Marca S, Pulvirenti A, Alaimo S, Pisano M, Terranova V, Corriere T, Ronsisvalle ML, Di Quattro R, Stancanelli B, Giordano M, Vancheri C, Malatino L. Neutrophil-To-Lymphocyte Ratio: An Emerging Marker Predicting Prognosis in Elderly Adults with Community-Acquired Pneumonia. J Am Geriatr Soc. 2017;65:1796-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 138] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 35. | Hu S, Zhu Y, Dong D, Wang B, Zhou Z, Wang C, Tian J, Peng Y. Chest Radiographs Using a Context-Fusion Convolution Neural Network (CNN): Can It Distinguish the Etiology of Community-Acquired Pneumonia (CAP) in Children? J Digit Imaging. 2022;35:1079-1090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 36. | Poddighe D. Extra-pulmonary diseases related to Mycoplasma pneumoniae in children: recent insights into the pathogenesis. Curr Opin Rheumatol. 2018;30:380-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 37. | Stelmach I, Podsiadłowicz-Borzecka M, Grzelewski T, Majak P, Stelmach W, Jerzyńska J, Popławska M, Dziadek J. Humoral and cellular immunity in children with Mycoplasma pneumoniae infection: a 1-year prospective study. Clin Diagn Lab Immunol. 2005;12:1246-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Aminjonovich AA. Treatment and diagnostic methods of pneumonia in children. J Adv Res Stab. 2022;16:560-566. |