Published online Sep 9, 2021. doi: 10.5409/wjcp.v10.i5.106
Peer-review started: March 24, 2021
First decision: April 29, 2021
Revised: April 29, 2021
Accepted: July 2, 2021
Article in press: July 2, 2021
Published online: September 9, 2021
Processing time: 165 Days and 8.9 Hours
As long as oral poliovirus vaccine (OPV) is used, the potential risk for the emer
We report a case of Sabin-like type 1 poliovirus infection in an immunocompetent 17-mo-old child after receiving four scheduled doses of OPV. Somehow, the four doses did not confer full protection, possibly because of interference created by other enteroviruses.
The surveillance of vaccine-related polioviruses has important implications for improving health policies and vaccination strategies. Missed cases of vaccine-related poliovirus infection might pose a potential risk to global poliovirus era
Core Tip: In this study, we report an unusual case of Sabin-like type 1 poliovirus infection in an immunocompetent 17-mo-old child after receiving four scheduled doses of oral poliovirus vaccine (OPV). Somehow, the four doses did not confer full pro
- Citation: Taherkhani R, Farshadpour F. Pediatric case with vaccine-related poliovirus infection: A case report. World J Clin Pediatr 2021; 10(5): 106-111
- URL: https://www.wjgnet.com/2219-2808/full/v10/i5/106.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v10.i5.106
The extensive use of trivalent oral poliovirus vaccine (tOPV) in routine and supplementary immunization schedules has led to the control and eradication of wild poliomyelitis in almost all parts of the world[1]. Despite inducing durable mucosal and humoral immunity, conferring immunity to unvaccinated individuals as well as low cost and easy oral administration, oral poliovirus vaccine (OPV) strains are genetically unstable[2]. On rare occasions, OPV might revert toward virulent strains by recombination with other enteroviruses in the human gut or reversion mutations under tropical conditions and with poor sanitation, hygiene and water quality, or under conditions of low vaccination coverage and poor population immunity[1,3]. Vaccine-related polioviruses (VRPVs) can cause vaccine-associated paralytic poliomyelitis (VAPP) in normal and immunodeficient vaccine recipients or their close contacts. However, the risk is much higher in immunodeficient individuals[4,5].
The emergence and spread of VRPVs are the biggest threats to the global poliovirus eradication program. A switch from live-attenuated OPV to inactivated poliovirus vaccine (IPV) seems to be the best option to eliminate the risk of VAPP emergence. However, in reality, OPV cessation is not feasible as long as global polio eradication is not achieved[5-7]. In polio-endemic regions or neighboring countries at risk of wild poliovirus importation and spread, OPV remains the vaccine of choice to block wild polio infection and transmission caused by induction of prolonged intestinal immunity even beyond its recipients[5,8]. Currently, we are on the horns of a dilemma. In these circumstances, timely detection and response to VRPVs need to be emphasized in countries using OPV to prevent paralysis development and community spread[6,9]. Here, we report a pediatric case of Sabin-like type 1 poliovirus infection at 17 mo of age after receiving four doses of tOPV.
A 17-mo-old girl from Bushehr city was admitted to Shohadaie Khalij-Fars Hospital with symptoms of fever (38.5°C-40°C), drowsiness, irritability, cough, rhinorrhea, vomiting, and generalized weakness.
On history, the child was immunocompetent and had no known illness. The immu
The child had no history of prior illness.
The child was immunocompetent and had no known illness.
A lumbar puncture (LP) was performed and antibiotic therapy with empiric antibiotics including vancomycin and ceftriaxone was initiated immediately. On the fourth day of hospitalization, her condition deteriorated, and the pediatrician referred her to the Pediatric Clinic of Namazi Hospital in Shiraz for further evaluation. On examination, reduced strength in all limbs, most notably in her lower extremities, and regression in her ability to sit and walk were noted. High-grade fever and conjunctivitis were the other clinical symptoms. An LP was repeated and cerebrospinal fluid (CSF) pleocyto
CSF analysis showed a clear appearance, lymphocytic pleocytosis, normal glucose, and a mild increase of protein levels. CSF bacterial culture was negative; viral culture and molecular assays were not performed. The diagnosis was aseptic meningitis.
There were no imaging examinations.
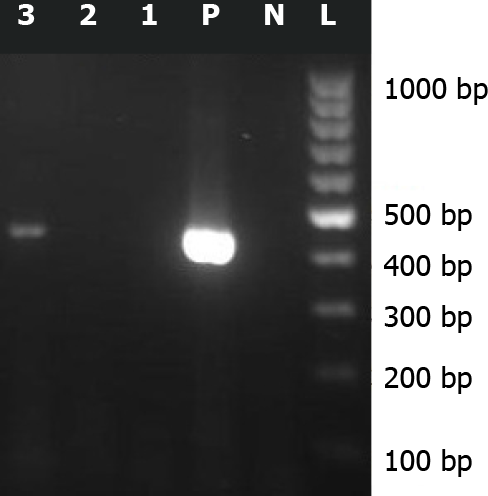
About 2 years after this event, a regional survey supported by Bushehr University of Medical Sciences (grant number 4359), was performed on leftover CSF samples of patients with a diagnosis of primary aseptic meningitis. The study was approved by the Ethical Committee of Bushehr University of Medical Sciences (reference number bpums.rec.1394.29). Sabin-like type 1 poliovirus was isolated from the CSF specimen of this patient by enterovirus reverse transcriptase-polymerase chain reaction assay (RT-PCR), targeting the 5’ untranslated region (5’ UTR) of the genome, followed by sequencing (Figure 1). The nucleotide sequence isolated from the CSF sample of this case was submitted to the GenBank sequence database (accession number: KX 011400.10).
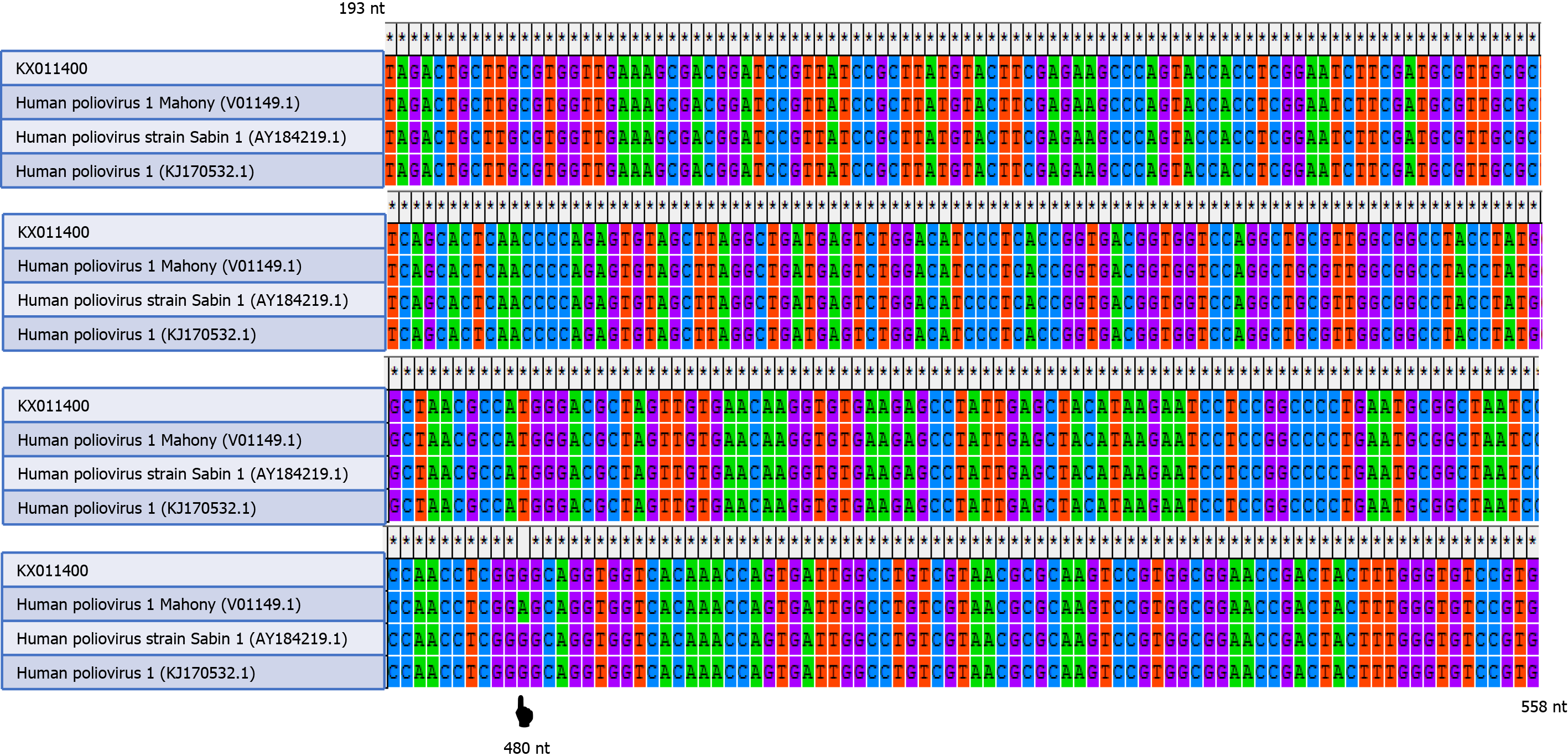
The nucleotide sequence of this case (KX011400.1) and the nucleotide sequences of wild-type poliovirus (human poliovirus 1 Mahoney), vaccine-derived poliovirus, and vaccine-strain poliovirus (Sabin type 1) were aligned by the ClustalW program in MEGA software version 4.0 (Biodesign Institute, Tempe, AZ, United States). A change of an A to a G was shown at position 480 of the 5’ UTR of the isolated sequence (Figure 2). The CSF sample was negative for nonpolio enteroviruses, mumps, herpes simplex virus types 1 and 2, cytomegalovirus, and varicella-zoster virus.
We present a case of Sabin-like type 1 poliovirus infection that was initially consistent with the diagnosis of aseptic meningitis. On further evaluation, a diagnosis of Ka
As Kawasaki disease was suspected, a single high-dose (2 g/kg) intravenous administration of immunoglobulin (IVIG) was given. However, the high-grade fever was not responsive to IVIG and persisted for approximately 8 d. Subsequently, the clinical symptoms were gradually improved. It is unclear whether immunoglobulin therapy facilitated the improvement of the clinical symptoms, or they improved spontaneously.
Following clinical improvement, the child was discharged from the hospital, but she had a mild fever, muscular weakness, and difficulty using her lower limbs for approximately 2 mo. At a 1-year follow-up, cardiac complications were not reported, and the strength of all her limbs was completely restored.
This is an unusual case of VRPV, as the child was immunocompetent and had received four doses of tOPV. Somehow, the four doses had not conferred full protection, possibly because of interference created by other enteroviruses. Of note, the child lives in a tropical area, where diarrheal diseases frequently occur. Neurovirulent reversion of OPV in the child’s gut is a possibility. However, the long interval between administration of the fourth dose of tOPV and onset of clinical symptoms, as well as the child’s immunocompetency make that unlikely. Other possibilities include the presence of a prolonged poliovirus excreter or the existence of circulating VRPVs in the environ
The VRPV surveillance has important implications for improving health policies and vaccination strategies. However, most cases of VRPV infection are captured through the acute flaccid paralysis surveillance system. Recognition of VRPVs remains an important challenge. Missed cases of VRPV infection pose a potential risk to global poliovirus eradication. As long as OPV is used, the potential risk of emergence of VRPVs remains[6]. VRPVs are clinically indistinguishable from wild polioviruses and are capable of causing paralytic poliomyelitis and circulating in society whenever the immunity coverage is reduced[2,6]. The emergence of VAPP is a health dilemma as devastating as wild polio. Therefore, the global withdrawal of OPV and shift toward the all-IPV schedule is the main objective of the polio eradication program[3].
In this study, we report an unusual case of Sabin-like type 1 poliovirus infection in an immunocompetent 17-mo-old child after receiving four scheduled doses of OPV. Somehow, the four doses did not confer full protection, possibly because of inter
Manuscript source: Invited manuscript
Specialty type: Virology
Country/Territory of origin: Iran
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Laassri M S-Editor: Wu YXJ L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Cassemiro KM, Burlandy FM, Barbosa MR, Chen Q, Jorba J, Hachich EM, Sato MI, Burns CC, da Silva EE. Molecular and Phenotypic Characterization of a Highly Evolved Type 2 Vaccine-Derived Poliovirus Isolated from Seawater in Brazil, 2014. PLoS One. 2016;11:e0152251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 2. | Burns CC, Diop OM, Sutter RW, Kew OM. Vaccine-derived polioviruses. J Infect Dis. 2014;210 Suppl 1:S283-S293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 236] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 3. | Pons-Salort M, Burns CC, Lyons H, Blake IM, Jafari H, Oberste MS, Kew OM, Grassly NC. Preventing Vaccine-Derived Poliovirus Emergence during the Polio Endgame. PLoS Pathog. 2016;12:e1005728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Shahmahmoodi S, Mamishi S, Aghamohammadi A, Aghazadeh N, Tabatabaie H, Gooya MM, Zahraei SM, Mousavi T, Yousefi M, Farrokhi K, Mohammadpour M, Ashrafi MR, Nategh R, Parvaneh N. Vaccine-associated paralytic poliomyelitis in immunodeficient children, Iran, 1995-2008. Emerg Infect Dis. 2010;16:1133-1136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Foiadelli T, Savasta S, Battistone A, Kota M, Passera C, Fiore S, Bino S, Amato C, Lozza A, Marseglia GL, Fiore L. Nucleotide variation in Sabin type 3 poliovirus from an Albanian infant with agammaglobulinemia and vaccine associated poliomyelitis. BMC Infect Dis. 2016;16:277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Li L, Ivanova O, Driss N, Tiongco-Recto M, da Silva R, Shahmahmoodi S, Sazzad HM, Mach O, Kahn AL, Sutter RW. Poliovirus excretion among persons with primary immune deficiency disorders: summary of a seven-country study series. J Infect Dis. 2014;210 Suppl 1:S368-S372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Aylward B, Yamada T. The polio endgame. N Engl J Med. 2011;364:2273-2275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Orenstein WA; Committee on Infectious Diseases. Eradicating polio: how the world's pediatricians can help stop this crippling illness forever. Pediatrics. 2015;135:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Farshadpour F, Taherkhani R. Molecular epidemiology of enteroviruses and predominance of echovirus 30 in an Iranian population with aseptic meningitis. J Neurovirol. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | McGoldrick A, Macadam AJ, Dunn G, Rowe A, Burlison J, Minor PD, Meredith J, Evans DJ, Almond JW. Role of mutations G-480 and C-6203 in the attenuation phenotype of Sabin type 1 poliovirus. J Virol. 1995;69:7601-7605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Georgescu MM, Balanant J, Macadam A, Otelea D, Combiescu M, Combiescu AA, Crainic R, Delpeyroux F. Evolution of the Sabin type 1 poliovirus in humans: characterization of strains isolated from patients with vaccine-associated paralytic poliomyelitis. J Virol. 1997;71:7758-7768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 61] [Article Influence: 2.2] [Reference Citation Analysis (0)] |