INTRODUCTION
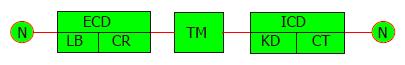
The epidermal growth factor receptor (EGFR) is nowadays being studied because of the possible role of using EGFR inhibitors in the cancer chemotherapy[1]. EGFR is the prototypal member of four homologous transmembrane proteins and was the first protein in this family to have been sequenced and identified to have tyrosine kinase activity[2-5]. EGFR is also referred to as HER (human EGF receptor) and c-erbB1and is encoded by the EGFR gene located on chromosome 7p12. This transmembrane glycoprotein comprises of 1186 amino acids having three main parts; extracellular domain (ECD), transmembrane pass (TM) and intracellular domain (ICD)[2] (Figure 1).
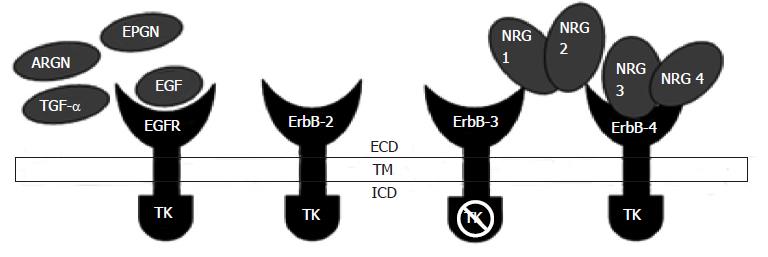
Figure 1 Epidermal growth factor receptor structure: Extracellular domain, transmembrane pass, intracellular domain, ligand binding, cysteine-rich domains.
Intracellular domain includes the kinase domain and cytoplasmic tail. EGFR: Epidermal growth factor receptor; ECD: Extracellular domain; TM: Transmembrane pass; ICD: Intracellular domain; ARGN: Amphiregulin; EPGN: Epiregulin; TGF-α: Transforming growth factor alpha; TK: Tyrosine kinase; NRG: Neuregulin.
In vertebrates, among the four EGFR family members (ErbB1, ErbB2, ErbB3 and ErbB4), overall similarity among the amino acids is about 50%[6]. The receptors EGFR family are together create an interacting system that receives and processes information that results in multiple cellular functions. EGFR binds to EGF, amphiregulin and TGF-α and ligands like betacellulin, heparin-binding EGF and epiregulin bind to the EGFR as well as ErbB4. The ErbB2/HER2/neu does not bind ligands and ErbB3/HER3 has an inactive kinase domain, and these receptors are thought to serve as co-receptors. Neuregulins 1 (NGR1) and Neuregulins 2 (NGR2) bind preferentially to ErbB3 and ErbB4 and the ligands NGR3 and NGR4 bind to ErbB4 (Figure 2). Ligand bonding initiates shape alteration that unmasks a “dimerization loop,” thereby triggering receptor homo-dimerization or hetero-dimerization which causes tyrosine trans-phosphorylation leading to activation of downstream signaling cascades[7]. These pathways are often functionally interlinked and ideally should not be considered in isolation; however, for the sake of simplicity most authors discuss them individually[7-10].
Figure 2 Epidermal growth factor receptor and its major ligands epidermal growth factor, transforming growth factor alpha, neuregulin, amphiregulin and epiregulin.
ECD: Extracellular domain; TM: Transmembrane pass; ICD: Intracellular domain; LB: Ligand binding; CR: Cysteine-rich; KD: Kinase domain; CT: Cytoplasmic tail.
The EGFR family is a diverse signaler and plays important physiological roles in determining cell lineage, organ morphogenesis, cell adaptation, motility, proliferation and apoptosis[5,7]. Damjanov et al[11] in 1986 conducted a study to identify EGFR in various tissues in human oral mucosa and suggested that membrane EGFR location depicts a more responsive cell than cytoplasmic EGFR localization This study said that it is likely that differential distribution of the EGFR to specific cell types and cellular compartments may signify adaptations that permit growth factor responsiveness in the surroundings of available ligand[11]. EGFR also interacts with RANK resulting in RANKL signaling pathways which helps in osteoclast differentiation and survival[12].
In general pathology EGFR plays a major role in human cancers. Aberrant EGFR signaling are initiated by several events, such as altered ligand production, receptor mutations or deletions and continuous signaling leading to uncontrolled cell multiplication, invasion, increased angiogenesis and metastasis[7,13-15]. EGFR also plays an important role in stopping autophagic cell death induced by death receptors which is one of the mechanisms that initiate cancer[14]. Another recent EGFR mechanism that was identified is the tyrosine kinase independent mode in which EGFR prevents cancer cells from apoptosis by regulating the basal intracellular glucose level via the sodium/glucose co transporter 1[15]. EGFR has varied effects in the prognosis of various cancers[13]. This is speculated to be an important reason for the aggressiveness and resistance to chemotherapy noticed in EGFR related epithelial tumors[16-19]. EGFR levels in normal cells usually ranges between 40000 and 100000 receptors per cell[19]. Enhanced EGFR expression are a notable characteristic of many epithelial carcinomas like glioblastoma, Non-small cell lung cancer, breast, colorectal, bladder, prostate and ovarian carcinomas[11,16-19].
EGFR IN ORAL PHYSIOLOGY
To understand EGFR related pathogenesis a proper understanding of its significance in physiology needed. EGFR plays important roles in the development and maintenance of various oral structures, tooth development, eruption and morphogenesis.
Hernández et al[20] in 1992 elicited the localization of epidermal growth factor and its receptor during tooth formation in rat embryos during embryonic days (E-16 to E-21) immunohistochemically. Another study by Heikinheimo et al[21] on role of EGFR in tooth development and few neoplastic odontogenic neoplasms concluded that that regulation of EGFR expression is developmentally determined in human odontogenesis. Furthermore, the odontogenic epithelium is the main target tissue for EGF, TGF-β and TGF-α and they may also be involved in odontogenic tumorigenesis[21]. Several authors like Wise et al[22], Shroff et al[23] and Cdhill et al[24] have proven that tooth follicle with the presence of EGFR and their ligands is essential for tooth eruption. EGFR and its ligands also mediates tooth morphogenesis as claimed by Hu et al[25].
On assessment of EGFR expression by Thesleff et al[26] it was seen that they intensely bind to the epithelial cell rests of Malassez concluding that they are responsive to the actions of EGF. Her study speculated that these epithelial rests may be activated whenever there is local rise of EGF ligand in that tissue milieu[26]. Another immunohistochemical analysis was performed in normal and pathological human gingival epithelia by Nordlund et al[27] which showed that basal layers of gingival proliferating cells in inflamed adult periodontitis cases, as well as the epithelial cell rests of Malassez bound to the antibody intensely signifying that EGF moderates epithelial growth and differentiation in periodontal tissues.
Also in recent research by O Häärä et al[28] it was evident that EGFR plays a role in formation of salivary gland by supporting the growth and development of the epithelium and survival of the mesenchyme.
EGFR IN ORAL PATHOLOGY
Various studies have analyzed EGFR expression in diverse oral lesions like squamous cell carcinomas[18,29-32], potentially malignant lesions[32-34], lichen planus[35,36] salivary gland tumors[37-39] and odontogenic cysts and tumours[40-44].
EGFR in head and neck squamous cell carcinoma
Head and neck squamous cell carcinoma (HNSCC) is increasing at an alarming rate with about 600000 patients being newly diagnosed annually. It was noted that most cases showing remission and metastasis are associated with poorer prognosis and a multitude of research is now being concentrated on understanding this disease and its relationship with EGFR pathways[18,19]. Heightened EGFR expression is observed in about 80%-90% HNSCC and often correlates with poorer prognosis, higher recurrence rate, advanced tumor stage and increased possibility of metastasis[13,18,19].
The HER 3 receptor was identified as most prognostic value in assessing tongue squamous cell carcinoma[31]. EGFR has been a recent target of anticancer therapies due to its critical roles in cellular homeostasis. EGFR mutations are found in four exons of the EGFR gene, exons 18 to 21. Exon 19 deletions and exon 21 mutations account for most of these mutations. On genetic analysis it is seen that the EGFR mutation of deletion in exon 19 was implicated with squamous cell carcinoma development in few of the cases[45].
EGFR in potentially malignant lesions
EGFR over expression is an initial event in the squamous cell carcinoma of the head and neck carcinogenesis. On investigation done by Rautava et al[34] in dysplastic, developing and malignant oral epithelium it was seen that all the four family of EGFR receptors was seen in developing oral epithelium and to a lesser extent in mature oral epithelium[34]. An increase in EGFR immuno-reactivity was seen in 61% and 54% of dysplasias and OSCC respectively. Its increased presence is also noted in “apparently normal” mucosa from cancer patients, when compared to healthy controls (field cancerization) and this over expression is observed to steadily increase analogous to observed histological abnormalities, from dysplasia to carcinoma in situ[34]. In normal mucosa EGFR positivity was seen only in the basal layers, whereas in leukoplakia the spinous and basal layers showed positivity and squamous cell carcinomas showed intense and increased positivity[46]. Another similarly designed study in dysplasias and SCC showed nearly all cells of the dysplastic epithelium showing positivity and in oral squamous cell carcinomas, the positivity in the tumor cells correlated inversely with cellular differentiation[47]. In other potentially malignant lesions like OSMF, it was found that there was a definite increase in EGFR expression along the differentiated layers of the oral epithelium[33]. In certain lesions like lichen planus, increased EGFR expression in the epithelial cells as well as the infiltrating lymphocytes are hypothesized to play a significant role in disease development[35,36].
EGFR expression in salivary gland lesions
EGFR expression has also been analyzed in various salivary gland lesions[37-39]. In a study done by Yamada et al[37] 1989 the immunohistochemical localization of EGFR was classified into two types, one the cell membrane-positive type found in epithelial tumor cells, and the other is the cytoplasm positivity seen in normal ductal cells and luminal tumor cells of pleomorphic adenomas and mucoepidermoid carcinomas. In a recent study[38] done on pleomorphic adenomas (PA), mucoepidermoid carcinoma (MC) and adenoid cystic carcinoma (ACC), it was found that all of them expressed EGFR family receptors. ErbB-2 was seen to be commonly expressed and both membrane and cytoplasmic staining is noted. Enhanced scores of ErbB-2 membrane were more common in MEC as compared to ACC and PA suggestive of their role in pathogenesis of salivary gland neoplasms. Another study done in carcinoma ex Pleomorphic adenoma (CXPA), showed intense EGFR expression in the outer borders of CXPA, indicating that this receptor may be related to cell detachment and invasive potential of CXPA[39].
EGFR expression in odontogenic lesions
Numerous investigations have been done in odontogenic epithelium and related lesions in reference to EGFR expression[40-44]. Based on the various studies conducted in odontogenic cysts and tumors and it has been suggested that EGFR is related to the proliferative mechanisms in these lesions. Shrestha was one of the first to study the expression of EGFR in odontogenic lesions and he found increased expression of EGFR in these odontogenic cysts and tumors but no positivity in ameloblastomas[42]. Based on these findings the author then concluded that the proliferative pathways in ameloblastomas were diverse. However, most of the studies done later in ameloblastoma showed diverse results with most ameloblastomas giving EGFR positive immunoexpression[41,43]. EGFR expression was studied in the physiological odontogenic epithelium represented by the pericoronal follicle by da Silva Baumgart et al[42] and he hypothesized that understanding the staining patterns of EGFR in the follicles could provide vital clues to the origin of various odontogenic cysts and tumors. Other studies by Vered et al[43] and de Vicente et al[44] also give diverse findings.
METHODS OF EVALUATION OF EGFR
EGFR quantification can be done at the DNA, RNA or protein level[45]. EGFR mutations are known to occur in various carcinomas and they are studied by analyzing the chromosomes and DNA[46]. EGFR amplification which is noted in various lesions can be studied by gene amplifications assay which analyze at the DNA, RNA and the protein levels in tissue[47,48]. mRNA based methods of detection are prone to problems with RNA degradation and contamination.
EGFR protein levels quantified by western blot analysis and enzyme immunoassay, measure total receptor protein and provide no data on their location in the cell[49]. Immunohistochemistry is commonly used to evaluate EGFR protein levels and is arguably the most convenient method for analyzing clinical samples and give an idea about the cellular localization. However, the main disadvantage of immunohistochemistry is its lack of sensitivity and specificity in comparison to other methods. Further there is still no consensus of standard scoring criteria for the quantifying EGFR positivity in tissue specimens. Downstream markers and their analysis may also provide EGFR related information. The EGFR molecule has various downstream pathways of action and these molecules are of significance in studying specific lesions. Some of the most common downstream markers of significance are EGFR, p-EGFR, p-Akt, p-Erk, p-STAT3[50,51].
EGFR AND ITS CLINICAL IMPLICATIONS
The identification of chemo-therapeutic agents in the treatment of specific malignancies like leukemias and lymphomas have simplified and inspired new treatment perspectives of neoplasms. Since the advent and success of these treatment strategies, researchers all over the world are trying to open more avenues in the treatment of other malignancies. Ever since the discovery of EGF in 1960 and the isolation of EGFR by Cohen et al[52] in 1980 numerous studies are done to elucidate its role in cancer pathogenesis. Based on the work of many pioneers on EGFR agents John Mendelsohn conducted research focusing on EGFR and proposed EGFR as an anticancer target, especially in various carcinomas[17,53]. Control of EGFR signaling is likely to open new avenues of treatment in three main areas that include cell yield, organ restoration and management of cancer.
EGFR is the receptor most often found up regulated and its gene mutations are evident in a wide variety of human tumors like head and neck cancers, renal carcinomas, breast carcinomas, gliomas, colon cancers, non-small-cell lung carcinomas and pancreatic carcinomas[7,13,18,19,30,45,54,55]. Herbst et al[55] in 2002 stated that EGFR is one of the most important receptors critical for cell proliferation, differentiation and survival and related its dysregulation to be of significance in suppressing apoptosis, mediating neoplastic angiogenesis, increasing metastatic ability and resisting chemo and radiotherapy[55]. Several ongoing clinical trials on humans are presently testing anti-EGFR antibodies with many of them showing promising results for the future. The rationale behind EGFR therapies is that they compete with endogenous growth factors like EGF and transforming growth factor-alpha, for binding sites. Once bound EGFR blocks crucial downstream pathways thereby interfering with the growth of neoplasms expressing EGFR.
The rationale of using anti-EGFR agents in head and neck cancers is that EGFR is expressed in more than 90% of head and neck carcinomas and studies have shown that EGFR over expression is associated with decreased survival[1,18,29-32,49]. Also it is noted that increased EGFR expression occurs initially in carcinogenesis and is present even in premalignant oral lesions[32-34]. Finally studies have also shown that inhibition of EGFR-TK pathway slows the growth of xenograft tumour models of head and neck. EGFR based chemotherapy can involve various methods[56]. This can be achieved by using directly acting anti-EGFR agents, by using tyrosine kinase inhibitors (TKIs) or agents that inhibit the downstream molecules in the EGFR pathway[56]. Amongst these, EGFR antibody Cetuximab and TKIs like Erlotinib and Gefitinib are being trialled in HNSCC. Understanding the molecular pathogenesis of the neoplasm will help in choosing the ideal therapeutic agent. For example EGFRvIII is caused by frame deletion mutations and is seen in 42% of HNSCC leading to growth of the tumor and provides resistance to antibody based treatment interventions.
In such situations, patients with EGFRvIII HNSCCs would possibly benefit better from tyrosine kinase inhibitors rather than EGFR antibody based treatment strategies[56,57].
Another important treatment possibility of the EGFR neoplasms is that anti-EGFR agents increase the radiosensitivity of several neoplasms. In a recent randomised phase III clinical trial attempted by Bonner et al[58] it was seen that simultaneous radiotherapy and chemotherapy with Cetuximab in head and neck cancer patients showed improved local tumour containment compared to radiotherapy alone[58]. The cause for increased radiation effects when combined with EGFR therapy are still obscure. Numerous other studies studying the efficacy of anti- EGFR agents are in the phase three trial[1,7,54,55,58]. Antisense oligonucleotides, ligand conjugates and immunoconjugates of EGFR are also used to inhibit EGFR activity. Other EGFR inhibitors like cetuximab-C225 are being extensively studied in head and neck carcinomas and are in the third phase of trial. However till date no drastic changes in treatment modalities have been experienced. Moreover, there are a lot of side effects associated with the use of EGFR which has rendered it use, unacceptable.
Vered in his article[43] mentions that adverse effects of anti-EGFR agents like C225 and ZD-1839 is usually observed in less than 15% of patients, and these effects may not occur with ameloblastoma, as these side effects could be avoided by intralesional administration of the anti-EGFR agents. Recently, a fully human anti-EGFR monoclonal antibody, vectibix-EGF was developed as a possible treatment for surgically compromised cases of ameloblastoma. However, clinical trials are yet to take place[43].
CONCLUSION
The EGF receptor is an important molecule in maintaining various pathways and homeostasis within an organism. It plays varied roles and its dysregulation is identified to be a key factor in various oral pathologies, especially HNSCC and ameloblastomas. Identifying ideal therapeutic target will enable the transition of treating these lesions using a non surgical modality thereby significantly reducing the mortality and morbidity of the patient. The receptor mediates it action through various pathways and the proper understanding of these will enable us to develop ideal treatment strategies to combat the various lesions. Several studies are now targeting these pathways, however, till now significant success has not been achieved in clinical trials using anti-EGFR agents in HNSCC. Several reasons suggested for this insensitivity are the multifactorial aetiology of head and neck carcinomas and lack of proper understanding of the various molecular pathways. More research needs to be focussed on the understanding of this molecule in future in order to bring the treatment of several debilitating neoplasms from the bench to the bedside.
P- Reviewer: Dereci O S- Editor: Ji FF L- Editor: A E- Editor: Jiao XK