Published online May 11, 2023. doi: 10.5317/wjog.v12.i3.17
Peer-review started: January 26, 2023
First decision: February 8, 2023
Revised: February 8, 2023
Accepted: April 4, 2023
Article in press: April 4, 2023
Published online: May 11, 2023
Processing time: 105 Days and 6 Hours
Preeclampsia (PE) is a pregnancy syndrome of undetermined etiology; inflammation was one of the proposed theories for its development.
To examine the platelet to lymphocyte ratio (PLR), an inflammatory biomarker, as a marker to predict poor maternal-neonatal outcomes in early-onset PE (EoPE).
A cross-sectional study enrolled 60 pregnant women with EoPE (at 32-30 wk of gestation) at a university hospital. Demographic criteria and hematological indices were collected, including platelet counts and indices (mean platelet volume and platelet distribution width), PLR, and the Doppler study, which calculated estimated fetal weight (EFW), amniotic fluid index (AFI), resistance index (RI), and pulsatility index (PI). Participants were followed until delivery, where maternal outcomes were recorded, including; delivery mode and reason for cesarean section, and neonatal outcomes, including fetal growth restriction (FGR), meconium-stained liquid, the 5-min Apgar score, and admission to the intensive care unit.
There was a trend of insignificant increases in cesarean sections. Sixty-one-point two percent (37/60) fetuses were admitted to the neonatal care unit; 70.0% of admitted fetuses were meconium-stained liquor, and 56.7% of them had FGR. PLR was positively correlated with AFI and EFW as r = 0.98, 0.97, P < 0.001; PLR showed negative correlations with PI and RI as r = -0.99, -0.98, P < 0.001. The Apgar score and the number of days admitted to the intensive care unit had a positive and negative correlation (0.69, -0.98), P < 0.0001, respectively. Receiver operating characteristic calculated a PLR cutoff value (7.49) that distinguished FGR at 100% sensitivity and 80% specificity.
Strong, meaningful relationships between PLR and FGR parameters and a poor neonatal outcome with a significant P value make it a recommendable biomarker for screening EoPE-related complications. Further studies are suggested to see the impact on maternal-neonatal health.
Core Tip: Women with preeclampsia (PE) suffer increased morbidity and mortality; their offspring endure higher risks in the early neonatal period and later life. Despite extensive research into PE, the only definitive treatment is to terminate the pregnancy. Many seek efficient prediction methods that may reduce expected risk. Platelet to lymphocyte ratio (PLR), an inflammatory biomarker, was studied in PE; however, little is known about its role in early-onset PE, a subtype with serious consequences for fetal and maternal health. Herein, we examine the role of PLR, which showed a strong, meaningful relationship between fetal growth restriction and poor neonatal outcome, making PLR a recommendable screening parameter.
- Citation: Akram W, Abdullah Hussein Z, Hameed Humadi M, Nori W. Clinical implication of platelet to lymphocyte ratio in early onset preeclampsia: A single-center experience. World J Obstet Gynecol 2023; 12(3): 17-27
- URL: https://www.wjgnet.com/2218-6220/full/v12/i3/17.htm
- DOI: https://dx.doi.org/10.5317/wjog.v12.i3.17
Preeclampsia (PE) is identified as new-onset hypertension in formerly normotensive pregnant women, combined with proteinuria, after the 20th wk of gestation; it affects 7% to 10% of all pregnancies. PE is primarily a placenta disease that can be early-onset or late-onset PE according to its onset below or above 32 wk of gestation[1,2]. Early-onset PE (EoPE) is a severe form of PE with a 0.35%-0.50% prevalence caused by inadequate recasting of the uterine spiral arteries and poor placental implantation. The hypoxic placenta produces excessive inflammatory mediators in maternal circulation as the pregnancy proceeds. As a result, vascular integrity is disrupted, and endothelial dysfunction occurs. The latter leads to hypertension, proteinuria, and other PE-related symptoms. The decreased perfusion to the fetus will reduce fetal growth rates; indeed, PE is a major cause of fetal growth restriction (FGR)[2-4].
FGR is linked to adverse obstetric effects, including higher cesarean sections (C-sections), poor neonatal outcomes; such as low Apgar scores, admission to the neonatal intensive care unit (NICU), and meconium-stained liquor, in addition to long-term health effects such as delayed neurodevelopmental milestones and adult cardiovascular disease[5-7].
Diagnosing FGR is made via serial ultrasound examination, which needs follow-up and patient compliance[3,8]. As a result, there was a necessity for prediction modules to assess current placental activity. Inflammation is a proposed cause of PE, and numerous inflammatory markers were examined to define PE severity and its related consequences[9-11]. Platelet turnover increases in the maternal circulation of PE women, which eventually reduces their numbers along with alterations in platelet size, and functionality; lymphocytes, on the other hand, will be increased in PE cases[12] owing to a maladaptive immune response and a hyper-inflammatory state in PE[13]. Therefore, the platelet to lymphocyte ratio (PLR) will decrease in severe PE cases as the numerator (platelets) decreases when divided by the increased denominator (lymphocyte). PLR has been studied for prognostic and predictive roles in various diseases, including PE and cancer prognosis. However, they presented inconsistent and sometimes contradicting results[11-13]. Moreover, PLR was not tested in EoPE.
We hypothesized that the reduction in PLR would predict poor obstetrical and neonatal outcomes in early-onset PE. We aimed to predict FGR through biological and ultrasonic markers for patients with EoPE as a primary goal. The secondary aim was to examine the correlation of PLR with predictors of maternal-fetal outcome.
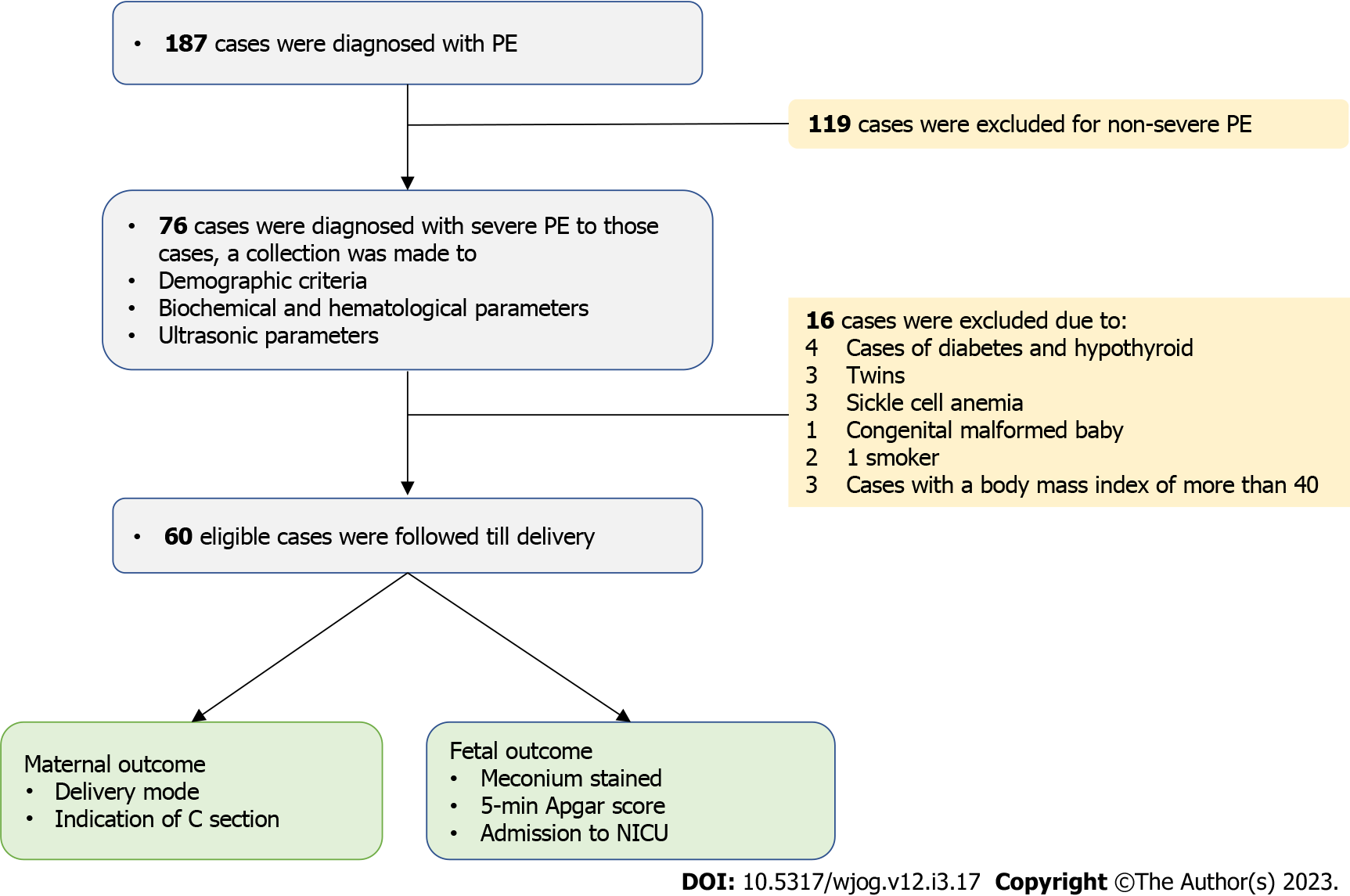
At the University Hospital in Baghdad, Iraq, a longitudinal study with a cross-sectional design recruited 60 eligible participants in June 2019 and ended in October 2020. The ethical committee of Mustansiriyah University approved the study (IRB 160, February 2019). The Declaration of Helsinki was followed in the study; all participants gave informed consent after we explained the study aims and methods prior to enrollment. The study participants were women with early-onset PE (less than 34 wk). Only women with severe PE were recruited (Figure 1).
Pregnant between the ages of 18 and 40, with a gestational age of 30-32 wk, as determined by history and/or confirmed by an early ultrasound scan. They should not have started treatment. We aimed to have a narrow window in recruitment to make the study’s demographics more uniform.
Pregnant women with gestational age outside reference gestational age (> 32 wk and < 30 wk). Medical disorders like kidney and liver diseases, a personal or family history of thyroid, diabetes, or cardiovascular diseases, a personal history of chronic hypertension, blood dyscrasias, and anemia. Those with twin pregnancies and congenital fetal anomalies. Smoking mothers and those on aspirin or steroids were also excluded. Non-severe PE cases have been excluded.
Our hospital is a tertiary center that receives many cases of PE in addition to referred cases from the periphery of Baghdad. Admission, assessment of fetomaternal wellbeing, blood pressure control, accelerated lung maturity, and close follow-up for any deterioration of a pregnant mom or her unborn baby necessitating pregnancy termination are all part of the policy for managing severe EoPE.
A detailed history and general and obstetrical exams were conducted on the day of admission. In every case, maternal age, weeks of gestation, and systolic and diastolic blood pressure (SBP, DBP) were recorded while the patients were at rest. Lab tests were ordered, including blood biochemistry (serum creatinine, blood urea, alanine aminotransferase, and aspartate aminotransferase); a complete blood count to assess hematological indices hematocrit (10%), platelet distribution width, mean platelet volume, platelet counts, lymphocyte count, where the PLR was created; and a urine sample for evaluating total protein excreted.
On the same day of admission, an ultrasound scan was arranged to assess fetal wellbeing [gestational age, amniotic fluid index (AFI), estimated fetal weight (EFW), signs of fetal growth resection (FGR)]. A color Doppler spectral study measured uterine artery pulsatility and resistance index (PI and RI).
Cases were followed until delivery, at which point the maternal outcomes, including; the mode of delivery and reason for a C-section, were recorded. In addition, the neonatal outcome included meconium-stained liquor, a 5-min Apgar score, and admission to the NICU. The information was saved on an Excel sheet so that it could be analyzed later.
PE was defined according to the ACOG technical bulletin No. 219 (American College of Obstetricians and Gynecologists), which includes patients with a systolic blood pressure of 140 mmHg or a diastolic blood pressure of 90 mmHg following 20 wk of gestation and albuminuria of > 300 mg or a sustained 1+ dipstick at 24 h. A gestational age of less than 32 wk defines EoPE[1].
Cases of severe PE had a systolic blood pressure of 160 mmHg or a diastolic blood pressure of 110 mmHg. In the absence of proteinuria, the diagnostic criteria for severe PE comprise hypertension plus thrombocytopenia, impaired liver function, pulmonary edema, new development of renal insufficiency, or new-onset cerebral or visual problems[2].
The early-onset FGR was defined as ultrasonic EFW or fetal abdomen circumference less than the 3rd percentile using a population chart, or EFW or fetal abdominal circumference less than the 10th percentile correlated with umbilical artery PI more than the 95th percentile, or cerebroplacental ratios less than the 5th percentile[3,8].
The sample size was calculated according to the following equation for a cross-sectional study with quantitative variables[14]: Z1-α/2 = is a standard normal variate which is equal to 1.96; SD = standard deviation of a variable calculated by earlier published works; d = the precision level decided by the operator; sample size = (1.96)2 (0.35)2/(0.1)2 = (3.84 × 0.1225)/0.01 = 43 patients. So, the sample size is 43 patients; we recruited 60 cases.
The statistical analysis of the data was carried out through Microsoft Office Excel 2016 and the SPSS 26 program. The numerical data were expressed as mean, standard deviation. Categorical data were presented as numbers and percentages. Linear regression assessed the correlation between PLR and the study parameters, including maternal and neonatal outcomes. The receiver operating characteristic (ROC) curve was constructed to calculate the PLR that correlates with FGR at the highest sensitivity and specificity. All tests were considered significant when the P value was 0.05.
This study examined 60 pregnant women diagnosed with severe PE at a gestational age of 32-30 wk. Table 1 shows the primary criteria of the study. The mean maternal age was 27.0 years ± 2.6 years, the PLR ratio was 7.86 ± 1.75, the EFW was 1.30 ± 0.08, the days of admission to the NICU were 7.09 d ± 2.05 d, and the mean Apgar score was 6.54 ± 1.60. Table 2 describes the maternal outcome in terms of cesarean section (C-section), which showed a trend toward a higher percentage vs vaginal delivery with no statistical significance (65% vs 35%). Table 3 describes the neonatal outcome of the delivered newborn. Of the total fetuses studied, 37/60 (61.2%) were admitted to the NICU. Seventy percent of admitted fetuses were meconium-stained liquor. The occurrence of meconium-stained liquor was significantly higher among admitted cases. The FGR was reported in 56.7% of the admitted fetuses, which is significantly higher in admitted fetuses. The lowest Apgar scores were found in the admitted cases to the NICU. A statistically significant difference was confirmed in the admission to the NICU between different Apgar scores. The percentage of dead fetuses is not statistically significant between admitted and unadmitted fetuses in Table 4. PLR, taken as an independent variable, was correlated with the FGR parameters (AFI, EFW, PI, and RI). All correlations were strongly significant, as the correlation coefficients (r) were (0.98, 0.97, -0.99, -0.98), respectively, with P < 0.001. As for newborn parameters, the Apgar score showed a positive correlation of 0.69, while admission days to the NICU showed a strong inverse correlation of -0.98; both had a P < 0.0001. The ROC curve calculated a PLR cutoff value < 7.49, an AUC of 0.8, and P < 0.001, which was correlated at 100% sensitivity and 80% specificity with FGR, as described in Table 5.
| Maternal demographic and biochemical parameter, n = 60 | |
| Maternal age in yr | 27.0 ± 2.6 |
| Mean systolic BP in mL/Hg | 160.2 ± 5.2 |
| Mean diastolic BP in mL/Hg | 105.2 ± 4.7 |
| Urine for albumin in gm/dL | 2.89 ± 0.09 |
| Serum creatinine in mg/dL | 0.86 ± 0.52 |
| Blood urea in mg/dL | 29.38 ± 14.04 |
| Alanine aminotransferase in U/L | 27.43 ± 5.32 |
| Aspartate aminotransferase in U/L | 21.80 ± 4.49 |
| Maternal hematological indices, n = 60 | |
| Hematocrit, 10% | 36.58 ± 2.81 |
| Platelet count as × 109 | 182.40 ± 47.42 |
| MPV in fL | 10.52 ± 0.23 |
| PDW in fL | 16.68 ± 1.37 |
| PLR ratio | 7.86 ± 1.75 |
| Fetal demographic criteria, n = 60 | |
| Fetal AFI in cm | 6.60 ± 1.19 |
| Estimated fetal weight FW in kg | 1.360 ± 0.08 |
| PI UA Doppler | 3.29 ± 0.59 |
| RI UA Doppler | 3.13 ± 0.55 |
| Admission to NICU in d | 7.09 ± 2.05 |
| Mean Apgar score | 6.54 ± 1.60 |
| Parameter | Study participants presented | n (%) | P value |
| Mode of delivery | Vaginal delivery | 21 (35) | < 0.407 |
| Cesarean delivery | 39 (65) | ||
| Indication for CS delivery | Previous scar | 13 (22.5) | < 0.190 |
| Fetal distress | 12 (20) | ||
| Failed induction | 11 (17.5) | ||
| Malpresentation | 3 (5) |
| Variable | Admitted, n = 37 | Not admitted, n = 23 | P value | |
| Meconium | Meconium stain | 26 (70%) | 0 (0%) | 0.000 |
| No meconium | 11 (30%) | 23 (100%) | ||
| Occurrence of FGR | FGR | 21 (56.7%) | 0 (0%) | 0.000 |
| No FGR | 16 (43.3%) | 23 (100%) | ||
| Apgar score | > 7 | 0 (0%) | 19 (51.3%) | 0.000 |
| 5-7 | 5 (21.7%) | 18 (48.7%) | ||
| < 5 | 23 (78.3%) | 0 (0%) | ||
| Occurrence of dead fetus | Dead fetus | 2 (5%) | 0 (0%) | 0.257 |
| Not dead fetus | 35 (95%) | 23 (100%) | ||
| PLR vs variables | Correlation coefficient | P value |
| AFI | 0.98 | < 0.001 |
| EFW | 0.97 | < 0.001 |
| PI | -0.99 | < 0.001 |
| RI | -0.98 | < 0.001 |
| Apgar score | 0.69 | < 0.0001 |
| Admission days to NICU | -0.98 | < 0.0001 |
| Parameter | Cutoff value | Sensitivity | Specificity | AUC | P value |
| PLR | < 7.49 | 100% | 80% | 0.9 | < 0.001 |
Analysis showed a strong, meaningful correlation of PLR to parameters that define FGR, which indicates PLR reliability in FGR prediction. The strong link between PLR and neonatal outcomes, such as Apgar score and number of days in the NICU, suggests that PLR is a good predictor of neonatal outcome.
According to Mannaerts et al[15], PLR was low among the EoPE group compared to healthy controls. They confirmed that PLR tends to decrease after 20 wk of gestation in patients destined to have PE. Their results were in good agreement with the Yücel et al[16], which confirmed a lower PLR among severe PE cases compared to mild PE and healthy controls. Sisti et al[17] examined PLR in a case-control study involving cases of HELLP syndrome and healthy controls. Their analysis confirmed lower PLR among affected patients. They suggested that the ratio be included in the HELLP syndrome prediction mode.
Our results showed a strong positive link between PLR and AFI. Less blood flow to the placenta and ischemia cause less blood flow to the fetal kidneys, which lowers AFI[10].
The PLR showed a significant positive correlation with the EFW. Likewise, Can et al[18] investigated PLR and NLR in relation to birth weight in healthy and malnourished term babies. Both ratios were significantly high in the malnourished study division; they were recommended as reliable markers.
Akgun et al[19] investigated PLR with birth weight and gestational age. Their results show a significant correlation with birth weight. Furthermore, a significant correlation was found between infants’ birth weight and gestational age. Kırmızı et al[20] examined PLR and NLR in late onset FGR in a case-control study. They did not recommend PLR as its levels were statistically insignificant compared to the NLR. Their study had a small sample size, which may explain the shortcomings of their results.
Both PI and RI were strongly correlated with PLR, which was consistent with previous research linking changes in PI and RI waveforms of uterine arteries to the development of PE and FGR[21]. A Cochrane review also showed that the use of a doppler can help reduce the number of C-sections, labor inductions, and perinatal deaths in FGR babies[22].
Platelet indices, along with PI and RI, were suggested by Abdel Razik et al[23] as a way to measure the severity of PE rather than predict its onset.
In terms of obstetric outcomes, 60.0% of cases were ended by C-section, 61.2% of fetuses were admitted to the neonatal care unit, 70.0% were meconium-stained, and 56.7% of the meconium-stained fetuses had FGR, which led to a lower Apgar score among admitted cases. Our results were in line with the study of Jha et al[24], where significant differences were seen in the PE groups they examined. In addition to low Apgar scores at 1st and 5th min and on admission days to the NICU[24].
In the current study, the Apgar score showed a significant P value among newborns with a positive correlation to PLR. Okoye et al[25] discussed lower PLR in neonates of PE women, which correlates with hypertension severity. PLR has also been linked to poor birth outcomes, as evidenced by low Apgar scores. Their study examined PLR and other blood indices in the cord blood of neonates born to PE mothers. No meaningful association was seen between PLR and neonatal birth weight; it only correlated with 1st and 5-min Apgar scores in newborns.
Kim et al[26] discussed a considerably low PLR in women with severe PE. It was most strongly related to the time of admission to the delivery interval.
According to the Özdemirci et al[27], PLR in late-onset FGR cases did not show a significant increase. They suggested that an exaggerated inflammatory response was proposed to be a cause for FGR and to be absent in late-onset FGR cases, emphasizing our findings and forming the novelty of our study.
PE is a major risk factor for growth restriction; insufficient spiral artery penetration during early implantation has been blamed for early-onset FGR. To supply the fetus with nutrients, the diseased placenta will develop a mechanism to overcome increased resistance to blood flow and decreased placenta perfusion[5]. Since blood is a primary interface between the fetus and mother, any stressful event will cause blood parameters passing through the placenta to be altered. Therefore, many researchers addressed blood indices, searching for biomarkers that correlate with PE and consequent FGR[10,28].
Different pathophysiologic mechanisms underlie PE sub-types. EoPE is the result of impaired placenta development and improper innate immune system activation that trigger a systemic inflammatory response as early as the second trimester. The injured endothelial cells secrete many cytokines and inflammatory markers into the circulation that cause changes in the complete blood film parameters in PE cases[29,30].
Platelet numbers will be reduced due to consumption. Lymphocyte numbers, key players in systemic inflammation, will be increased. These changes are thought to be responsible for maternal and fetal complications[11]. Hence, PLR forecasts an impending or ongoing inflammatory pathology.
The current study result may have a clinical implication by preventing PE-related complications. Patients with known inflammatory biomarkers may benefit from prophylactic doses of low molecular weight heparin, which has immunomodulatory and anti-inflammatory properties. Low-molecular-weight heparin was recommended to prevent adverse obstetrical problems[31].
We have to acknowledge some of the inconsistencies in earlier studies regarding the value of PLR. Morisaki et al[32] technical report explained that different blood ratios were caused by different gestational ages, attributing these inconsistencies to different maternal criteria, gestational age, and inflammatory responses among pregnant women. Our findings clearly demonstrated that there was no statistical correlation between the PLR and the mode of delivery or the indication of the delivery, which was consistent with previous studies that criticized the insignificant role of blood ratios in predicting maternal outcomes[33,34]. Since delivery is the only treatment for PE progression, we must evaluate maternal risk against newborn problems. For that, early and accurate detection is necessary[35,36].
The cross-sectional nature of this study is one since the causal effect cannot be elucidated[37]. A case-control study may perform better in confirming the link between PLR and EoPE. We aimed to collect a higher sample size; however, the COVID-19 pandemic limited many work aspects. It is worth mentioning that risk analysis for the prevention of PE was not done; we think that the current analysis served our aim well. The fact that the current study was a single-center experience may limit the globalization of its results.
Although PLR was examined in late-onset PE, its role in EoPE was not addressed earlier. This paper emphasizes the significance of PLR in predicting early-onset PE associated with FGR[38-40]. PLR was intimately linked to FGR parameters; moreover, it correlated with important predictors of neonatal outcome; its significant correlation to FGR EoPE with high sensitivity and specificity (100%, 80%) and a significant AUC of 0.9, P < 0.001 makes it a valuable predictor. Since PLR was already validated for PE and its related co-morbidities, we needed no external validation. FGR is responsible for 50% of unexplained stillbirths. Its implications extend beyond postpartum, as it increases neonatal morbidity and the risk of cardiovascular diseases in the offspring[41]. PLR is simple, inexpensive, and can guide clinicians and assist with the timely referral of affected women to tertiary care centers to halt adverse fetal outcomes. Further studies are needed to explore the future implications of PLR on fetal and maternal health and their predictive value for early childhood and adult-onset diseases.
PLR is a reliable predictor of adverse fetal outcomes, including FGR parameters, a poor Apgar score, and admission to the neonatal care unit among pregnant women with EoPE. PLR had high sensitivity and specificity with no added expanders, making it a recommendable marker in their prediction. In light of the promising role of anti-coagulant use in preventing obstetrical-related complications, PLR may be used in predicting, categorizing, and preventing early-onset PE-related complications.
Preeclampsia (PE) is a pregnancy condition with an unknown origin that includes two subtypes based on 34 wk of gestation: Early and late onset PE; inflammation was postulated as an explanation. The platelet to lymphocyte ratio (PLR), an inflammatory biomarker, was investigated as a predictor of poor maternal-neonatal outcome in patients with early-onset PE (EoPE).
Much research has shown that inflammation may be an underlying pathology that triggers PE development. There is an increased need for new methods with enhanced predictive ability. Demonstrating changes in blood indices, PLR seems an appealing option given the promising results declared by earlier work.
To ascertain if PLR in cases with early-onset PE can be linked to essential predictors of fetomaternal wellbeing during the intrapartum period. The second goal is to analyze the reliability of PLR as a helpful marker for monitoring prenatal predictors in women with early-onset PE.
Cross-sectional research at University Hospital involved 60 pregnant women with EoPE (at 32-30 wk of gestation). Platelet counts and indices (mean platelet volume and platelet distribution width), PLR, Doppler study, which produced estimated fetal weight (EFW), amniotic fluid index (AFI), resistance index (RI), and pulsatility index (PI) were all gathered. Participants were tracked until birth, when maternal outcomes such as delivery style and reason for cesarean section were documented, as well as newborn outcomes such as fetal growth restriction (FGR), meconium-stained fluids, five-minute Apgar score, and admission to the critical care unit.
A cesarean section trend has been noted. Sixty-one-point two percent (37/60) fetuses were hospitalized to the newborn care unit, 70% had meconium-stained liquid, and 56.7% had FGR. PLR was shown to be favorably connected with AFI and EFW (r = 0.98, 0.97, P < 0.001), and negatively correlated with PI and RI (r = -0.99, -0.98, P < 0.001). The Apgar score and the number of days admitted to the critical care unit had a positive and negative connection (r = 0.69, -0.98, P < 0.001), respectively. The PLR cutoff value derived by receiver operating characteristic (7.49) differentiated FGR with 100% sensitivity and 80% specificity.
PLR had substantial P value associations with FGR measures and poor neonatal outcomes, making it a promising biomarker for screening EoPE-related problems. More research is needed to determine the influence on maternal-neonatal health.
Defining reliable biomarkers that are antenatal clinics based with no added expense can be a promising option, especially for low-resource settings.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, general and internal
Country/Territory of origin: Iraq
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Imbeah EG, Ghana; Pahlavani HA, Iran S-Editor: Chen YL L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1452] [Cited by in RCA: 2175] [Article Influence: 197.7] [Reference Citation Analysis (0)] |
| 2. | He Y, Xu B, Song D, Yu F, Chen Q, Zhao M. Correlations between complement system's activation factors and anti-angiogenesis factors in plasma of patients with early/late-onset severe preeclampsia. Hypertens Pregnancy. 2016;35:499-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Audette MC, Kingdom JC. Screening for fetal growth restriction and placental insufficiency. Semin Fetal Neonatal Med. 2018;23:119-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 129] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 4. | Bellos I, Fitrou G, Pergialiotis V, Papantoniou N, Daskalakis G. Mean platelet volume values in preeclampsia: A systematic review and meta-analysis. Pregnancy Hypertens. 2018;13:174-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Pastén V, Tapia-Castillo A, Fardella CE, Leiva A, Carvajal CA. Aldosterone and renin concentrations were abnormally elevated in a cohort of normotensive pregnant women. Endocrine. 2022;75:899-906. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Womersley K, Ripullone K, Hirst JE. Tackling inequality in maternal health: Beyond the postpartum. Future Healthc J. 2021;8:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Andraweera PH, Lassi ZS. Cardiovascular Risk Factors in Offspring of Preeclamptic Pregnancies-Systematic Review and Meta-Analysis. J Pediatr. 2019;208:104-113.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 8. | Gordijn SJ, Beune IM, Thilaganathan B, Papageorghiou A, Baschat AA, Baker PN, Silver RM, Wynia K, Ganzevoort W. Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet Gynecol. 2016;48:333-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 727] [Cited by in RCA: 1010] [Article Influence: 112.2] [Reference Citation Analysis (0)] |
| 9. | Perucci LO, Corrêa MD, Dusse LM, Gomes KB, Sousa LP. Resolution of inflammation pathways in preeclampsia-a narrative review. Immunol Res. 2017;65:774-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Nori W, Ali IA, Akram W. The Value of Serum Fibrinogen/Uric Acid Ratio as a Novel Marker of Fetal Growth Restriction in Preeclampsia at 34 wk. Current Women’s Health Reviews. 2023;19:e010322201543. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Nori W, Roomi AB, Akram W. Platelet indices as predictors of fetal growth restriction in Preeclamptic Women. Revista Latinoamericana de Hipertension. 2020;15:280-285. [DOI] [Full Text] |
| 12. | Triggianese P, Perricone C, Chimenti MS, De Carolis C, Perricone R. Innate Immune System at the Maternal-Fetal Interface: Mechanisms of Disease and Targets of Therapy in Pregnancy Syndromes. Am J Reprod Immunol. 2016;76:245-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Harmon AC, Cornelius DC, Amaral LM, Faulkner JL, Cunningham MW Jr, Wallace K, LaMarca B. The role of inflammation in the pathology of preeclampsia. Clin Sci (Lond). 2016;130:409-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 393] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 14. | Chow SC, Shao J, Wang H, Lokhnygina Y. Sample size calculations in clinical research. Biometrics. 2017;64:1307-1308. [RCA] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Mannaerts D, Heyvaert S, De Cordt C, Macken C, Loos C, Jacquemyn Y. Are neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), and/or mean platelet volume (MPV) clinically useful as predictive parameters for preeclampsia? J Matern Fetal Neonatal Med. 2019;32:1412-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 16. | Yücel B, Ustun B. Neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, mean platelet volume, red cell distribution width and plateletcrit in preeclampsia. Pregnancy Hypertens. 2017;7:29-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 17. | Sisti G, Faraci A, Silva J, Upadhyay R. Neutrophil-to-Lymphocyte Ratio, Platelet-to-Lymphocyte Ratio, and Routine Complete Blood Count Components in HELLP Syndrome: A Matched Case Control Study. Medicina (Kaunas). 2019;55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Can E, Can C. The value of neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) parameters in analysis with fetal malnutrition neonates. J Perinat Med. 2019;47:775-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Akgun N, Namli Kalem M, Yuce E, Kalem Z, Aktas H. Correlations of maternal neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) with birth weight. J Matern Fetal Neonatal Med. 2017;30:2086-2091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Kırmızı DA, Baser E, Onat T, Caltekin MD, Kara M, Yalvac ES. Can Inflammatory Hematological Parameters be a Guide to Late-onset Fetal Growth Restriction? Z Geburtshilfe Neonatol. 2020;224:262-268. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Cnossen JS, Morris RK, ter Riet G, Mol BW, van der Post JA, Coomarasamy A, Zwinderman AH, Robson SC, Bindels PJ, Kleijnen J, Khan KS. Use of uterine artery Doppler ultrasonography to predict pre-eclampsia and intrauterine growth restriction: a systematic review and bivariable meta-analysis. CMAJ. 2008;178:701-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 458] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 22. | Dall'Asta A, Brunelli V, Prefumo F, Frusca T, Lees CC. Early onset fetal growth restriction. Matern Health Neonatol Perinatol. 2017;3:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 23. | Abdel Razik M, Mostafa A, Taha S, Salah A. Combined Doppler ultrasound and platelet indices for prediction of preeclampsia in high-risk pregnancies. J Matern Fetal Neonatal Med. 2019;32:4128-4132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Jha UC, Dangal G. Fetal Outcome in Cesarean Versus Normal Deliveries in Pregnancy with Meconium-stained Liquor: A Cross-sectional Study. J Nepal Health Res Counc. 2021;19:107-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Okoye HC, Madu AJ, Korubo K, Efobi C, Eze OE, Obodo O, Okereke K, Ilechukwu G. Correlates of neutrophil/lymphocyte, platelet/lymphocyte, and platelet/neutrophil ratios of neonates of women with hypertensive disease of pregnancy with neonatal birth outcomes. Hypertens Pregnancy. 2019;38:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (1)] |
| 26. | Kim MA, Han GH, Kwon JY, Kim YH. Clinical significance of platelet-to-lymphocyte ratio in women with preeclampsia. Am J Reprod Immunol. 2018;80:e12973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Özdemirci Ş, Başer E, Kasapoğlu T, Karahanoğlu E, Kahyaoglu I, Yalvaç S, Tapısız Ö. Predictivity of mean platelet volume in severe preeclamptic women. Hypertens Pregnancy. 2016;35:474-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Nori W, Hamed RM, Roomi AB, Akram W. Alpha-1antitrypsin in pre-eclampsia; from a clinical perspective. J Pak Med Assoc. 2021;71 Suppl 8:S53-S56. [PubMed] |
| 29. | Aneman I, Pienaar D, Suvakov S, Simic TP, Garovic VD, McClements L. Mechanisms of Key Innate Immune Cells in Early- and Late-Onset Preeclampsia. Front Immunol. 2020;11:1864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 136] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 30. | Örgül G, Aydın Haklı D, Özten G, Fadiloğlu E, Tanacan A, Beksaç MS. First trimester complete blood cell indices in early and late onset preeclampsia. Turk J Obstet Gynecol. 2019;16:112-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 31. | Beksac MS, Tanacan A, Ozten G, Cakar AN. Low-dose low-molecular-weight heparin prophylaxis against obstetrical complications in pregnancies with metabolic and immunological disorder-associated placental inflammation. J Matern Fetal Neonatal Med. 2022;35:1546-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Morisaki N, Piedvache A, Nagata C, Michikawa T, Morokuma S, Kato K, Sanefuji M, Shibata E, Tsuji M, Shimono M, Ohga S, Kusuhara K; Japan Environment and Children’s Study Group. Maternal blood count parameters of chronic inflammation by gestational age and their associations with risk of preterm delivery in the Japan Environment and Children's Study. Sci Rep. 2021;11:15522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 33. | Yakiştiran B, Tanaçan A, Altinboğa O, Erol A, Şenel S, Elbayiyev S, Yücel A. Role of derived neutrophil-to-lymphocyte ratio, uric acid-to-creatinine ratio and Delta neutrophil index for predicting neonatal outcomes in pregnancies with preeclampsia. J Obstet Gynaecol. 2022;42:1835-1840. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 34. | Aydoğan Kırmızı D, Onat T, Başer E, Demir Çaltekin M. Letter to Editor. Turk J Obstet Gynecol. 2019;16:278-279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 35. | Melamed N, Baschat A, Yinon Y, Athanasiadis A, Mecacci F, Figueras F, Berghella V, Nazareth A, Tahlak M, McIntyre HD, Da Silva Costa F, Kihara AB, Hadar E, McAuliffe F, Hanson M, Ma RC, Gooden R, Sheiner E, Kapur A, Divakar H, Ayres-de-Campos D, Hiersch L, Poon LC, Kingdom J, Romero R, Hod M. FIGO (international Federation of Gynecology and obstetrics) initiative on fetal growth: best practice advice for screening, diagnosis, and management of fetal growth restriction. Int J Gynaecol Obstet. 2021;152 Suppl 1:3-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 272] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 36. | Helou A, Walker S, Stewart K, George J. Management of pregnancies complicated by hypertensive disorders of pregnancy: Could we do better? Aust N Z J Obstet Gynaecol. 2017;57:253-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 37. | Solem RC. Limitation of a cross-sectional study. Am J Orthod Dentofacial Orthop. 2015;148:205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 38. | Cornelius DC. Preeclampsia: From Inflammation to Immunoregulation. Clin Med Insights Blood Disord. 2018;11:1179545X17752325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 113] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 39. | Stojkovic Lalosevic M, Pavlovic Markovic A, Stankovic S, Stojkovic M, Dimitrijevic I, Radoman Vujacic I, Lalic D, Milovanovic T, Dumic I, Krivokapic Z. Combined Diagnostic Efficacy of Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR), and Mean Platelet Volume (MPV) as Biomarkers of Systemic Inflammation in the Diagnosis of Colorectal Cancer. Dis Markers. 2019;2019:6036979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 111] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 40. | Biswas M, Belle VS, Maripini N, Prabhu K. Neutrophil-lymphocyte ratio in pregnancy-associated maternal complications: A review. Asian Pac J Reproduct. 2021;10:252. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 41. | Nardozza LM, Caetano AC, Zamarian AC, Mazzola JB, Silva CP, Marçal VM, Lobo TF, Peixoto AB, Araujo Júnior E. Fetal growth restriction: current knowledge. Arch Gynecol Obstet. 2017;295:1061-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 372] [Article Influence: 46.5] [Reference Citation Analysis (0)] |