Peer-review started: July 29, 2014
First decision: August 14, 2014
Revised: August 27, 2014
Accepted: September 3, 2014
Article in press: September 4, 2014
Published online: September 28, 2014
Processing time: 87 Days and 10.9 Hours
Cerebral ageing is a complex biological process associated with progressing cerebrovascular disease and neuronal death. It does not always, however, associate with a functional decline, as the ageing mammalian brain retains considerable functional plasticity which supports successful cerebral ageing where age-related cognitive decline is modest. On the contrary, pathological cerebral ageing results in memory impairment and cognitive deterioration, with Alzheimer’s disease (AD) being a florid example. Trophic/growth factors promote brain plasticity; among them are peptides which belong to the insulin family. Preclinical research suggests that the evolutionarily conserved brain insulin/insulin-like growth factor-1 (IGF-1) signalling system controls lifespan and protects against some features of AD such as neurodegeneration-related accumulation of toxic proteins and cognitive deficiencies, as observed in animal models. Insulin and IGF-1 activate cell signalling mechanisms which play protective and regenerative roles; abnormalities in the insulin/IGF-1 system may trigger a cascade of neurodegeneration in AD. AD patients show cerebral resistance to insulin which associates with IGF-I resistance and dysregulation of insulin/IGF-1 receptors as well as cognitive deterioration. This review is focused on the roles of the insulin/IGF-1 signalling system in cerebral ageing and its potential involvement in neurodegeneration in the human brain as seen against the background of preclinical evidence.
Core tip: Age itself is a major risk factor for the development of age-related cognitive decline, Alzheimer’s and cerebrovascular diseases. Increased life expectancy necessitates the need to understand the processes that underlie successful vs pathological brain ageing in order to develop early interventions which may assist in delaying if not reversing the detrimental effects of brain ageing. This review focuses on the signalling system of insulin and insulin-like growth factor-1 (IIS) and its roles in cerebral ageing; it highlights some conflicting literature opinions and incomplete understandings of the roles and mechanisms of the IIS system.
- Citation: Romain G, Opacka-Juffry J. Cerebral ageing-the role of insulin and insulin-like growth factor signalling: A review. World J Neurol 2014; 4(3): 12-22
- URL: https://www.wjgnet.com/2218-6212/full/v4/i3/12.htm
- DOI: https://dx.doi.org/10.5316/wjn.v4.i3.12
Ageing is a complex biological process that affects all species ranging from invertebrates through to non-human mammals and humans, being underpinned by alterations at molecular and cellular levels[1,2] that compromise the organism’s physiological homeostasis, induce susceptibility to disease and accelerate death[3-5]. The cellular and anatomical changes that occur in the course of brain ageing contribute to cerebrovascular disease[6], impact motor performance and learning and memory functions[7,8], thus leading to cognitive decline and dementia[9]. As human life span is growing and the proportion of the elderly is increasing in most societies, the prevalence of age-dependent diseases such as Alzheimer’s disease (AD), Parkinson’s disease and stroke is also on the increase world-wide. Consequently, understanding the biological and molecular mechanisms of what constitutes “successful”vs pathological cerebral ageing is critical to both delaying the ageing process, enhancing the quality of life in the elderly, and alleviating the already overburdened health care systems, from the cost component involved in treating age-related diseases.
In view of the above points, research on brain-ageing has generated a pool of information regarding the potential mechanisms, which underpin cognitive decline. Advancements in molecular and cell technology, have been instrumental in identifying factors such as oxidative stress[10,11], epigenetic changes[12], mitochondrial dysfunction, inflammatory response[13], impaired cell signalling and gene expressions[14], autophagy and protein turnover, target of rapamycin (TOR) and insulin/ insulin-like growth factor (IGF) signalling as potential mechanisms that contribute to alterations observed in brain ageing[9].
This review will first discuss what constitutes “successful”vs pathological brain ageing. Within this context, the review will then focus predominantly on the evolutionarily conserved insulin/IGF-signalling (IIS) system, and its roles in cognitive decline, dementia, AD and neuroinflammation.
It has been postulated that the IIS system plays a regulatory role in organismal ageing, lifespan and longevity[15,16], and a reduced expression of its components under experimental conditions has been linked to amelioration of amyloid-β accumulation, the latter being one of the key features of AD[17-19], and cognitive impairment[20]. The IIS system merits an arduous and in depth investigation into the role and the extent of its involvement in cerebral aging, as targeting its components may pave the way to designing novel pharmacological approaches to early interventions and facilitate reversal or delay of cognitive decline in human patients.
Defining successful cerebral ageing has been both challenging and controversial and to date there is no scientific consensus defining normal ageing in older age. Despite the lack of an operational definition, it is generally agreed that successful cerebral ageing is a multi-dimensional process, characterized by the absence of cognitive impairment and preservation of mental faculties, which allows for social functioning and independence in older age[21]. This suggests that beyond the neurophysiological and psychological functions, equally vital are some esoteric elements such as wisdom and resilience, which together with lifestyle factors may contribute towards the variability, detected in cognitive abilities amongst “successful”vs“unsuccessful” elderly individuals and groups[22,23].
Current research postulates that the ageing mammalian brain retains a considerable functional plasticity, which is activity-related and thus it depends on the lifestyle of the individual (e.g.,[24]). There is evidence that human age-related diseases can be delayed by a healthy lifestyle which includes stress management, physical exercise and caloric restriction[24,25]. Thus, although genes are important determinants of longevity, an individual’s lifestyle is a powerful instrument that can delay the development of age-related diseases and lead to the path of ageing successfully[25-27].
Functional imaging studies on ageing human brains, suggest that in the absence of pathology, age-related cognitive decline is rather modest and varies amongst individuals[9]. It is characterized by anatomical and functional changes, which are associated with neuronal-synaptic molecular substrates specific to brain area[28,29]. These changes may be attributed to synaptic connectivity rather than neuronal and white matter losses[30,31].
In contrast, the pathologically ageing brain, as that in AD, exhibits marked cognitive decline, which is associated with a significant loss of synapses[32]. Although the molecular mechanisms underlying this synaptic impairment are not fully understood, dysfunction of γ-secretase is evident in many cases of early onset of AD[33], and the gamma-secretase-mediated EphA4 signalling system may be involved in the synaptic pathogenesis of AD[32,33]. Equally, apolipoprotein E4 can increase the presence of amyloid beta (aβ) oligomers in the brain, which in turn may increase the loss of dendritic spines and accelerate memory decline in AD[34].
Of the regions associated with memory and learning, the hippocampal formation exhibit age-related decrease in volume, which may be a consequence of a decrease in neuronal and synaptic density[28,30]; prefrontal cortex (PFC) also shows reductions in grey matter[35,36]. The PFC is implicated in higher executive functions, involving explicit, implicit and spatial memories[28,37,38]. Its decreased grey matter diffusivity may be a potential biomarker for early AD[39].
Similarly, the posterior cingulate, which is both anatomically and functionally connected to PFC and medial temporal lobe (MTL)[40,41], and which plays a vital role in encoding and retrieval of information[42,43] is also affected in ageing. Interestingly, it is one of the first structures to be affected in AD[44,45] but has not yet been explicitly studied in conjunction with PFC to establish associations between task-related and cognitive task activation[46].
These associations may be modifiable in healthily aged individuals[46-48] in contrast to AD patients[48-50]. PFC grey matter loss may trigger plasticity, which is dependent on the MTL function for memory tasks[51], and consequently, those individuals with functionally intact grey matter/MTL ratio, may make a greater use of the PFC[52].
The hippocampal region implicated in memory function shows age-related atrophy[53], and a decline of working memory function is often observed in healthy ageing[54]. In AD pathology, impaired hippocampal function is detectable even before the formation and accumulation of plaques[55] and volume analysis by means of MRI has been used as diagnostic tool in distinguishing AD patients and healthy age-matched subjects by measuring the grey matter volumes in the lateral temporal and parietal cortices[56]. Furthermore, the AD brain is characterized by ventricular enlargement[57-60], consistent with a considerable loss of grey and white matter[61].
The above alterations may be attributed to age-related neuronal loss, and/or compensatory plasticity, and future studies are needed to test how these three way structure-function-behaviour associations impact the grey matter loss and PFC activation in successful ageing[46]. Equally, stress-related hormonal changes[9] or compromised calcium homeostasis[38,62] can play a role too as prolonged increases of intracellular calcium concentrations may cause neurite degeneration and cell death in ageing[63].
In addition to the observed anatomical, functional and cellular changes, the brain’s neurochemistry is also affected, with dopaminergic, noradrenergic and cholinergic systems exhibiting deficits[60,64-69]. Studies on human and rhesus monkey PFC indicate that the balance between inhibitory and excitatory neurotransmission is decreased[70] as an effect of reduced gene expression, which may compromise neural activity resulting in excitotoxicity and neurodegenerative pathology. Positron emission tomography scans in ageing humans show a reduction in dopamine synthesis in the striatum, of relevance to frontal lobe cognitive function[71], and a marked decrease in dopamine receptor binding within caudate and putamen nuclei[66,72].
Reductions in serotonin synthesis, reuptake and receptor binding have also been noted in the caudate nucleus, putamen and PFC of ageing brains[67], and glutamate decreased levels in grey and white matter, basal ganglia have been reported[69,73].
It is of significance that all the pathological features of AD such as neuronal loss, neurofibrillary tangles and plagues may be present in the brains of elderly who may never show the full extent of cognitive deterioration observed in AD[9]. This resilience to cognitive decline in the presence of AD pathology may be attributed to “cognitive reserve”, which may reduce the risk of dementia in ageing[74]. It further suggests that the hallmarks of AD may be secondary to ageing.
One of the cellular mechanisms regulating ageing processes is the insulin and IIS system, which is described below with regard to its role and those of its components in cerebral ageing. This system, extensively studied in model organisms, appears to underpin the innate resilience that is essential in successful ageing; it may also present therapeutic potential in the treatment of debilitating neurodegenerative and cerebrovascular diseases.
Insulin and the IGF-1 and IGF-2 constitute a family of structurally similar peptides[75,76], which have been preserved in most organisms through evolution[77]. Peripherally, insulin is synthesized and secreted into blood by pancreatic cells, whereas IGF-1 and IGF-2 by the liver in response to the pituitary growth hormone[78].
Insulin is a powerful player in glucose homeostasis, e.g.,[79,80], which targets the liver, muscle, and adipose tissue[81], and also the vasculature and the brain[82]. The IGF-1 in contrast, is implicated in foetal and postnatal development, with a role in cellular survival of adult tissues[82]. The circulation and delivery of IGF-1 to the tissues is aided by IGF-1 binding proteins 1-6 in contrast to insulin, which circulates freely[82].
The transportation of insulin and IGF 1 into the brain is achieved through a saturable mechanism within the blood-brain barrier (BBB)[78,82,83], although there is evidence of their de novo synthesis in the central nervous system (CNS)[84-86]. Insulin’s ability to cross the BBB[83,87-89] depends on a number of factors such as age, fasting or obesity[88]. Under experimental conditions, insulin administered directly into the CNS, decreases body weight by suppressing appetite, lowers serum insulin levels and increases serum glucose[90,91]. An increase in peripheral insulin levels leads to increased cerebrospinal fluid (CSF) insulin, whereas chronic insulin resistance impairs cerebral transportation by down regulating insulin receptors (IR) at BBB[92]. Brain activity in healthy individuals subjected to direct determination of insulin sensitivity with the hyperinsulinemic-euglycemic clamp technique, has been shown to be affected by increased levels of circulating insulin[93].
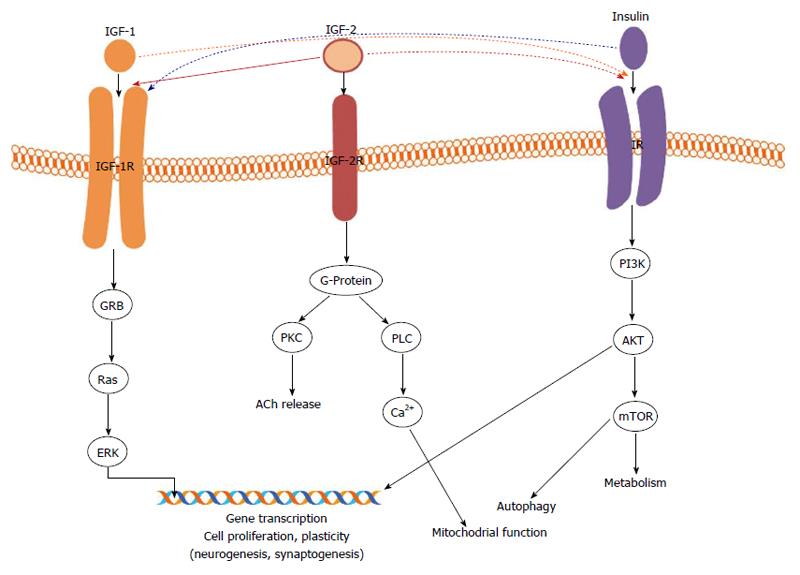
BBB uptake of IGF involves a lipoprotein receptor-related protein 1, the respective receptor (IGF-1R) and other transport mechanisms, enabling IGF access to CSF, and hypothalamic and hippocampal regions[82]. Insulin and IGFs activate signalling systems through their respective receptors, which belong to the tyrosine kinase receptor family[77]: IR, IGF-1R and IGF-2R (Figure 1). Low affinity binding can take place between insulin and IGF-1R, and IGF-1 and IR using the phosphoinositide 3-kinase (PI3K)/AKT pathway, while IGF-2 signals downstream not only via IGF-2R but also IGF-1R[77]. It should be mentioned that IGF-2R mainly controls the uptake and activation/destruction of extracellular of IGF-1/2 IGF2[94]; the present review is focused on IGF-1R (Figure 1).
IR and IGF-1R differ in their respective functions and tissue expressions[82] being present in the brain within neuron rich structures[95-97] and glial cells[18,98,99]; they are different entities expressed in diverse brain regions. The IR is highly expressed in the anterior thalamic and hypothalamic nuclei, olfactory bulb, hippocampus, cerebral cortex[100,101] and promotes plasticity through supporting synaptogenesis and synaptic remodelling[102,103], and metabolic homeostasis[75,104]. IGF-1R signalling supports normal brain development with its neurogenesis, and successful ageing, consistent with the roles of its agonist neurotrophins (see review[77]). A genetic modification, which results in IGF-1R deletion, causes microcephaly and death in experimental animals[105,106].
The IIS system has been probably the most widely studied conserved mechanism that extends lifespan in worms, flies and mammals[16], linking longevity and successful ageing across the species[107,108]. It has been suggested that although insulin does not directly influence cerebral glucose transport, it appears to influence regionally the distribution of glucose transporter (GLUT) isoforms such as GLUT 4 and GLUT 8; the former being expressed in the cerebellum, hippocampus, pituitary and hypothalamus[109] whereas the latter in the hippocampus and hypothalamus[110]. This selective stimulation of glucose uptake in the brain areas implicated in learning and memory renders the hormone a potent player in potential therapeutic use to restore or enhance impaired cognitive functions[111]. In addition, insulin’s indirect role in hippocampal functioning via the long-term potentiation cascade, involving the N-methyl-D-aspartate receptor[112] further suggests that insulin can be implicated in synaptic remodelling which is vital for the formation of new memories.
Similarly, insulin’s ability to modulate CNS levels of acetylcholine and norepinephrine, known to influence cognitive function[113,114], may further substantiate its role in neural activity and potential neural protection against the effects of oxidative stress[115].
Studies in animals and humans have suggested that deficiency or reduced effectiveness of insulin is a contributing factor to cognitive decline, impacting memory functions and brain ageing[116-118]. Insulin resistance has been positively correlated with neurodegeneration observed in AD and mild cognitive impairment[118,119]. Patients with type II diabetes mellitus, hypertension and chronic hyperinsulinemia show impaired verbal memory while enhanced memory function has been observed upon intranasal insulin administration[120,121].
In preclinical studies, liver-specific IGF-1-deficient (LID) mice, deficient in liver-derived IGF-1 exhibit impaired spatial learning and memory functions[122], demonstrating that this peptide is vital in mediating exercise-induced effects on the adult brain, thus suggesting promotion of neurogenesis[123,124]. In addition, IGF-1 appears to possess neurotrophic properties and plays a role in the amelioration of age-related reduction in hippocampal neurogenesis and behavioural deficits[125,126]; it also improves regional cerebral blood flow in normal rats[127]. It targets brain neurones and glia implying a trophic action on glutamatergic synapses, modulating hippocampal circuitries that are involved in learning and memory[116].
Within this context, research on brain ageing suggests that IGF-1 has potent effects on brain function, and that its reduced signalling during ageing may contribute to cognitive deterioration and compromise the organism’s ability to deal with age-associated cerebral pathologies[19]. Similarly, impaired insulin signalling in the brain has been linked to cognitive decline associated with pathological brain ageing[111,128]. Epidemiological studies suggest that individuals with type II diabetes and obesity may be at higher risk of developing vascular dementia[129].
On the other hand, centenarian studies provide evidence of correlation between reduced IIS activity and extreme human longevity. Ashkenazi centenarians have been found to have mutations in the IGF-1R that leads to lower activity of the respective signalling pathway[107], and centenarians’ offspring had lower peripheral IGF-1 activity when compared with appropriately matched-controls[130]. Of significance, in the same sample of centenarians’ offspring, IGF-1 was inversely related to insulin sensitivity[130], and an Italian cohort study reported an IR variant to be associated with longevity[131]. The above findings suggest that in humans the IIS system is a complex determinant of lifespan[132].
The importance of IR, IGF-1R and their respective agonist peptides has been underscored by data postulating the involvement of the IIS system in AD, which is characterized by amyloid-dependent neurodegeneration and late onset progressive cognitive decline. AD sufferers display impaired cerebral glucoregulation[133], reduced brain insulin receptor activity, reduced insulin concentrations in cerebrospinal fluid, peripheral hyperinsulinemia[134] and reduced insulin and IGF-1 expression[135], together with synaptic loss associated with the accumulation and formation of aggregate amyloid plagues (aβ) and neurofibrillary tangles (tau protein) as measured post mortem[136-138]. The above suggests that the IIS system may play a significant role in the loss of memory functions associated with AD and a reduction of its activity may reduced toxicity, delay aβ accumulation and improve cognitive functions[18,139-143].
In the preclinical approaches, mouse models of AD with a knockout of IGF-1 receptor exhibit reduced cognitive impairment, neurodegeneration and longer lifespan. The findings from the above studies, point to aβ oligomers as the toxic species associated with AD, and the ageing process to be associated with the organism’s exposure to their toxicity, leading to age-related neurodegenerative diseases[132]. Consequently, the IIS system was mechanistically linked to neurone-associated toxic protein accumulation and ageing, as reduced signalling is thought to protect the brain and slow the progression of AD[132]. It has been shown to activate the PI3K/AKT and Ras pathways (Figure 1). The former leads to the activation of mammalian TOR (mTOR), and rapamycin-treated mice have been shown to increase their lifespan and ameliorate age-related cognitive deficits[141,144]. The latter activates extracellular signal-regulated kinase-1/-2, which has been implicated in plasticity, including long-term potentiation, and memory formation in the CNS[145]. Although no data as yet exist to support the involvement of both pathways in the pathogenesis of AD, it may present an interesting direction for future research.
A number of current preclinical studies on the animal models of AD suggest that genetic reduction of the signalling pathway may protect against the AD pathology[18,20] while older studies seem to postulate that its reduction is associated with the age-related pathologies[146]. These conflicting views may arise from the use of different experimental approaches[19], or as it has been suggested, reduced IGF-1 peripheral bioactivity may not necessarily induce the same results in brain IGF-1 levels[147], postulating an independent regulatory activity.
It is important to appreciate the complexity of this relationship as human studies which try to correlate peripheral levels of IGF-1/2 with cognitive functioning in health and disease, report disparate finding, as illustrated below. Of the most recent publications in this area, a large scale long-term study on a community sample of over 3500 participants of middle and old ages has demonstrated that lower serum levels of IGF-1 associate with an increased risk of developing AD dementia while higher levels of IGF-1 associate with greater brain volumes in middle-aged participants free of stroke and dementia. The authors conclude that elevated levels of IGF-1 may protect against neurodegeneration[148]. On the other hand, the Caerphilly Prospective Study on 746 men has not found associations between age-related cognitive decline and IGF-1, contrary to IGF-2 which was associated with both normal age-related and pathological cognitive decline[149]. Furthermore, offspring from families with a parental history of AD appear to have higher serum IGF-1 levels in middle age when compared with appropriate controls, leading to a conclusion that elevated peripheral IGF-1 associates with an increased risk of AD[150].
Despite the existence of opposing views in the field, epidemiological evidence postulates a strong association between type II diabetes (T2D) and AD occurrence as AD patients exhibit higher rates of diabetes and impaired fasting glucose levels[151-153], and although the molecular mechanisms underlying this association are not yet clearly understood[82] impaired insulin signalling, amyloid-genesis and inflammation appear to be heavily implicated in the aetiology of diabetes, AD and consequently cerebral ageing[154].
Inflammation is seen a key player in obesity, insulin resistance and diabetes, as based on elevated levels of pro-inflammatory cytokines in the circulation and pancreatic islets of T2D patients[155]. Similarly, elevated levels of pro-inflammatory proteins and chemokines have been detected in post-mortem AD patients’ brains, e.g.,[156]. This was further substantiated by findings from studies on AD mouse models suggestive of inflammation as key to early and/or intermediate stages of the neurodegenerative condition[157]. There is consensus that cerebrovascular inflammation and neuroinflammation, along with an increased accumulation of toxic aβ, all result in a disruption of synaptic activity, which according to some theories is a trigger in AD pathophysiology, e.g.,[158].
Studies investigating IGF-1 and IGF-2 peptides’ expression in human microglia in vivo and in vitro suggest that both peptides are expressed in microglia, conferring vital protection against cytokine-mediated neuronal death. It should be mentioned here that microglial activation is associated with increased activities of inflammatory cytokines, e.g., interleukin (IL)-1β and IL-6 which itself can disrupt neural signalling, e.g.,[159].
Chronic inflammation increases the production of inflammatory cytokines in the long-term, which contributes to the suppression of neurotrophic factors, including the IGFs, and leads to progressive tissue damage, thus accelerating the onset of clinical manifestations of AD and metabolic disorders including T2D[160,161], and may contribute to neurodegeneration[162].
Research on brain ageing suggests that age itself is a major risk factor for the development of age-related cognitive decline, Alzheimer’s and cerebrovascular diseases. The increased life expectancy observed in most societies has further necessitated the need to understand the processes that underlie successful vs pathological brain ageing such that early interventions through lifestyle modifications or pharmacological agents may assist in delaying if not reversing the detrimental effects on brain pathology.
Within this context, this review examined the role of an evolutionarily conserved signalling pathway, IIS, with the focus on insulin and insulin-like growth factor IGF-1 and their roles in cerebral ageing. Translation of data derived from animal models allow for linking the IIS pathway with its supporting longevity, protein homeostasis, learning and memory, and delayed ageing. The above is also consistent with the human studies, which find evidence of reduced messaging for insulin, IGF-1 and their receptors in post mortem brains of patients with AD. While the link between insulin as such and brain ageing has been recognised, the IIS pathway in its entirety deserves more attention; our still incomplete understanding of the roles and mechanisms of this pathway calls for more translational research to explore novel treatments for cognitive decline through delaying cerebral ageing.
Some conflicting literature opinions and incomplete understanding of the roles and mechanisms of the IIS system demand novel approaches and directions in this field. The IIS system clearly lends itself to the ongoing search for modifiable physiological factors which may delay the onset of cognitive decline and cerebral ageing.
P- Reviewer: Cavanna AE, Lee YJ, Lichtor T S- Editor: Qi Y L- Editor: A E- Editor: Liu SQ
| 1. | de Magalhães JP. From cells to ageing: a review of models and mechanisms of cellular senescence and their impact on human ageing. Exp Cell Res. 2004;300:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 2. | de Magalhães JP, Faragher RG. Cell divisions and mammalian aging: integrative biology insights from genes that regulate longevity. Bioessays. 2008;30:567-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Kirkwood TB. The origins of human ageing. Philos Trans R Soc Lond B Biol Sci. 1997;352:1765-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 48] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Partridge L, Mangel M. Messages from mortality: the evolution of death rates in the old. Trends Ecol Evol. 1999;14:438-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 69] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Sharpless NE, DePinho RA. How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol. 2007;8:703-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 654] [Cited by in RCA: 626] [Article Influence: 34.8] [Reference Citation Analysis (1)] |
| 6. | Sonntag WE, Lynch C, Thornton P, Khan A, Bennett S, Ingram R. The effects of growth hormone and IGF-1 deficiency on cerebrovascular and brain ageing. J Anat. 2000;197 Pt 4:575-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 96] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Mattay VS, Fera F, Tessitore A, Hariri AR, Das S, Callicott JH, Weinberger DR. Neurophysiological correlates of age-related changes in human motor function. Neurology. 2002;58:630-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 411] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 8. | Koivisto K, Reinikainen KJ, Hänninen T, Vanhanen M, Helkala EL, Mykkänen L, Laakso M, Pyörälä K, Riekkinen PJ. Prevalence of age-associated memory impairment in a randomly selected population from eastern Finland. Neurology. 1995;45:741-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 113] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Bishop NA, Lu T, Yankner BA. Neural mechanisms of ageing and cognitive decline. Nature. 2010;464:529-535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1050] [Cited by in RCA: 935] [Article Influence: 62.3] [Reference Citation Analysis (0)] |
| 10. | Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H. Trends in oxidative aging theories. Free Radic Biol Med. 2007;43:477-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 752] [Cited by in RCA: 706] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 11. | Wolf FI, Fasanella S, Tedesco B, Cavallini G, Donati A, Bergamini E, Cittadini A. Peripheral lymphocyte 8-OHdG levels correlate with age-associated increase of tissue oxidative DNA damage in Sprague-Dawley rats. Protective effects of caloric restriction. Exp Gerontol. 2005;40:181-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH. Recovery of learning and memory is associated with chromatin remodelling. Nature. 2007;447:178-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 840] [Cited by in RCA: 895] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 13. | McGeer EG, McGeer PL. Neuroinflammation in Alzheimer’s disease and mild cognitive impairment: a field in its infancy. J Alzheimers Dis. 2010;19:355-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 264] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 14. | Freitas AA, de Magalhães JP. A review and appraisal of the DNA damage theory of ageing. Mutat Res. 2011;728:12-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 145] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 15. | Blalock EM, Geddes JW, Chen KC, Porter NM, Markesbery WR, Landfield PW. Incipient Alzheimer’s disease: microarray correlation analyses reveal major transcriptional and tumor suppressor responses. Proc Natl Acad Sci USA. 2004;101:2173-2178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 710] [Cited by in RCA: 755] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 16. | Broughton S, Partridge L. Insulin/IGF-like signalling, the central nervous system and aging. Biochem J. 2009;418:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 175] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 17. | Moloney AM, Griffin RJ, Timmons S, O’Connor R, Ravid R, O’Neill C. Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer’s disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiol Aging. 2010;31:224-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 598] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 18. | Freude S, Hettich MM, Schumann C, Stöhr O, Koch L, Köhler C, Udelhoven M, Leeser U, Müller M, Kubota N. Neuronal IGF-1 resistance reduces Abeta accumulation and protects against premature death in a model of Alzheimer’s disease. FASEB J. 2009;23:3315-3324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 162] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 19. | Piriz J, Muller A, Trejo JL, Torres-Aleman I. IGF-I and the aging mammalian brain. Exp Gerontol. 2011;46:96-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 20. | Cohen E, Paulsson JF, Blinder P, Burstyn-Cohen T, Du D, Estepa G, Adame A, Pham HM, Holzenberger M, Kelly JW. Reduced IGF-1 signaling delays age-associated proteotoxicity in mice. Cell. 2009;139:1157-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 392] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 21. | Hendrie HC, Albert MS, Butters MA, Gao S, Knopman DS, Launer LJ, Yaffe K, Cuthbert BN, Edwards E, Wagster MV. The NIH Cognitive and Emotional Health Project. Report of the Critical Evaluation Study Committee. Alzheimers Dement. 2006;2:12-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 217] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 22. | Lamond AJ, Depp CA, Allison M, Langer R, Reichstadt J, Moore DJ, Golshan S, Ganiats TG, Jeste DV. Measurement and predictors of resilience among community-dwelling older women. J Psychiatr Res. 2008;43:148-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 277] [Cited by in RCA: 223] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 23. | Jeste DV, Ardelt M, Blazer D, Kraemer HC, Vaillant G, Meeks TW. Expert consensus on characteristics of wisdom: a Delphi method study. Gerontologist. 2010;50:668-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 24. | Stranahan AM, Mattson MP. Recruiting adaptive cellular stress responses for successful brain ageing. Nat Rev Neurosci. 2012;13:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 124] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 25. | Gitler AD. Another reason to exercise. Science. 2011;334:606-607. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Abbott A. Ageing: growing old gracefully. Nature. 2004;428:116-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Mora F, Segovia G, Del Arco A, de Blas M, Garrido P. Stress, neurotransmitters, corticosterone and body-brain integration. Brain Res. 2012;1476:71-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 153] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 28. | Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5:87-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1329] [Cited by in RCA: 1423] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 29. | Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7:30-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 971] [Cited by in RCA: 1059] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 30. | Morrison JH, Baxter MG. The ageing cortical synapse: hallmarks and implications for cognitive decline. Nat Rev Neurosci. 2012;13:240-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 678] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 31. | Balietti M, Tamagnini F, Fattoretti P, Burattini C, Casoli T, Platano D, Lattanzio F, Aicardi G. Impairments of synaptic plasticity in aged animals and in animal models of Alzheimer’s disease. Rejuvenation Res. 2012;15:235-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Matsui C, Inoue E, Kakita A, Arita K, Deguchi-Tawarada M, Togawa A, Yamada A, Takai Y, Takahashi H. Involvement of the γ-secretase-mediated EphA4 signaling pathway in synaptic pathogenesis of Alzheimer’s disease. Brain Pathol. 2012;22:776-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Inoue E, Deguchi-Tawarada M, Togawa A, Matsui C, Arita K, Katahira-Tayama S, Sato T, Yamauchi E, Oda Y, Takai Y. Synaptic activity prompts gamma-secretase-mediated cleavage of EphA4 and dendritic spine formation. J Cell Biol. 2009;185:551-564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 34. | Hashimoto T, Serrano-Pozo A, Hori Y, Adams KW, Takeda S, Banerji AO, Mitani A, Joyner D, Thyssen DH, Bacskai BJ. Apolipoprotein E, especially apolipoprotein E4, increases the oligomerization of amyloid beta peptide. J Neurosci. 2012;32:1581-1592. [RCA] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 213] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 35. | Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thornton AE, Acker JD. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex. 1997;7:268-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 854] [Cited by in RCA: 866] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 36. | Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Morris JC, Dale AM, Fischl B. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14:721-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1274] [Cited by in RCA: 1382] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 37. | Woodruff-Pak DS, Thompson RF. Classical conditioning of the eyeblink response in the delay paradigm in adults aged 18-83 years. Psychol Aging. 1988;3:219-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 90] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 38. | Kenessary A, Zhumadilov Z, Nurgozhin T, Kipling D, Yeoman M, Cox L, Ostler E, Faragher R. Biomarkers, interventions and healthy ageing. N Biotechnol. 2013;30:373-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 39. | Jacobs HI, van Boxtel MP, Gronenschild EH, Uylings HB, Jolles J, Verhey FR. Decreased gray matter diffusivity: a potential early Alzheimer’s disease biomarker? Alzheimers Dement. 2013;9:93-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 40. | Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19:72-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1813] [Cited by in RCA: 1633] [Article Influence: 102.1] [Reference Citation Analysis (0)] |
| 41. | Dörfel D, Werner A, Schaefer M, von Kummer R, Karl A. Distinct brain networks in recognition memory share a defined region in the precuneus. Eur J Neurosci. 2009;30:1947-1959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 42. | Vannini P, O’Brien J, O’Keefe K, Pihlajamäki M, Laviolette P, Sperling RA. What goes down must come up: role of the posteromedial cortices in encoding and retrieval. Cereb Cortex. 2011;21:22-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 43. | Huijbers W, Vannini P, Sperling RA, C M P, Cabeza R, Daselaar SM. Explaining the encoding/retrieval flip: memory-related deactivations and activations in the posteromedial cortex. Neuropsychologia. 2012;50:3764-3774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 44. | Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol. 1997;42:85-94. [PubMed] |
| 45. | Pengas G, Hodges JR, Watson P, Nestor PJ. Focal posterior cingulate atrophy in incipient Alzheimer’s disease. Neurobiol Aging. 2010;31:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 46. | Maillet D, Rajah MN. Association between prefrontal activity and volume change in prefrontal and medial temporal lobes in aging and dementia: a review. Ageing Res Rev. 2013;12:479-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 47. | Brassen S, Büchel C, Weber-Fahr W, Lehmbeck JT, Sommer T, Braus DF. Structure-function interactions of correct retrieval in healthy elderly women. Neurobiol Aging. 2009;30:1147-1156. [PubMed] |
| 48. | Trivedi MA, Stoub TR, Murphy CM, George S, deToledo-Morrell L, Shah RC, Whitfield-Gabrieli S, Gabrieli JD, Stebbins GT. Entorhinal cortex volume is associated with episodic memory related brain activation in normal aging and amnesic mild cognitive impairment. Brain Imaging Behav. 2011;5:126-136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 49. | Rémy F, Mirrashed F, Campbell B, Richter W. Verbal episodic memory impairment in Alzheimer’s disease: a combined structural and functional MRI study. Neuroimage. 2005;25:253-266. [PubMed] |
| 50. | Meulenbroek O, Rijpkema M, Kessels RP, Rikkert MG, Fernández G. Autobiographical memory retrieval in patients with Alzheimer’s disease. Neuroimage. 2010;53:331-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 51. | Greenwood PM. Functional plasticity in cognitive aging: review and hypothesis. Neuropsychology. 2007;21:657-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 191] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 52. | Rosen AC, Gabrieli JD, Stoub T, Prull MW, O’Hara R, Yesavage J, deToledo-Morrell L. Relating medial temporal lobe volume to frontal fMRI activation for memory encoding in older adults. Cortex. 2005;41:595-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 53. | Du AT, Schuff N, Chao LL, Kornak J, Jagust WJ, Kramer JH, Reed BR, Miller BL, Norman D, Chui HC. Age effects on atrophy rates of entorhinal cortex and hippocampus. Neurobiol Aging. 2006;27:733-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 168] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 54. | Wang M, Gamo NJ, Yang Y, Jin LE, Wang XJ, Laubach M, Mazer JA, Lee D, Arnsten AF. Neuronal basis of age-related working memory decline. Nature. 2011;476:210-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 335] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 55. | Marchetti C, Marie H. Hippocampal synaptic plasticity in Alzheimer’s disease: what have we learned so far from transgenic models? Rev Neurosci. 2011;22:373-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 56. | Hänggi J, Streffer J, Jäncke L, Hock C. Volumes of lateral temporal and parietal structures distinguish between healthy aging, mild cognitive impairment, and Alzheimer’s disease. J Alzheimers Dis. 2011;26:719-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 57. | Carmichael OT, Kuller LH, Lopez OL, Thompson PM, Dutton RA, Lu A, Lee SE, Lee JY, Aizenstein HJ, Meltzer CC. Ventricular volume and dementia progression in the Cardiovascular Health Study. Neurobiol Aging. 2007;28:389-397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 58. | Carmichael OT, Kuller LH, Lopez OL, Thompson PM, Dutton RA, Lu A, Lee SE, Lee JY, Aizenstein HJ, Meltzer CC. Cerebral ventricular changes associated with transitions between normal cognitive function, mild cognitive impairment, and dementia. Alzheimer Dis Assoc Disord. 2007;21:14-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 342] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 59. | Nestor SM, Rupsingh R, Borrie M, Smith M, Accomazzi V, Wells JL, Fogarty J, Bartha R; Alzheimer’s Disease Neuroimaging Initiative. Ventricular enlargement as a possible measure of Alzheimer’s disease progression validated using the Alzheimer’s disease neuroimaging initiative database. Brain. 2008;131:2443-2454. |
| 60. | Apostolova LG, Mosconi L, Thompson PM, Green AE, Hwang KS, Ramirez A, Mistur R, Tsui WH, de Leon MJ. Subregional hippocampal atrophy predicts Alzheimer’s dementia in the cognitively normal. Neurobiol Aging. 2010;31:1077-1088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 239] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 61. | Salat DH, Kaye JA, Janowsky JS. Prefrontal gray and white matter volumes in healthy aging and Alzheimer disease. Arch Neurol. 1999;56:338-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 182] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 62. | Toescu EC, Verkhratsky A, Landfield PW. Ca2+ regulation and gene expression in normal brain aging. Trends Neurosci. 2004;27:614-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 164] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 63. | Kapogiannis D, Mattson MP. Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer’s disease. Lancet Neurol. 2011;10:187-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 462] [Cited by in RCA: 426] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 64. | Rinne JO, Lönnberg P, Marjamäki P. Age-dependent decline in human brain dopamine D1 and D2 receptors. Brain Res. 1990;508:349-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 148] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 65. | Iyo M, Yamasaki T. The detection of age-related decrease of dopamine D1, D2 and serotonin 5-HT2 receptors in living human brain. Prog Neuropsychopharmacol Biol Psychiatry. 1993;17:415-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 66. | Wang Y, Chan GL, Holden JE, Dobko T, Mak E, Schulzer M, Huser JM, Snow BJ, Ruth TJ, Calne DB. Age-dependent decline of dopamine D1 receptors in human brain: a PET study. Synapse. 1998;30:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 67. | Yamamoto M, Suhara T, Okubo Y, Ichimiya T, Sudo Y, Inoue M, Takano A, Yasuno F, Yoshikawa K, Tanada S. Age-related decline of serotonin transporters in living human brain of healthy males. Life Sci. 2002;71:751-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 68. | Ota M, Yasuno F, Ito H, Seki C, Nozaki S, Asada T, Suhara T. Age-related decline of dopamine synthesis in the living human brain measured by positron emission tomography with L-[beta-11C]DOPA. Life Sci. 2006;79:730-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 69. | Chang L, Jiang CS, Ernst T. Effects of age and sex on brain glutamate and other metabolites. Magn Reson Imaging. 2009;27:142-145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 70. | Loerch PM, Lu T, Dakin KA, Vann JM, Isaacs A, Geula C, Wang J, Pan Y, Gabuzda DH, Li C. Evolution of the aging brain transcriptome and synaptic regulation. PLoS One. 2008;3:e3329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 245] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 71. | Braskie MN, Wilcox CE, Landau SM, O’Neil JP, Baker SL, Madison CM, Kluth JT, Jagust WJ. Relationship of striatal dopamine synthesis capacity to age and cognition. J Neurosci. 2008;28:14320-14328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 72. | Kaasinen V, Vilkman H, Hietala J, Någren K, Helenius H, Olsson H, Farde L, Rinne J. Age-related dopamine D2/D3 receptor loss in extrastriatal regions of the human brain. Neurobiol Aging. 2000;21:683-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 248] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 73. | Sailasuta N, Ernst T, Chang L. Regional variations and the effects of age and gender on glutamate concentrations in the human brain. Magn Reson Imaging. 2008;26:667-675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 74. | Honer WG, Barr AM, Sawada K, Thornton AE, Morris MC, Leurgans SE, Schneider JA, Bennett DA. Cognitive reserve, presynaptic proteins and dementia in the elderly. Transl Psychiatry. 2012;2:e114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 75. | Bondy CA, Cheng CM. Signaling by insulin-like growth factor 1 in brain. Eur J Pharmacol. 2004;490:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 257] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 76. | Schulingkamp RJ, Pagano TC, Hung D, Raffa RB. Insulin receptors and insulin action in the brain: review and clinical implications. Neurosci Biobehav Rev. 2000;24:855-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 333] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 77. | Fernandez AM, Torres-Alemán I. The many faces of insulin-like peptide signalling in the brain. Nat Rev Neurosci. 2012;13:225-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 662] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 78. | Skottner A. Biosynthesis of Growth Hormone and Insulin-Like Growth Factor-I and the Regulation of their Secretion. Open Endocrinol J. 2012;6:3-12. |
| 79. | Banting FG, Best CH, Collip JB, Campbell WR, Fletcher AA. Pancreatic Extracts in the Treatment of Diabetes Mellitus. Can Med Assoc J. 1922;12:141-146. [PubMed] |
| 80. | Zhao WQ, Chen H, Quon MJ, Alkon DL. Insulin and the insulin receptor in experimental models of learning and memory. Eur J Pharmacol. 2004;490:71-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 354] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 81. | Fulop T, Larbi A, Douziech N. Insulin receptor and ageing. Pathol Biol (Paris). 2003;51:574-580. [PubMed] |
| 82. | Werner H, LeRoith D. Insulin and insulin-like growth factor receptors in the brain: Physiological and pathological aspects. Eur Neuropsychopharmacol. 2014;24:1947-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 83. | Schechter R, Whitmire J, Holtzclaw L, George M, Harlow R, Devaskar SU. Developmental regulation of insulin in the mammalian central nervous system. Brain Res. 1992;582:27-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 84. | Lupu F, Terwilliger JD, Lee K, Segre GV, Efstratiadis A. Roles of growth hormone and insulin-like growth factor 1 in mouse postnatal growth. Dev Biol. 2001;229:141-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 85. | Sun LY, Al-Regaiey K, Masternak MM, Wang J, Bartke A. Local expression of GH and IGF-1 in the hippocampus of GH-deficient long-lived mice. Neurobiol Aging. 2005;26:929-937. [PubMed] |
| 86. | Costantini C, Scrable H, Puglielli L. An aging pathway controls the TrkA to p75NTR receptor switch and amyloid beta-peptide generation. EMBO J. 2006;25:1997-2006. [PubMed] |
| 87. | Margolis RU, Altszuler N. Insulin in the cerebrospinal fluid. Nature. 1967;215:1375-1376. [PubMed] |
| 88. | Banks WA. The source of cerebral insulin. Eur J Pharmacol. 2004;490:5-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 340] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 89. | Burns JM, Donnelly JE, Anderson HS, Mayo MS, Spencer-Gardner L, Thomas G, Cronk BB, Haddad Z, Klima D, Hansen D. Peripheral insulin and brain structure in early Alzheimer disease. Neurology. 2007;69:1094-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 90. | Florant GL, Singer L, Scheurink AJ, Park CR, Richardson RD, Woods SC. Intraventricular insulin reduces food intake and body weight of marmots during the summer feeding period. Physiol Behav. 1991;49:335-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 91. | Foster LA, Ames NK, Emery RS. Food intake and serum insulin responses to intraventricular infusions of insulin and IGF-I. Physiol Behav. 1991;50:745-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 81] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 92. | Moreira PI, Duarte AI, Santos MS, Rego AC, Oliveira CR. An integrative view of the role of oxidative stress, mitochondria and insulin in Alzheimer’s disease. J Alzheimers Dis. 2009;16:741-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 135] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 93. | Freude S, Plum L, Schnitker J, Leeser U, Udelhoven M, Krone W, Bruning JC, Schubert M. Peripheral hyperinsulinemia promotes tau phosphorylation in vivo. Diabetes. 2005;54:3343-3348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 110] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 94. | Hawkes C, Kar S. The insulin-like growth factor-II/mannose-6-phosphate receptor: structure, distribution and function in the central nervous system. Brain Res Brain Res Rev. 2004;44:117-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 116] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 95. | de la Monte SM, Wands JR. Review of insulin and insulin-like growth factor expression, signaling, and malfunction in the central nervous system: relevance to Alzheimer’s disease. J Alzheimers Dis. 2005;7:45-61. [PubMed] |
| 96. | Hill JM, Lesniak MA, Pert CB, Roth J. Autoradiographic localization of insulin receptors in rat brain: prominence in olfactory and limbic areas. Neuroscience. 1986;17:1127-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 291] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 97. | Banks WA, Jaspan JB, Huang W, Kastin AJ. Transport of insulin across the blood-brain barrier: saturability at euglycemic doses of insulin. Peptides. 1997;18:1423-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 226] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 98. | Broughton SK, Chen H, Riddle A, Kuhn SE, Nagalla S, Roberts CT, Back SA. Large-scale generation of highly enriched neural stem-cell-derived oligodendroglial cultures: maturation-dependent differences in insulin-like growth factor-mediated signal transduction. J Neurochem. 2007;100:628-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 99. | Zeger M, Popken G, Zhang J, Xuan S, Lu QR, Schwab MH, Nave KA, Rowitch D, D’Ercole AJ, Ye P. Insulin-like growth factor type 1 receptor signaling in the cells of oligodendrocyte lineage is required for normal in vivo oligodendrocyte development and myelination. Glia. 2007;55:400-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 144] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 100. | Havrankova J, Roth J, Brownstein M. Insulin receptors are widely distributed in the central nervous system of the rat. Nature. 1978;272:827-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 804] [Cited by in RCA: 790] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 101. | Unger JW, Livingston JN, Moss AM. Insulin receptors in the central nervous system: localization, signalling mechanisms and functional aspects. Prog Neurobiol. 1991;36:343-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 282] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 102. | Chiu SL, Cline HT. Insulin receptor signaling in the development of neuronal structure and function. Neural Dev. 2010;5:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 158] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 103. | Abbott MA, Wells DG, Fallon JR. The insulin receptor tyrosine kinase substrate p58/53 and the insulin receptor are components of CNS synapses. J Neurosci. 1999;19:7300-7308. [PubMed] |
| 104. | McNay EC, Ong CT, McCrimmon RJ, Cresswell J, Bogan JS, Sherwin RS. Hippocampal memory processes are modulated by insulin and high-fat-induced insulin resistance. Neurobiol Learn Mem. 2010;93:546-553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 315] [Cited by in RCA: 298] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 105. | Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73-82. [PubMed] |
| 106. | Vicario-Abejón C, Yusta-Boyo MJ, Fernández-Moreno C, de Pablo F. Locally born olfactory bulb stem cells proliferate in response to insulin-related factors and require endogenous insulin-like growth factor-I for differentiation into neurons and glia. J Neurosci. 2003;23:895-906. [PubMed] |
| 107. | Suh Y, Atzmon G, Cho MO, Hwang D, Liu B, Leahy DJ, Barzilai N, Cohen P. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc Natl Acad Sci USA. 2008;105:3438-3442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 573] [Cited by in RCA: 521] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 108. | Flachsbart F, Caliebe A, Kleindorp R, Blanché H, von Eller-Eberstein H, Nikolaus S, Schreiber S, Nebel A. Association of FOXO3A variation with human longevity confirmed in German centenarians. Proc Natl Acad Sci USA. 2009;106:2700-2705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 428] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 109. | Reagan LP, Gorovits N, Hoskin EK, Alves SE, Katz EB, Grillo CA, Piroli GG, McEwen BS, Charron MJ. Localization and regulation of GLUTx1 glucose transporter in the hippocampus of streptozotocin diabetic rats. Proc Natl Acad Sci USA. 2001;98:2820-2825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 72] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 110. | Brant AM, Jess TJ, Milligan G, Brown CM, Gould GW. Immunological analysis of glucose transporters expressed in different regions of the rat brain and central nervous system. Biochem Biophys Res Commun. 1993;192:1297-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 111] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 111. | Cholerton B, Baker LD, Craft S. Insulin resistance and pathological brain ageing. Diabet Med. 2011;28:1463-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 112. | Skeberdis VA, Lan J, Zheng X, Zukin RS, Bennett MV. Insulin promotes rapid delivery of N-methyl-D- aspartate receptors to the cell surface by exocytosis. Proc Natl Acad Sci USA. 2001;98:3561-3566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 260] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 113. | Figlewicz DP, Szot P, Israel PA, Payne C, Dorsa DM. Insulin reduces norepinephrine transporter mRNA in vivo in rat locus coeruleus. Brain Res. 1993;602:161-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 90] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 114. | Kopf D, Baratti M. Effects of postraining administration of insulin on retention of a habituation response in mice: participation of a central cholinergic mechanism. Neurobiol Learn Mem. 1999;71:50-61. [RCA] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 115. | Duarte AI, Santos MS, Seiça R, Oliveira CR. Oxidative stress affects synaptosomal gamma-aminobutyric acid and glutamate transport in diabetic rats: the role of insulin. Diabetes. 2004;53:2110-2116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 116. | Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci. 2001;21:1628-1634. [PubMed] |
| 117. | Zhao WQ, Alkon DL. Role of insulin and insulin receptor in learning and memory. Mol Cell Endocrinol. 2001;177:125-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 362] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 118. | Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, Fuino RL, Kawaguchi KR, Samoyedny AJ, Wilson RS. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012;122:1316-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1129] [Cited by in RCA: 1408] [Article Influence: 108.3] [Reference Citation Analysis (0)] |
| 119. | Frölich L, Blum-Degen D, Bernstein HG, Engelsberger S, Humrich J, Laufer S, Muschner D, Thalheimer A, Türk A, Hoyer S. Brain insulin and insulin receptors in aging and sporadic Alzheimer’s disease. J Neural Transm. 1998;105:423-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 526] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 120. | Wallum BJ, Taborsky GJ, Porte D, Figlewicz DP, Jacobson L, Beard JC, Ward WK, Dorsa D. Cerebrospinal fluid insulin levels increase during intravenous insulin infusions in man. J Clin Endocrinol Metab. 1987;64:190-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 189] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 121. | Reger MA, Watson GS, Frey WH, Baker LD, Cholerton B, Keeling ML, Belongia DA, Fishel MA, Plymate SR, Schellenberg GD. Effects of intranasal insulin on cognition in memory-impaired older adults: modulation by APOE genotype. Neurobiol Aging. 2006;27:451-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 491] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 122. | Svensson J, Diez M, Engel J, Wass C, Tivesten A, Jansson JO, Isaksson O, Archer T, Hökfelt T, Ohlsson C. Endocrine, liver-derived IGF-I is of importance for spatial learning and memory in old mice. J Endocrinol. 2006;189:617-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 123. | O’Kusky JR, Ye P, D’Ercole AJ. Insulin-like growth factor-I promotes neurogenesis and synaptogenesis in the hippocampal dentate gyrus during postnatal development. J Neurosci. 2000;20:8435-8442. [PubMed] |
| 124. | Torres-Aleman I. Serum growth factors and neuroprotective surveillance: focus on IGF-1. Mol Neurobiol. 2000;21:153-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 125. | Lichtenwalner RJ, Forbes ME, Bennett SA, Lynch CD, Sonntag WE, Riddle DR. Intracerebroventricular infusion of insulin-like growth factor-I ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience. 2001;107:603-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 319] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 126. | Markowska AL, Mooney M, Sonntag WE. Insulin-like growth factor-1 ameliorates age-related behavioral deficits. Neuroscience. 1998;87:559-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 212] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 127. | Gillespie CM, Merkel AL, Martin AA. Effects of insulin-like growth factor-I and LR3IGF-I on regional blood flow in normal rats. J Endocrinol. 1997;155:351-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 128. | Hölscher C. Diabetes as a risk factor for Alzheimer’s disease: insulin signalling impairment in the brain as an alternative model of Alzheimer’s disease. Biochem Soc Trans. 2011;39:891-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 129. | MacKnight C, Rockwood K, Awalt E, McDowell I. Diabetes mellitus and the risk of dementia, Alzheimer’s disease and vascular cognitive impairment in the Canadian Study of Health and Aging. Dement Geriatr Cogn Disord. 2002;14:77-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 206] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 130. | Vitale G, Brugts MP, Ogliari G, Castaldi D, Fatti LM, Varewijck AJ, Lamberts SW, Monti D, Bucci L, Cevenini E. Low circulating IGF-I bioactivity is associated with human longevity: findings in centenarians’ offspring. Aging (Albany NY). 2012;4:580-589. [PubMed] |
| 131. | Barbieri M, Rizzo MR, Papa M, Boccardi V, Esposito A, White MF, Paolisso G. The IRS2 Gly1057Asp variant is associated with human longevity. J Gerontol A Biol Sci Med Sci. 2010;65:282-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 132. | Dillin A, Cohen E. Ageing and protein aggregation-mediated disorders: from invertebrates to mammals. Philos Trans R Soc Lond B Biol Sci. 2011;366:94-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 133. | Mosconi L, Mistur R, Switalski R, Brys M, Glodzik L, Rich K, Pirraglia E, Tsui W, De Santi S, de Leon MJ. Declining brain glucose metabolism in normal individuals with a maternal history of Alzheimer disease. Neurology. 2009;72:513-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 153] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 134. | Zhao L, Teter B, Morihara T, Lim GP, Ambegaokar SS, Ubeda OJ, Frautschy SA, Cole GM. Insulin-degrading enzyme as a downstream target of insulin receptor signaling cascade: implications for Alzheimer’s disease intervention. J Neurosci. 2004;24:11120-11126. [PubMed] |
| 135. | Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR, de la Monte SM. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease--is this type 3 diabetes? J Alzheimers Dis. 2005;7:63-80. [PubMed] |
| 136. | Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18:351-357. [PubMed] |
| 137. | Benilova I, Karran E, De Strooper B. The toxic Aβ oligomer and Alzheimer’s disease: an emperor in need of clothes. Nat Neurosci. 2012;15:349-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1387] [Cited by in RCA: 1581] [Article Influence: 121.6] [Reference Citation Analysis (0)] |
| 138. | Haass C. Initiation and propagation of neurodegeneration. Nat Med. 2010;16:1201-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 139. | Franke TF. PI3K/Akt: getting it right matters. Oncogene. 2008;27:6473-6488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 545] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 140. | Cohen E, Bieschke J, Perciavalle RM, Kelly JW, Dillin A. Opposing activities protect against age-onset proteotoxicity. Science. 2006;313:1604-1610. [PubMed] |
| 141. | Caccamo A, Majumder S, Richardson A, Strong R, Oddo S. Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: effects on cognitive impairments. J Biol Chem. 2010;285:13107-13120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 685] [Cited by in RCA: 707] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 142. | Majumder S, Caccamo A, Medina DX, Benavides AD, Javors MA, Kraig E, Strong R, Richardson A, Oddo S. Lifelong rapamycin administration ameliorates age-dependent cognitive deficits by reducing IL-1β and enhancing NMDA signaling. Aging Cell. 2012;11:326-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 168] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 143. | Killick R, Scales G, Leroy K, Causevic M, Hooper C, Irvine EE, Choudhury AI, Drinkwater L, Kerr F, Al-Qassab H. Deletion of Irs2 reduces amyloid deposition and rescues behavioural deficits in APP transgenic mice. Biochem Biophys Res Commun. 2009;386:257-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 144. | Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392-395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3003] [Cited by in RCA: 2849] [Article Influence: 178.1] [Reference Citation Analysis (0)] |
| 145. | Sweatt JD. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J Neurochem. 2001;76:1-10. [PubMed] |
| 146. | Carro E, Trejo JL, Gomez-Isla T, LeRoith D, Torres-Aleman I. Serum insulin-like growth factor I regulates brain amyloid-beta levels. Nat Med. 2002;8:1390-1397. [PubMed] |
| 147. | Adams MM, Elizabeth Forbes M, Constance Linville M, Riddle DR, Sonntag WE, Brunso-Bechtold JK. Stability of local brain levels of insulin-like growth factor-I in two well-characterized models of decreased plasma IGF-I. Growth Factors. 2009;27:181-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 148. | Westwood AJ, Beiser A, Decarli C, Harris TB, Chen TC, He XM, Roubenoff R, Pikula A, Au R, Braverman LE. Insulin-like growth factor-1 and risk of Alzheimer dementia and brain atrophy. Neurology. 2014;82:1613-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 165] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 149. | Green CJ, Holly JM, Bayer A, Fish M, Ebrahim S, Gallacher J, Ben-Shlomo Y. The role of IGF-I, IGF-II, and IGFBP-3 in male cognitive aging and dementia risk: the Caerphilly Prospective Study. J Alzheimers Dis. 2014;41:867-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 150. | van Exel E, Eikelenboom P, Comijs H, Deeg DJ, Stek ML, Westendorp RG. Insulin-like growth factor-1 and risk of late-onset Alzheimer’s disease: findings from a family study. Neurobiol Aging. 2014;35:725.e7-725.10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 151. | Kopf D, Frölich L. Risk of incident Alzheimer’s disease in diabetic patients: a systematic review of prospective trials. J Alzheimers Dis. 2009;16:677-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 163] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 152. | Biessels GJ, De Leeuw FE, Lindeboom J, Barkhof F, Scheltens P. Increased cortical atrophy in patients with Alzheimer’s disease and type 2 diabetes mellitus. J Neurol Neurosurg Psychiatry. 2006;77:304-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 153. | Janson J, Laedtke T, Parisi JE, O’Brien P, Petersen RC, Butler PC. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes. 2004;53:474-481. [PubMed] |
| 154. | Yang Y, Song W. Molecular links between Alzheimer’s disease and diabetes mellitus. Neuroscience. 2013;250:140-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 156] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 155. | Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012;18:363-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1082] [Cited by in RCA: 1171] [Article Influence: 90.1] [Reference Citation Analysis (0)] |
| 156. | Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21:383-421. [PubMed] |
| 157. | de la Monte SM, Tong M. Brain metabolic dysfunction at the core of Alzheimer’s disease. Biochem Pharmacol. 2014;88:548-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 342] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 158. | Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med. 2006;12:1005-1015. [PubMed] |
| 159. | Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387-1394. [PubMed] |
| 160. | Ferreira ST, Clarke JR, Bomfim TR, De Felice FG. Inflammation, defective insulin signaling, and neuronal dysfunction in Alzheimer’s disease. Alzheimers Dement. 2014;10:S76-S83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 265] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 161. | De Felice FG. Alzheimer’s disease and insulin resistance: translating basic science into clinical applications. J Clin Invest. 2013;123:531-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 254] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 162. | Suh HS, Zhao ML, Derico L, Choi N, Lee SC. Insulin-like growth factor 1 and 2 (IGF1, IGF2) expression in human microglia: differential regulation by inflammatory mediators. J Neuroinflammation. 2013;10:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 177] [Article Influence: 14.8] [Reference Citation Analysis (0)] |