Peer-review started: May 28, 2014
First decision: July 30, 2014
Revised: October 10, 2014
Accepted: December 29, 2014
Article in press: December 31, 2014
Published online: February 2, 2015
Processing time: 120 Days and 14 Hours
The present paper is in the same time an overview of the literature concerning the alterations of lipids in the stratum corneum (SC) of atopic dogs and a review of data based on our publications. Knowing the importance of the SC barrier function for against pathogens in atopic dermatitis, we show for the first time a detailed biochemical analysis of lipids corresponding to the same amount of proteins in the successive layers of canine SC taken using tape stripping and their specificity as compared to humans. Also we show new results concerning the changes in the composition for protein-bound ceramides, and for the other lipids in involved and non-involved skin areas in atopic dogs. We show how a topical or oral treatment can restore the SC lipid composition and reconstruct the barrier integrity by up-regulating the biosynthesis of protein-bound ceramides.
Core tip: The review concerns the literature on modifi-cations of sphingolipids in the stratum corneum (SC) of atopic dogs. We gave for the first time a detailed biochemical analysis of dog lipids in the successive layers of the SC take by tape stripping, and discussed their specificity by comparison to humans. We showed also the specific composition in protein-bound ceramides and the importance of CerOS in dog skin for barrier integrity. The lipid composition of involved and non-involved skin areas in atopic dogs was described and we showed how a topical or oral treatment can restore the lipid composition of SC and reconstruct the barrier integrity.
- Citation: Popa I, Portoukalian J, Haftek M. Specificity in the alteration of lesional and non-lesional skin lipids in atopic dogs. World J Dermatol 2015; 4(1): 1-7
- URL: https://www.wjgnet.com/2218-6190/full/v4/i1/1.htm
- DOI: https://dx.doi.org/10.5314/wjd.v4.i1.1
Stratum corneum (SC) is the outmost layer of the epidermis. It comprises a unique structure made of protein-enriched corneocytes embedded in a lipid-enriched extracellular matrix.
SC lipids are composed of polar lipids such as cholesterol-sulphate, neutral lipids such as sterols, fatty acids, triglycerides, wax esters, squalene and extremely hydrophobic species such as ceramides (Table 1). The lipid extracellular matrix containing in few amount the omega-hydroxyceramides (CerOS, CerOP and CerOH), is replacing the plasma membrane of the living cells and it constitutes a scaffold for the intercellular lipid lamellae[1].
| Sphingoid basefatty acid | Sphingosine(S) | Phytosphingosine(P) | 6-Hydroxy-sphingosine(H) |
| Normal FA (N) | NS Cer 2 | NP Cer 3 | NH Cer 8 |
| Alpha-Hydroxy FA (A) | AS Cer 5 | AP Cer 6 | AH Cer 7 |
| Omega-Hydroxy FA (O) | OS Cer A | OP | OH Cer B |
| Omega Esterified FA (E) | EOS Cer 1 | EOP Cer 9 | EOH Cer 4 |
Between the terrestrial mammals, the SC thickness and the organisation are variable.
Regarding the canine skin samples, the intercellular lipid content was less than in other terrestrial species[2] and humans[3]. The lipid content also varies among the breeds. The Labrador retriever and Siberian husky are presenting a higher skin lipid content than the poodle[3].
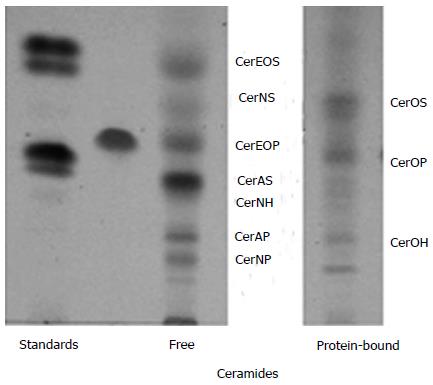
As shown in a previous paper the results performed on the SC of five healthy dogs showed similar molecular species of ceramides as in Figure 1, but in different quantity concerning free and protein-bound lipids[4]. For example, in the Labrador breed, 6-hydroxysphingosine was the major long-chain base of ceramides, The other dogs presented mostly sphingosine- based ceramides and low amounts of phytosphingosine and 6-hydroxysphingosine ceramides.
Within the depth in the SC, we observed a variation in free ceramides but not for protein-bound ceramides as well as it was describes in other studies where it was used the cyanoacrylate method[5,6].
As can be seen from Figure 1, we identified ceramides with omega-hydroxylated long chain fatty acids such as CerOS, CerOP and CerOH, and we showed that CerOS contains omega hydroxy fatty acids species in decreasing order from omega -OH C30:0, omega -OH C29:0, omega -OH C28:0, to omega -OH C32:0.
In conclusion, due to its structure, the Stratum Corneum of healthy dogs show an important role in the skin barrier protection towards environmental factors and trans-epidermal water loss.
Halliwell[7] describes for the first time that from the clinical point of view that the canine atopic dermatitis (AD) show many similarities with the human skin[8]. After 1999, when the American College of Veterinary Dermatology established that any review of AD should be supported by the medically based evidence of atopy, the term “allergic inhalant dermatitis” was replaced by “AD” because of the lack of asthma sign developing in dogs. In this respect, AD is defined as a genetically determined or allergic or inflammatory pathology resulting into a pruritic skin disorder. Environmental allergens[9] or cutaneous Malassezia and Staphylococcus infections[10] are well known to trigger inflammatory changes that are commonly associated with IgE antibodies.
Recently, in a skin model Leiden epidermal model (LEM)[11], it was used IL-4, IL-13, IL-31, and TNF-α to induce AD-like cytokines disorder in skin (spongiosis and alterations of early stage and terminal stage of expression in differentiation-protein in reconstructed skin). Another effect of TNF-α alone or together with Th2 cytokines consisted in a decrease of the level of long chain free fatty acids (FFAs) and ester linked omega-hydroxy (EO) ceramides, leading to an abnormal lipid organization and a defect in skin barrier integrity. Another cause of exacerbating the pathological changes and the impairment of the synthesis of ultra long-chain ceramides in AD results into a higher amount of the interferon gamma which decreases the very long chain fatty acids and ceramide synthase 3 enzymes necessary for the synthesis of the very long-chain ceramides[12].
AD in dog species affects up to 10% of dogs and it has an important breed prevalence[13]. If in humans, mutations in the filaggrin gene accounted for the predisposition to develop AD, in dogs there is no known direct link[14] and a total absence of correlation has been reported in West Highland White Terriers[15].
Previously, it was shown that in AD, the genetic defects in proteins structure or enzymes could impair the synthesis of SC lipids by incomplete extrusion of lipid bodies (LBs)[16,17]. The LBs composition in lipid catabolic enzymes is also changed[18], resulting in an impairment of lateral packing of the inter-corneocytes lamellar lipids (LL) as observed in humans with AD[19]. Compared to normal skin, it was observed in the non-lesional skin of atopic dogs, by transmission electron microscopy (TEM), some ultrastructural changes in SC morphology such as larger intercorneocyte spaces,as well as severely disorganised, sporadic and incomplete lamellar lipids (20).
After topical treatment with SLC[20], or a ceramide-like moisturizer[21], or other extrinsic lipids[22] that would be integrated into the nascent lamellar bilayers, it was observed by TEM a new formation of the SC compactum, an increase in the LLs and an improvement of the SC integrity.
An inherent abnormal lamellar structure will cause disorders in cornification as in many ichtyosis, due to a reduced level in protein-bound omega-hydroxyceramides[4], or alteration in the content of other sphingolipids as in psoriasis[23] and AD[5,24-26]. These covalently bound ceramides were first described by Wertz et al[27] in 1989.
Recently, a reduction in the free fatty acid chain length was reported in non-lesional and lesional SC of atopic eczema patients[28], associated with a reduced ceramide chain length, suggesting a common synthetic pathway.
This finding could be sustained by the results of Haller et al[29] who found that the loss of Abca12 function results in a failure of interaction between glucocerebrosidase and its GlcCer substrate and an accumulation of GlcCer species in SC.
Regarding glucosylceramide, our results[30] show in atopic dogs a near absence of CerOP, a protein-bound ceramide, with the concomitant presence of glucosyceramide in large amount in the SC.
Moreover, Reiter et al[5] showed that the ratio cholesterol to ceramide in atopic dog SC is higher than in normal SC dogs, in uninvolved as well as in lesional areas. Also, some results from Sugiura et al[31] support the notion that AD in non-lesional skin is associated with an impaired homeostasis in a ceramide-generating process.
Concerning our work, we reported in 2011 the lipid patterns in non-involved SC of atopic dogs vs normal dogs SC that suggested an impaired biosynthesis of the long chain bases of ceramides[30,32]. Here we give the complete analysis of free and protein-bound lipids of lesional and non-lesional SC of 5 atopic dogs.
As shown in Table 2, the amount of proteins taken by tape stripping in non-lesional areas of atopic dogs SC was not significantly different from that of normal dogs, but the protein content was reduced by half compared to the non-lesional areas. The lower protein content of lesional areas was likely to be due to the limited sticking of the tapes on inflammatory areas.
The free lipid content of normal and non-lesional and lesional atopic dog SC is given in Table 3. As compared to their respective contents in normal dog SC, cholesterol and fatty acids showed a moderate (10% to 15%) decrease in both non-lesional and lesional SC of atopic dogs. Free ceramides were reduced by 30% to 40%. However, for glucosylceramides which were absent in normal dog SC, large amounts were detected in non-lesional SC of atopic dogs, with a significant concentration also present in lesional ares, showing a deficient activity of the glucocerebrosidase in the atopic dogs skin.
Table 4 shows the protein-bound lipids of the SC. The amount of Cholesterol, fatty acids and omega hydroxy ceramides of the protein-bound lipids show an important decreasing in atopic dog SC, even higher than that of free lipids. The cholesterol amount was reduced by 30%, the fatty acids by 50% and the omega hydroxyceramides, only 20% compare to the amount found in healthy dog SC. Compare to the normal dog SC which does not contain covalently-bound glucosylceramides, we found an important amount in both non-lesional and lesional atopic dog SC.
In a recent study it was shown that a neutral ceramidase isolated from Pseudomonas aeruginosa (PaCDase) isolated from a patient with AD could degrade the ceramides in the presence of Staphylococcus aureus-derived lipids or neutral detergents in a keratinocyte model[33].
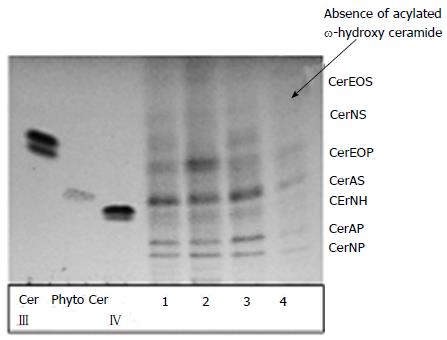
To illustrate the data shown previously[34] and the values from Tables 3 and 4, we analysed comparatively in depth by chromatographic plates the free ceramides of SC from lesional (line 4) and non-lesional (line 3) spots of atopic dog compared to the heathy one (line 1 and 2) (Figure 2).
It is noticeable that in non-lesional areas (4) the proportion of Cer NP and Cer AS dropped as well as in the lesional side, and moreover, Cer EOS (omega-hydroxy ceramide) is totally absent from lesional areas.
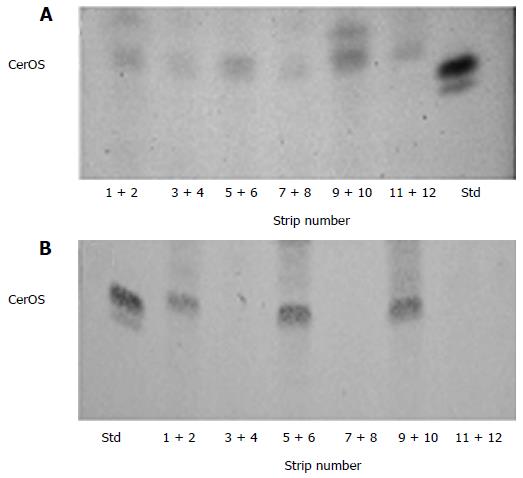
We may noticed an heterogenic distribution in SC depth of protein-bound omega hydroxy ceramides, Cer OS and CerOP, in non-lesional (Figure 3A) and lesional (Figure 3B) SC of atopic dog. Compare to non-lesional SC (Figure 3A), in lesional SC (Figure 3B) an important absence of protein-bound céramides, one reason why the SC integrity is markedly altered.
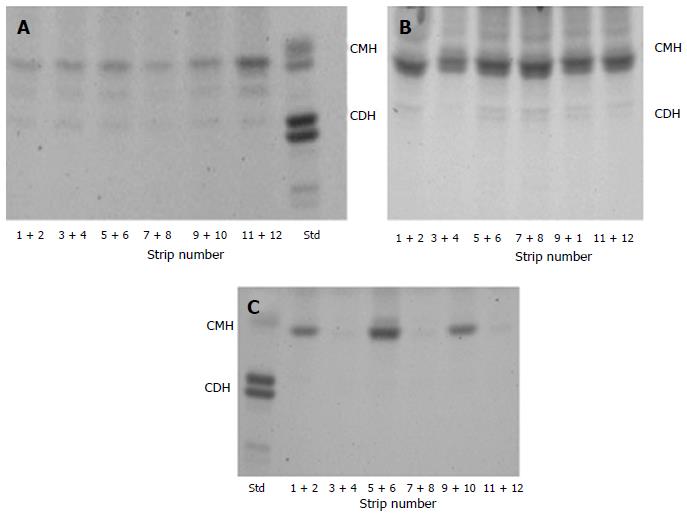
Figure 4 shows comparatively the presence of glucosy-ceramide in SC of healthy dog, the precursor of ceramides, (Figure 4A) and its presence in non-lesional (Figure 4B) and the lesional (Figure 4C) SC of atopic dog.
Compare to the healthy dog’s SC (Figure 4A), in the successive strips of lesional areas of atopic dogs (Figure 4C), the omega hydroxy ceramides are absent in one sample out of two whereas this does not appear so clearly in non-lesional areas (Figure 4B), responding to question why the alteration of the SC integrity occurs.
Table 4 presents the comparison concerning the whole lipids content of protein-bound lipids of Sc of healty dog compare to atopic dog. We may notice that in atopic dog SC, the whole lipids are strongly reduced as compared to normal dogs. The decreasing in CerOS, which accounts for about 75% of the total omega hydroxy ceramides in normal dog SC, is the only species present in atopic dog SC. Table 4 shows also the amount of glucosylceramide in non-lesional and in lesional areas which confirm the heterogenic profile (Figure 4B and C).
This accumulation of glucosyceramide in SC of atopic dogs compared to the normal SC dogs is due to the deficient activity of beta-glucocerebrosidase. This enzyme is known to be essential in acquiring a good SC barrier[18].
Concerning the free glucosylceramide type of lipids, they are completely absent in the normal dog SC. In a similar way, the free glucosylceramides were present in all layers of non-lesional dog SC in an important quantity. In lesional areas, the concentration of glucosylceramides was much lower and there were some variations in the molecular species visualized in these samples, as glucosylceramides with long-chain fatty acids were prominent in pooled strips 5 + 6.
We found in our studies on dog SC, the cholesterol in the protein-bound fraction, as it was reported in human skin[35], Its presence was observed in the successive layers in a striking heterogeneity. In the case of the atopic dogs in non-lesional layers, some layers were more enriched and in lesional layers the content was lowered.
One key fact is to restore the barrier function and this requires a decrease to allergen exposure.
Several treatments based on topical application of lipids were designed for patients with allergic contact dermatitis, irritant dermatitis and atopic dermatitits[22]. For exemple, the improvement of the skin barrier in children with AD was accelerated after treatment with a pseudoceramides-based moisturizer[21].
Another study suggested that the application of an emulsion based on an physiological lipid granules would restore the barrier of atopic patients and reduce the clinical symptoms and any side effects[36].
Concerning the dogs, frequent washing and rinsing of the contact zones may help to decrease allergen exposure. In this respect it was shown previously[20] that a mixture of lipid complex SLC® based mainly on ceramides and cholesterol (Allerderm/Derm-1 Spot-on, Virbac Laboratory) would restructure the SC lipid lamellae. The treatment resulted[32] into an important increase in lipid biosynthesis of keratinocytes (i.e., protein-bound ceramides CerOS and CerOP, and normal and omega-hydroxy fatty acids) and an efficient barrier formation. Another treatment, Megaderm®, designed by Virbac for atopic dogs was a food supplement based on essential fatty acids and vitamin E[33].
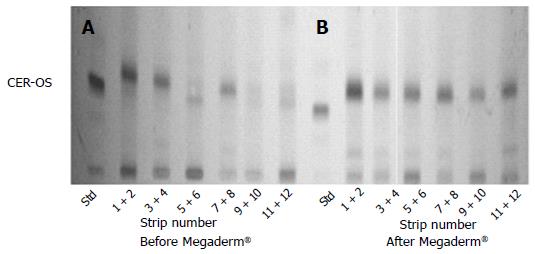
Figure 5 shows, as most of our previous publications[30], that the lower lipid content and the marked deficit in protein-liked ceramides (Figure 5A) in atopic dogs may be reversed with the feed supplementation with Megaderm® (Figure 5B). This is the most remarkable feature, accounting for the observed accumulation of the intercorneocyte lamellar lipids[37].
Although it was observed that after feeding for about two months with Megaderm®, the deepest layers of the SC presented several imperfections compared to the SC of healthy dogs, the overall improvement in the lamellar lipid organization and normalization of the protein-bound lipid content did occur, just as with SLC® treatment[20].
It was recently demonstrated with Fourier-transformed infrared spectroscopy and Raman imaging spectroscopy that the stability of the crystalline structure of free fatty acid, ceramide and cholesterol mixtures strongly depends on the length of the fatty acids built into ceramides[38]. It can be that the optimal molecular proportions can be best achieved when living epidermal cells are sufficiently supplied with the essential building bricks provided through the treatments.
Of course, besides the dietary supplementation such as Megaderm® or topical treatment such as SLC®, new treatments used in humans suffering from AD may be also applied in dogs.
These are topical ceramide formulations[39] including targeted CerAP microemulsions[40], that may contain inhibitors of calcineurin[41]. They are all aiming at increasing the epidermal lipid content, supplying filaggrin degradation products[42], regulating the environmental pH[43,44] and the glucosylceramidase activity[45,46], and resulting in a decrease of the transepidermal water loss[47] and inflammation.
The authors thank Virbac (Hugues Gatto) and National Veterinary School (Didier Pin) for their previous collaboration and support.
P- Reviewer: Firooz A, Garcia-Elorriaga G S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Jensen JM, Fölster-Holst R, Baranowsky A, Schunck M, Winoto-Morbach S, Neumann C, Schütze S, Proksch E. Impaired sphingomyelinase activity and epidermal differentiation in atopic dermatitis. J Invest Dermatol. 2004;122:1423-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 221] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 2. | Olivry T, Hill PB. The ACVD task force on canine atopic dermatitis (VIII): is the epidermal lipid barrier defective? Vet Immunol Immunopathol. 2001;81:215-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 93] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Dustan RW, Herdt TH, Oliver T. Age- and breed-related differences in canine skin surface lipids and pH. Oxford: UK, Blackwell 2002; 37-42. |
| 4. | Popa I, Thuy LH, Colsch B, Pin D, Gatto H, Haftek M, Portoukalian J. Analysis of free and protein-bound ceramides by tape stripping of stratum corneum from dogs. Arch Dermatol Res. 2010;302:639-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Reiter LV, Torres SM, Wertz PW. Characterization and quantification of ceramides in the nonlesional skin of canine patients with atopic dermatitis compared with controls. Vet Dermatol. 2009;20:260-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Shimada K, Yoon JS, Yoshihara T, Iwasaki T, Nishifuji K. Increased transepidermal water loss and decreased ceramide content in lesional and non-lesional skin of dogs with atopic dermatitis. Vet Dermatol. 2009;20:541-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 7. | Halliwell RE. Atopic disease in the dog. Vet Rec. 1971;89:209-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Griffin CE, DeBoer DJ. The ACVD task force on canine atopic dermatitis (XIV): clinical manifestations of canine atopic dermatitis. Vet Immunol Immunopathol. 2001;81:255-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 128] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Hill PB, Hillier A, Olivry T. The ACVD task force on canine atopic dermatitis (VI): IgE-induced immediate and late-phase reactions, two inflammatory sequences at sites of intradermal allergen injections. Vet Immunol Immunopathol. 2001;81:199-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Leung D, Eichenfield F and Boguniewicz M, Atopic dermatitis (atopic eczema). In: Freeedberg IM, Eisen AZ, Wolff K, et al., eds. Fitzpatrick’s dermatology in general medicine. Ed.6. New York: McGraw-Hill 2003; 1181-1194. |
| 11. | Danso MO, van Drongelen V, Mulder A, van Esch J, Scott H, van Smeden J, El Ghalbzouri A, Bouwstra JA. TNF-α and Th2 cytokines induce atopic dermatitis-like features on epidermal differentiation proteins and stratum corneum lipids in human skin equivalents. J Invest Dermatol. 2014;134:1941-1950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 247] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 12. | Feingold KR. The adverse effect of IFN gamma on stratum corneum structure and function in psoriasis and atopic dermatitis. J Invest Dermatol. 2014;134:597-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Sousa CA, Marsella R. The ACVD task force on canine atopic dermatitis (II): genetic factors. Vet Immunol Immunopathol. 2001;81:153-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Chervet L, Galichet A, McLean WH, Chen H, Suter MM, Roosje PJ, Müller EJ. Missing C-terminal filaggrin expression, NFkappaB activation and hyperproliferation identify the dog as a putative model to study epidermal dysfunction in atopic dermatitis. Exp Dermatol. 2010;19:e343-e346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Barros Roque J, O’Leary CA, Kyaw-Tanner M, Latter M, Mason K, Shipstone M, Vogelnest L, Duffy DL. Haplotype sharing excludes canine orthologous Filaggrin locus in atopy in West Highland White Terriers. Anim Genet. 2009;40:793-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Inman AO, Olivry T, Dunston SM, Monteiro-Riviere NA, Gatto H. Electron microscopic observations of stratum corneum intercellular lipids in normal and atopic dogs. Vet Pathol. 2001;38:720-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 63] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Nemes Z, Steinert PM. Bricks and mortar of the epidermal barrier. Exp Mol Med. 1999;31:5-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 415] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 18. | Holleran WM, Takagi Y, Menon GK, Legler G, Feingold KR, Elias PM. Processing of epidermal glucosylceramides is required for optimal mammalian cutaneous permeability barrier function. J Clin Invest. 1993;91:1656-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 201] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 19. | Pilgram GS, Vissers DC, van der Meulen H, Pavel S, Lavrijsen SP, Bouwstra JA, Koerten HK. Aberrant lipid organization in stratum corneum of patients with atopic dermatitis and lamellar ichthyosis. J Invest Dermatol. 2001;117:710-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 149] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 20. | Piekutowska A, Pin D, Rème CA, Gatto H, Haftek M. Effects of a topically applied preparation of epidermal lipids on the stratum corneum barrier of atopic dogs. J Comp Pathol. 2008;138:197-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Chamlin SL, Frieden IJ, Fowler A, Williams M, Kao J, Sheu M, Elias PM. Ceramide-dominant, barrier-repair lipids improve childhood atopic dermatitis. Arch Dermatol. 2001;137:1110-1112. [PubMed] |
| 22. | Mao-Qiang M, Brown BE, Wu-Pong S, Feingold KR, Elias PM. Exogenous nonphysiologic vs physiologic lipids. Divergent mechanisms for correction of permeability barrier dysfunction. Arch Dermatol. 1995;131:809-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 110] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Macheleidt O, Kaiser HW, Sandhoff K. Deficiency of epidermal protein-bound omega-hydroxyceramides in atopic dermatitis. J Invest Dermatol. 2002;119:166-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 178] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 24. | Yamamoto A, Serizawa S, Ito M, Sato Y. Stratum corneum lipid abnormalities in atopic dermatitis. Arch Dermatol Res. 1991;283:219-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 194] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 25. | Di Nardo A, Wertz P, Giannetti A, Seidenari S. Ceramide and cholesterol composition of the skin of patients with atopic dermatitis. Acta Derm Venereol. 1998;78:27-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 340] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 26. | Jungersted JM, Scheer H, Mempel M, Baurecht H, Cifuentes L, Høgh JK, Hellgren LI, Jemec GB, Agner T, Weidinger S. Stratum corneum lipids, skin barrier function and filaggrin mutations in patients with atopic eczema. Allergy. 2010;65:911-918. [PubMed] |
| 27. | Wertz PW, Madison KC, Downing DT. Covalently bound lipids of human stratum corneum. J Invest Dermatol. 1989;92:109-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 139] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 28. | van Smeden J, Janssens M, Kaye EC, Caspers PJ, Lavrijsen AP, Vreeken RJ, Bouwstra JA. The importance of free fatty acid chain length for the skin barrier function in atopic eczema patients. Exp Dermatol. 2014;23:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 203] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 29. | Haller JF, Cavallaro P, Hernandez NJ, Dolat L, Soscia SJ, Welti R, Grabowski GA, Fitzgerald ML, Freeman MW. Endogenous β-glucocerebrosidase activity in Abca12⁻/⁻epidermis elevates ceramide levels after topical lipid application but does not restore barrier function. J Lipid Res. 2014;55:493-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Popa I, Remoue N, Hoang LT, Pin D, Gatto H, Haftek M, Portoukalian J. Atopic dermatitis in dogs is associated with a high heterogeneity in the distribution of protein-bound lipids within the stratum corneum. Arch Dermatol Res. 2011;303:433-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Sugiura A, Nomura T, Mizuno A, Imokawa G. Reevaluation of the non-lesional dry skin in atopic dermatitis by acute barrier disruption: an abnormal permeability barrier homeostasis with defective processing to generate ceramide. Arch Dermatol Res. 2014;306:427-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Popa I, Remoue N, Osta B, Pin D, Gatto H, Haftek M, Portoukalian J. The lipid alterations in the stratum corneum of dogs with atopic dermatitis are alleviated by topical application of a sphingolipid-containing emulsion. Clin Exp Dermatol. 2012;37:665-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 33. | Popa I, Pin D, Remoué N, Osta B, Callejon S, Videmont E, Gatto H, Portoukalian J, Haftek M. Analysis of epidermal lipids in normal and atopic dogs, before and after administration of an oral omega-6/omega-3 fatty acid feed supplement. A pilot study. Vet Res Commun. 2011;35:501-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Oizumi A, Nakayama H, Okino N, Iwahara C, Kina K, Matsumoto R, Ogawa H, Takamori K, Ito M, Suga Y. Pseudomonas-derived ceramidase induces production of inflammatory mediators from human keratinocytes via sphingosine-1-phosphate. PLoS One. 2014;9:e89402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Serizawa S, Ito M, Hamanaka S, Otsuka F. Bound lipids liberated by alkaline hydrolysis after exhaustive extraction of pulverized clavus. Arch Dermatol Res. 1993;284:472-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 36. | Na JI, Hwang JS, Park HJ, Kim DH, Park WS, Youn SW, Huh CH, Park KC. A new moisturizer containing physiologic lipid granules alleviates atopic dermatitis. J Dermatolog Treat. 2010;21:23-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Ponec M, Boelsma E, Weerheim A. Covalently bound lipids in reconstructed human epithelia. Acta Derm Venereol. 2000;80:89-93. [PubMed] |
| 38. | Oguri M, Gooris GS, Bito K, Bouwstra JA. The effect of the chain length distribution of free fatty acids on the mixing properties of stratum corneum model membranes. Biochim Biophys Acta. 2014;1838:1851-1861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 39. | Kircik L, Hougeir F, Bikowski J. Atopic dermatitis, and the role for a ceramide-dominant, physiologic lipid-based barrier repair emulsion. J Drugs Dermatol. 2013;12:1024-1027. [PubMed] |
| 40. | Sahle FF, Metz H, Wohlrab J, Neubert RH. Lecithin-based microemulsions for targeted delivery of ceramide AP into the stratum corneum: formulation, characterizations, and in vitro release and penetration studies. Pharm Res. 2013;30:538-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 41. | Hon KL, Leung AK, Barankin B. Barrier repair therapy in atopic dermatitis: an overview. Am J Clin Dermatol. 2013;14:389-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 42. | Del Rosso JQ, Kircik LH. The integration of physiologically-targeted skin care in the management of atopic dermatitis: focus on the use of a cleanser and moisturizer system incorporating a ceramide precursor, filaggrin degradation products, and specific “skin-barrier-friendly” excipients. J Drugs Dermatol. 2013;12:s85-s91. [PubMed] |
| 43. | Hachem JP, Crumrine D, Fluhr J, Brown BE, Feingold KR, Elias PM. pH directly regulates epidermal permeability barrier homeostasis, and stratum corneum integrity/cohesion. J Invest Dermatol. 2003;121:345-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 328] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 44. | Rippke F, Schreiner V, Doering T, Maibach HI. Stratum corneum pH in atopic dermatitis: impact on skin barrier function and colonization with Staphylococcus Aureus. Am J Clin Dermatol. 2004;5:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 119] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 45. | Alessandrini F, Pfister S, Kremmer E, Gerber JK, Ring J, Behrendt H. Alterations of glucosylceramide-beta-glucosidase levels in the skin of patients with psoriasis vulgaris. J Invest Dermatol. 2004;123:1030-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 46. | Hachem JP, Man MQ, Crumrine D, Uchida Y, Brown BE, Rogiers V, Roseeuw D, Feingold KR, Elias PM. Sustained serine proteases activity by prolonged increase in pH leads to degradation of lipid processing enzymes and profound alterations of barrier function and stratum corneum integrity. J Invest Dermatol. 2005;125:510-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 190] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 47. | Meguro S, Arai Y, Masukawa Y, Uie K, Tokimitsu I. Relationship between covalently bound ceramides and transepidermal water loss (TEWL). Arch Dermatol Res. 2000;292:463-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 95] [Article Influence: 3.8] [Reference Citation Analysis (0)] |