Published online Sep 18, 2017. doi: 10.5312/wjo.v8.i9.719
Peer-review started: February 12, 2017
First decision: March 28, 2017
Revised: April 19, 2017
Accepted: May 12, 2017
Article in press: May 13, 2017
Published online: September 18, 2017
Processing time: 225 Days and 13.1 Hours
To investigate the possible relationship of adiponectin (ADIPOQ) gene polymorphisms, plasma adiponectin, and the risk of knee osteoarthritis (OA).
A total of 398 subjects, 202 knee OA patients and 196 healthy individuals, were enrolled in the case-control study. Genotyping at +45T/G (rs2241766) and +276G/T (rs1501299) loci was performed using polymerase chain reaction-restriction fragment length polymorphism. Plasma adiponectin levels were assessed using enzyme-linked immunosorbent assay. OA severity was determined using the Kellgren-Lawrence (KL) grading system.
No significant associations were observed in the genotype distributions and allele frequencies at two loci of +45T/G and +276G/T polymorphisms in the ADIPOQ between knee OA patients and control subjects. There was a significant association between genotype distribution of +276G/T polymorphism and KL grade 2, 3 or 4 (P = 0.037, P = 0.046, P = 0.016, respectively). At +45T/G locus, the percentage of GG genotype was notably greater in control subjects (13.40%) compared with OA subjects (1.70%) (P = 0.023). Plasma adiponectin was markedly decreased in OA subjects compared with control subjects (P = 0.03). Likewise, circulating adiponectin in OA subjects was notably lesser than that in control subjects in GG genotype of +45T/G (P = 0.029) and +276G/T polymorphisms (P = 0.012).
Polymorphisms +45T/G and +276G/T of the ADIPOQ gene might not be responsible for OA susceptibility among Thais.
Core tip: Plasma adiponectin levels were significantly lower in knee osteoarthritis (OA) than controls. No significant associations were observed in the genotype distributions and allele frequencies of ADIPOQ +45T/G and +276G/T polymorphisms between knee OA subjects and controls. There was a significant association between genotype distribution of +276G/T polymorphism and OA severity. In addition, plasma adiponectin in OA subjects was seemingly lower than that in control subjects in GG genotype of +45T/G and +276G/T polymorphisms. Polymorphisms +45T/G and +276G/T of the ADIPOQ gene might not be responsible for the susceptibility to knee OA in the Thai population.
- Citation: Zhan D, Thumtecho S, Tanavalee A, Yuktanandana P, Anomasiri W, Honsawek S. Association of adiponectin gene polymorphisms with knee osteoarthritis. World J Orthop 2017; 8(9): 719-725
- URL: https://www.wjgnet.com/2218-5836/full/v8/i9/719.htm
- DOI: https://dx.doi.org/10.5312/wjo.v8.i9.719
Osteoarthritis (OA), also known as degenerative joint disorder, is characterized by progressive cartilagenous damage, chronic synovial inflammation, development of bone spurs, subchondral cyst formation, and osteosclerosis, leading to joint disability. OA of the knee remains a main cause of mobility impairment, particularly in the elderly population and has been recognized as a major global health problem. A wide variety of potential factors including environments, biomechanics, biochemical processes and/or genetics have been demonstrated to play substantial parts in the progression of OA. Nonetheless, the cause of OA is still a mystery. Numerous single-nucleotide polymorphisms (SNPs) related to OA have been previously investigated.
Besides storing-energy, adipogenous tissue is also recognized as a metabolic and endocrine organ with significance, complication and high activity. Hormones secreted from adipose tissue are named after adipokines that associate with metabolic processes and inflammatory reaction as well as performance cytokine-like function including anti- and pro-inflammatory effects[1-3]. In human chromosome 3q27, ADIPOQ gene encodes one essential adipokine - adiponectin which contains 244 amino acid residues. It is synthesized in differentiated adipocytes and maintains high levels in blood circulation. The function and effect of adiponectin have been clearly elaborated in anti-diabetic and anti-atherogenic properties. It is still controversial whether adiponectin may have a contributing role in the development of OA. Recently, adiponectin was identified in cartilage, osteophytes, meniscus, synovial membrane and infrapatellar fat pad taken from the knees of OA patients, with the highest concentrations found in the last two[4]. Previous investigations demonstrated that circulating and synovial adiponectin concentrations were negatively correlated with the radiographic severity in OA subjects[5,6]. In chondrocytes, adiponectin could modulate cartilage destruction through increasing tissue inhibitor of metalloproteinase-2 and decreasing interleukin-1β (IL-1β)[7]. Accumulating documentation proposes that adiponectin might act as a protective cytokine in OA.
As an essential component of the etiology of OA, candidate genes encoding proteins about metabolism of the articular cartilage and inflammation of synovial membrane have been proved with the pathogenesis of OA. It is ascertained that a number of SNPs involving in OA surrounding genes of estrogen receptor alpha[8], interleukin-6[9] and matrix metalloproteinase-3 (MMP-3)[10]. However, until recently, the study of adiponectin gene polymorphisms in OA patients has received little attention. There are many genetic variations of the human adiponectin gene reported, including several non-synonymous mutations. Some metabolic disorders have been recognized to be related with the two most commonly investigated polymorphisms of ADIPOQ, +45T/G and +276G/T SNPs[11,12]. Additionally, Qi et al[13] found that greater circulating adiponectin concentration in control subjects carried more T allele at +276G/T locus. We hypothesized that the adiponectin gene would play a part in the development of OA. Thus, the objective of the present investigation is to determine the association between +45T/G or +276G/T ADIPOQ polymorphisms and OA susceptibility and plasma adiponectin in knee OA subjects.
This study was approved by the Institutional Review Board on Human Research of the Faculty of Medicine, Chulalongkorn University. The present study was conducted in compliance with the guidelines of the Declaration of Helsinki. All subjects gave written informed consent prior to their participation in the study.
The current study recruited 202 primary knee OA patients (average age 68.80 ± 7.80 years, range from 50-84 years), including 136 female and 66 male subjects. Diagnostic criteria of the American College of Rheumatology were used to identify knee OA subjects. We precluded individuals who had other chronic inflammatory diseases or immunological abnormalities, or preceding knee trauma or surgery. Kellgren-Lawrence (KL) classification system was assigned to determine the severity of knee OA into KL grade 1, 2, 3, or 4 corresponding to radiographic examination[14]. Furthermore, 196 healthy individuals (average age 65.20 ± 6.20 years, 128 female and 68 male) without any symptoms and signs and previous history of OA were used as control subjects.
Peripheral venous blood specimens of 3 mL were collected from each participant by standard venipuncture. Genomic DNA was extracted from buffy coats by using the commercially available Illustra Blood Genomic Prep Midi Flow Kit (GE Healthcare, Buckinghamshire, United Kingdom) and was maintained at -20 °C until analysed. +45T/G and +276G/T polymorphisms of adiponectin gene were detected by polymerase chain reaction (PCR) restriction fragment length polymorphism (PCR-RFLP). PCR amplifications were conducted for the +45T/G (rs2241766) SNP by using the published primer set[15]: forward, 5’-TCCTTTGTAGGTCCCAACT-3’ and reverse, 5’GCAGCAAAGCCAAAGTCTTC-3’. The PCR for +45T/G SNP was performed with the following protocols: 95 °C for 15 min, repeated by 35 amplification cycles at 95 °C for 30 s, 56 °C for 30 s, and 72 °C for 1 min, and a last extension at 72 °C for 7 min. After digestion with the restriction enzyme BspH1 (New England Biolabs, Beverly, MA) in 37 °C water bath for 16 h, the PCR amplified 503 base pair length sequence was cleaved into 375 and 128 base pair segments (T allele of +45T/G). PCR amplifications were conducted for the +276G/T (rs1501299) SNP by using the published primers set[15]: Forward primer 5’-ACACTGATATAAACGCCATGAA-3’ and reverse primer 5’-GCAGCAAAGCCAAAGTCTTC-3’. The PCR for +276G/T (rs1501299) SNP was performed with the following protocols: 95 °C for 10 min, repeated by 40 amplification cycles at 95 °C for 30 s, 48 °C for 1 min and 72 °C for 1 min, and a last extension at 72 °C for 7 min. After digestion with the restriction enzyme Bgl1 (New England Biolabs, Beverly, MA) in 37 °C water bath for 16 h, the PCR amplified 168 base pair length sequence was cleaved into 147 and 21 base pair segments (G allele of +276G/T). The digested sequences were resolved by electrophoresis in 2.5% agarose gel or 12% polyacrylamide gel. The gels were stained with ethidium bromide and analysed by exposure to ultraviolet light on a transilluminator.
Following blood sample collection, the plasma were centrifuged and kept promptly at -20 °C till analysis. Plasma adiponectin concentrations were assessed by a commercially available sandwich enzyme-linked immunosorbent assay kit (DuoSet ELISA Development kit for human adiponectin, R and D Systems, Minneapolis, MN). Based on the guidelines of manufacturer, 100 μL of samples or standards in reagent diluent were added into a 96-well plate which was precoated with capture antibody overnight at room temperature (RT). After incubating for 2 h at RT and washing three times with washing buffer, 100 μL of the specific detection antibody was pipetted and kept for 2 h at RT. After thoroughly four washes with washing buffer, 100 μL of streptavidin-HRP (1:200) was pipetted to each well and kept for 20 min at RT to avoid in direct light. One hundred slightly of substrate solution was pipetted and kept for another 20 min. Finally, 50 μL of stop solution was pipetted to terminate reactions. The optical density (OD) of each well was determined immediately using a micro-plate reader. The readings at 450 nm were subtracted at 570 nm to correct for optical imperfections in the plate. Adiponectin value was assessed using a linear standard calibration curve constructed from a series of adiponectin standard.
All data were analysed were with SPSS version 22.0 software (SPSS Inc., Chicago, IL) and GraphPad Prism (GraphPad Software, Inc., La Jolla, CA). The Hardy-Weinberg equilibrium analyses of two SNPs were determined by the χ2 test to examine the differences in allele frequency and genotype distribution between OA group and control group. Odds ratios (ORs) and 95% confidence intervals (CIs) of genotypes and alleles were assessed by using the Medcalc® (Medcalc® Software, Mariakerke, Belgium) statistical software program. Their haplotypes and linkage disequilibrium (LD), D’ and r2 were conducted with Haploview software version 4.1 (Broad Institute Cambridge, MA). Unpaired Student’s t-test and one-way analysis of variance were utilised to analyse quantitative data of two and more than two independent groups. Genotype distribution and allele frequency of ADIPOQ in OA patients and control subjects was calculated by the χ2 test. The statistical review of the study was performed by a biomedical statistician. P values < 0.05 were considered as statistical difference.
The distributions of the genotypes in the control and OA groups conformed to the Hardy-Weinberg equilibrium. The genotype and allele frequency of +45T/G ADIPOQ polymorphisms were present in Table 1. No statistically significant differences were observed in the genotype and allele frequencies between knee OA and control groups. The T allele frequency was 68.11% in control group and 64.60% in OA group, and the G allele frequency was 31.89% in control subjects and 35.40% in OA group (P = 0.295). For the +276G/T polymorphism, there was no difference in the genotypic distribution and allelic frequency between knee OA participants and control subjects (Table 2). The G allele frequency was 71.68% in control group and 71.29% in OA group, and the T allele frequency was 28.32% in control group and 28.71% in OA group. There were no remarkable differences in the +45T/G and +276G/T loci haplotype distributions. The correlation coefficient of the frequencies r2 is 0.033 in Linkage disequilibrium (LD) in these two polymorphisms.
| +45T/G SNP (rs2241766) | Control n (%) | OA n (%) | OR (95%CI) | P | |
| Genotype | TT | 96 (48.98) | 84 (41.6) | 1 | - |
| TG | 75 (38.27) | 93 (46) | 1.417 (0.929-2.162) | 0.106 | |
| GG | 25 (12.75) | 25 (12.4) | 1.143 (0.611-2.139) | 0.676 | |
| Allele | T | 267 (68.11) | 261 (64.6) | 1 | |
| G | 125 (31.89) | 143 (35.4) | 1.170 (0.872-1.571) | 0.295 | |
| +276G/T SNP (rs1501299) | Control n (%) | OA n (%) | OR (95%CI) | P | |
| Genotype | GG | 102 (52) | 106 (52.5) | 1 | - |
| GT | 77 (39.3) | 76 (37.6) | 0.950 (0.626-1.442) | 0.809 | |
| TT | 17 (8.7) | 20 (9.9) | 1.132 (0.561-2.283) | 0.729 | |
| Allele | G | 281 (71.68) | 288 (71.29) | 1 | |
| T | 111 (28.32) | 116 (28.71) | 1.020 (0.750-1.387) | 0.901 | |
The association between genotypes of the +45T/G ADIPOQ gene polymorphism and radiographic severity of OA patients was shown in Table 3. The genotypic distribution and allelic frequency of the +45T/G SNP was not significantly different among various groups of OA severity. Corresponding to the genotypes of the +276G/T ADIPOQ SNP, however, there were significantly different between KL grade 2 and KL grade 3 at +276G/T genotypes (P = 0.037), as well as between KL grade 2 and KL grade 4 (P = 0.046) (Table 4). The allele frequency of +276G/T polymorphism was not significantly different.
| OA severity | Genotype | P | aP | ||
| KL system | TT | TG | GG | ||
| Grade 2 | 27 | 30 | 8 | ||
| Grade 3 | 29 | 29 | 8 | NS | |
| Grade 4 | 28 | 34 | 9 | NS | NS |
| OA severity | Genotype | P | aP | ||
| KL system | GG | GT | TT | ||
| Grade 2 | 20 | 29 | 5 | ||
| Grade 3 | 41 | 22 | 8 | 0.037 | |
| Grade 4 | 45 | 25 | 7 | 0.046 | NS |
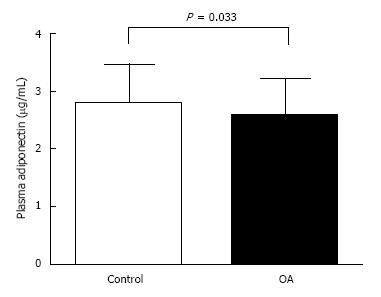
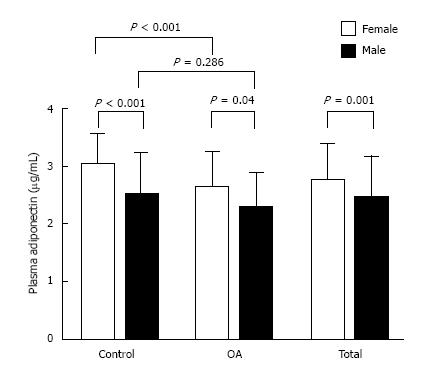
Circulating adiponectin concentrations of control group and knee OA group were shown in Figure 1. Circulating adiponectin values in OA group were notably lesser than those of the control group (2.58 ± 0.60 μg/mL vs 2.78 ± 0.68 μg/mL, P = 0.033). Further analysis of plasma adiponectin based on gender was shown in Figure 2. Plasma adiponectin of female subjects was seemingly greater than that of male subjects in both controls and OA patients (P < 0.001).
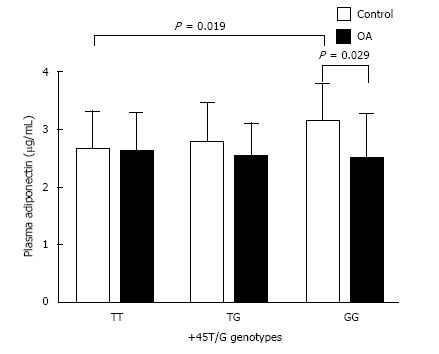
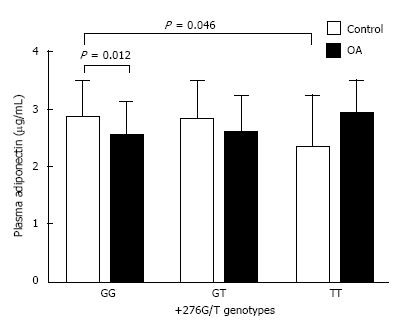
Figure 3 demonstrates plasma adiponectin concentrations of various genotypes of +45T/G and +276G/T loci. Plasma adiponectin levels of GG genotype were statistically higher than those of the TT genotype at the +45T/G polymorphism of control group (3.16 ± 0.63 μg/mL vs 2.66 ± 0.66 μg/mL, P = 0.019). In the GG genotype of the +45T/G locus, the circulating adiponectin levels of control group were significantly greater than those of OA group (P = 0.029). In control group, the mean value of plasma adiponectin in TT of +276 G/T was lowest among three genotypes (2.34 ± 0.88, 2.81 ± 0.66, 2.84 ± 0.63 μg/mL, respectively). In the +276G/T polymorphism, plasma adiponectin levels were more elevated in GG genotype when compared with those in TT genotype of healthy individuals (P = 0.046). In GG genotype of +276 G/T locus, the plasma adiponectin of control group was significantly greater than that of OA group (P = 0.012) (Figure 4).
Adiponectin is a noval adipocyte-derived hormone with various biological functions. Most previous studies have suggested that circulating adiponectin levels have been found to be decreased in patients with OA. The genetic mechanism of low adiponectin level and its significance in the pathogenesis of OA needed to be determined. The purpose of the current investigation was to investigate the relationship between 2 single nucleotide polymorphisms, +45T/G (rs2241766) and +276G/T (rs1501299), in ADIPOQ gene with the risk of OA in Thai population. Moreover, we empasized on the impact of the 2 SNPs on plasma adiponectin values. We postulated that the ADIPOQ SNPs could serve as genetic parameters that affected the risk of OA.
This study is the first to explore the possible relationship between +45T/G and +276G/T polymorphisms of the ADIPOQ with the susceptibility of knee OA. The population of this study was ethnically homogeneous according to the Hardy-Weinberg equilibrium, which makes the possibility of confounding ethnic heterogeneity less possible. Compared with other diseases, OA is a polygenic disease on the basis of the epidemiologic and genetic studies.
Adiponectin is derived from adipocytes, has anti-inflammatory and anti-atherogeneic effects as well as multiple beneficial effects on metabolism[16]. Studies indicate that adiponectin modulates the function and phenotypes of macrophages in chronic inflammation[3], suppressed the production of TNF-alpha[2]. Moreover, it was shown that adiponectin up-regulated tissue inhibitor of metalloproteinases-2 (TIMP-2)[17] and down-regulated IL-1β-induced MMP-13[7]. Until now, there are two-loci polymorphisms in adiponectin gene have been researched extensively, +45T/G SNP located in exon 2 and +276G/T SNP located in intron 2. The two loci polymorphisms have been identified to associate with amount of diseases related with metabolism and inflammation. Our findings indicated that the percentage of alleles and the genotypic distributions were not statistically different between knee OA participants and control subjects. Interestingly, based on knee OA severity, ADIPOQ genotype at +276G/T was significant difference between KL grade 2 and grade 3 or 4, suggesting that OA patients with GG genotype are more likely to develop, or be more severe OA than those with GT and TT genotype. The association of ADIPOQ polymorphisms with circulating adiponectin concentration is in line with the previous finding that +276G/T polymorphism was significantly associated with serum adiponectin in Chingford study by Kyriakou et al[18].
It has been widely studied that the relationship between plasma adiponectin levels and the +45T/G and +276 G/T polymorphisms. Our study revealed that plasma adiponectin level in OA group was significantly lower than control group. Additionally, the GG genotype at +45T/G and +276 G/T polymorphisms in knee OA patients was associated with lower circulating adiponectin concentration. Different body fat distribution may have a contributory role on adiponectin expression and response in obesity individuals with low-grade inflammatory reaction[19]. Therefore, genetic variation in the ADIPOQ could regulate adiponectin level in the circulation.
How the +276G/T polymorphism affects the ADIPOQ gene function and expression remains questionable. The changed genotype at a specific polymorphism locus could not alter amino acid sequence or structure of the protein. In other words, no obviously biological function might not be precluded. As a matter of fact, it has been demostrated by Yang et al[20] in ADIPOQ gene. On the other hand, linkage disequilibrium could exist at this SNP to influence its gene with other mutation sites. A recent study reported that the single nucleotide mutation at +276G/T locus arised linkage disequilibrium with inserted “A” nucleotide at +2019 SNP of adiponectin gene three prime untranslated region (3’ UTR) which was known as an important part to affect synthesis and degradation of adiponectin mRNA[21]. Furthermore, it has been demonstrated that mRNA stability would be affected by 3’UTR polymorphisms of other reseached genes[22]. The discrepancy persists in several studies regarding to the association of the SNPs with OA. The susceptibility of candidate genes for OA has previously been demonstrated by some studies, but variants will be controversial by other researchers. This study included a relatively small number of participants in this single-center trial study. It is necessary to conduct additional observations under administration of multiple centers with a larger increased sample size. Multiple risk factors contribute to OA including mechanical stress, inflammation, obesity, aging, and genetic alteration. The susceptibility of OA could vary in different populations. Environmental factors may influence the genetic contributions to the susceptibility of OA.
Taken together, our study suggested that the +45T/G and +276G/T polymorphisms were not related with the risk of knee OA in our Thai population. The knee OA patients with the GG genotype at the +276G/T locus seemed to have a higher potential risk in the severity of OA than those having the GT and TT genotypes. The GG genotypes at SNP +45T/G and +276G/T loci were associated with plasma adiponectin concentration in healthy controls and knee OA patients. Further studies will be needed to clarify the relationship of two single nucleotide polymorphisms in larger sample size and different ethnic cohort on knee joint or other joints to yield a better understanding of these polymorphisms in the development of OA.
The authors thank the Research Chair from the National Science and Technology Development Agency, and the 100th Anniversary Chulalongkorn University for Doctoral Scholarship to DZ, National Research University Project through the Ageing Cluster, Chulalongkorn University. The authors are also grateful to Dr. Wanvisa Udomsinprasert, Research Core Facility of Department of Biochemistry and Chulalongkorn Medical Research Center for providing technical assistance.
The understanding of genetic factors in the pathogenesis of osteoarthritis (OA) is still incomplete. There is growing awareness of the role of adiponectin in knee OA. Understanding the polymorphisms of adiponectin might help explain why these polymorphisms play roles in the development of knee OA.
Adiponectin +45T/G and +276G/T polymorphisms and plasma adiponectin levels have been studied in patients with knee OA, including healthy controls.
This is a novel study in that it addresses the polymorphisms and plasma of adiponectin in patients with knee OA, including healthy controls. The authors found that Plasma adiponectin levels were significantly lower in knee OA than controls. There were no significant differences in the genotype distributions and allele frequencies of ADIPOQ +45T/G and +276G/T polymorphisms between patients with knee OA and controls.
Understanding the role of adiponectin +45T/G and +276G/T polymorphisms in OA could help find possible biomarkers of susceptibility of OA. It could also serve as predictive parameter for disease severity of knee OA.
The study is interesting.
Manuscript source: Invited manuscript
Specialty type: Orthopedics
Country of origin: Thailand
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Lee NJG, Mavrogenis AF, Unver B S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Meier U, Gressner AM. Endocrine regulation of energy metabolism: review of pathobiochemical and clinical chemical aspects of leptin, ghrelin, adiponectin, and resistin. Clin Chem. 2004;50:1511-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 648] [Cited by in RCA: 623] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 2. | Ouchi N, Walsh K. Adiponectin as an anti-inflammatory factor. Clin Chim Acta. 2007;380:24-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 664] [Cited by in RCA: 639] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 3. | Ouchi N, Kihara S, Arita Y, Nishida M, Matsuyama A, Okamoto Y, Ishigami M, Kuriyama H, Kishida K, Nishizawa H. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation. 2001;103:1057-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 897] [Cited by in RCA: 893] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 4. | Gegout PP, Francin PJ, Mainard D, Presle N. Adipokines in osteoarthritis: friends or foes of cartilage homeostasis? Joint Bone Spine. 2008;75:669-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Honsawek S, Chayanupatkul M. Correlation of plasma and synovial fluid adiponectin with knee osteoarthritis severity. Arch Med Res. 2010;41:593-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 6. | Cuzdan Coskun N, Ay S, Evcik FD, Oztuna D. Adiponectin: is it a biomarker for assessing the disease severity in knee osteoarthritis patients? Int J Rheum Dis. 2015; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Chen TH, Chen L, Hsieh MS, Chang CP, Chou DT, Tsai SH. Evidence for a protective role for adiponectin in osteoarthritis. Biochim Biophys Acta. 2006;1762:711-718. [PubMed] |
| 8. | Bergink AP, van Meurs JB, Loughlin J, Arp PP, Fang Y, Hofman A, van Leeuwen JP, van Duijn CM, Uitterlinden AG, Pols HA. Estrogen receptor alpha gene haplotype is associated with radiographic osteoarthritis of the knee in elderly men and women. Arthritis Rheum. 2003;48:1913-1922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 97] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Valdes AM, Arden NK, Tamm A, Kisand K, Doherty S, Pola E, Cooper C, Tamm A, Muir KR, Kerna I. A meta-analysis of interleukin-6 promoter polymorphisms on risk of hip and knee osteoarthritis. Osteoarthritis Cartilage. 2010;18:699-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Honsawek S, Malila S, Yuktanandana P, Tanavalee A, Deepaisarnsakul B, Parvizi J. Association of MMP-3 (-1612 5A/6A) polymorphism with knee osteoarthritis in Thai population. Rheumatol Int. 2013;33:435-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Hara K, Boutin P, Mori Y, Tobe K, Dina C, Yasuda K, Yamauchi T, Otabe S, Okada T, Eto K. Genetic variation in the gene encoding adiponectin is associated with an increased risk of type 2 diabetes in the Japanese population. Diabetes. 2002;51:536-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 518] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 12. | Jang Y, Lee JH, Chae JS, Kim OY, Koh SJ, Kim JY, Cho H, Lee JE, Ordovas JM. Association of the 276G-& gt; T polymorphism of the adiponectin gene with cardiovascular disease risk factors in nondiabetic Koreans. Am J Clin Nutr. 2005;82:760-767. [PubMed] |
| 13. | Qi L, Li T, Rimm E, Zhang C, Rifai N, Hunter D, Doria A, Hu FB. The +276 polymorphism of the APM1 gene, plasma adiponectin concentration, and cardiovascular risk in diabetic men. Diabetes. 2005;54:1607-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 14. | Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9311] [Cited by in RCA: 8886] [Article Influence: 130.7] [Reference Citation Analysis (0)] |
| 15. | Nakatani K, Noma K, Nishioka J, Kasai Y, Morioka K, Katsuki A, Hori Y, Yano Y, Sumida Y, Wada H. Adiponectin gene variation associates with the increasing risk of type 2 diabetes in non-diabetic Japanese subjects. Int J Mol Med. 2005;15:173-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Ukkola O, Santaniemi M. Adiponectin: a link between excess adiposity and associated comorbidities? J Mol Med (Berl). 2002;80:696-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 255] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 17. | Kumada M, Kihara S, Ouchi N, Kobayashi H, Okamoto Y, Ohashi K, Maeda K, Nagaretani H, Kishida K, Maeda N. Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation. 2004;109:2046-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 390] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 18. | Kyriakou T, Collins LJ, Spencer-Jones NJ, Malcolm C, Wang X, Snieder H, Swaminathan R, Burling KA, Hart DJ, Spector TD. Adiponectin gene ADIPOQ SNP associations with serum adiponectin in two female populations and effects of SNPs on promoter activity. J Hum Genet. 2008;53:718-727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Park KG, Park KS, Kim MJ, Kim HS, Suh YS, Ahn JD, Park KK, Chang YC, Lee IK. Relationship between serum adiponectin and leptin concentrations and body fat distribution. Diabetes Res Clin Pract. 2004;63:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 161] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 20. | Yang WS, Tsou PL, Lee WJ, Tseng DL, Chen CL, Peng CC, Lee KC, Chen MJ, Huang CJ, Tai TY. Allele-specific differential expression of a common adiponectin gene polymorphism related to obesity. J Mol Med (Berl). 2003;81:428-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 110] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Menzaghi C, Ercolino T, Di Paola R, Berg AH, Warram JH, Scherer PE, Trischitta V, Doria A. A haplotype at the adiponectin locus is associated with obesity and other features of the insulin resistance syndrome. Diabetes. 2002;51:2306-2312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 318] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 22. | Jupe ER, Badgett AA, Neas BR, Craft MA, Mitchell DS, Resta R, Mulvihill JJ, Aston CE, Thompson LF. Single nucleotide polymorphism in prohibitin 3’ untranslated region and breast-cancer susceptibility. Lancet. 2001;357:1588-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 2.3] [Reference Citation Analysis (0)] |