Published online Mar 18, 2017. doi: 10.5312/wjo.v8.i3.256
Peer-review started: July 29, 2016
First decision: September 2, 2016
Revised: November 10, 2016
Accepted: December 27, 2016
Article in press: December 28, 2016
Published online: March 18, 2017
Processing time: 234 Days and 18.6 Hours
To describe, using gait analysis, the development of spinal motion in the growing child.
Thirty-six healthy children aged from 3 to 16 years old were included in this study for a gait analysis (9 m-walk). Various kinematic parameters were recorded and analyzed such as thoracic angle (TA), lumbar angle (LA) and sagittal vertical axis (SVA). The kinetic parameters were the net reaction moments (N.m/kg) at the thoracolumbar and lumbosacral junctions.
TA and LA curves were not statistically correlated to the age (respectively, P = 0.32 and P = 0.41). SVA increased significantly with age (P < 0.001). Moments in sagittal plane at the lumbosacral junction were statistically correlated to the age (P = 0.003), underlining the fact that sagittal mechanical constraints at the lumbosacral junction increase with age. Moments in transversal plane at the thoracolumbar and lumbosacral junctions were statistically correlated to the age (P = 0.0002 and P = 0.0006), revealing that transversal mechanical constraints decrease with age.
The kinetic analysis showed that during growth, a decrease of torsional constraint occurs while an increase of sagittal constraint is observed. These changes in spine biomechanics are related to the crucial role of the trunk for bipedalism acquisition, allowing stabilization despite lower limbs immaturity. With the acquisition of mature gait, the spine will mainly undergo constraints in the sagittal plane.
Core tip: Many postural changes occur during childhood, including the adaptation of the spine to maintain an erect posture. The aim was to describe, using gait analysis, the development of spinal motion during growth. Various kinematic parameters were recorded in 36 healthy children. Thoracic kyphosis and lumbar lordosis were not found to increase during childhood whereas sagittal vertical axis increased with age. The kinetic analysis showed a decrease of torsional constraint while sagittal constraint increased. These changes in spine biomechanics are related to the crucial role of the trunk for bipedalism acquisition, allowing stabilization despite lower limbs immaturity.
- Citation: Pesenti S, Blondel B, Peltier E, Viehweger E, Pomero V, Authier G, Fuentes S, Jouve JL. Spinal alignment evolution with age: A prospective gait analysis study. World J Orthop 2017; 8(3): 256-263
- URL: https://www.wjgnet.com/2218-5836/full/v8/i3/256.htm
- DOI: https://dx.doi.org/10.5312/wjo.v8.i3.256
With the acquisition of bipedalism, many anatomical and postural changes occurred in humans[1-3]. Among these changes, an adaptation of the spine has been necessary to maintain an erect position, in combination with an adaptation of the pelvis and the lower limbs[4-6]. Although gait acquisition is apparently complete by the age of 3, adaptation to erect posture continues until the end of growth. According to Peterson et al[7], mature gait patterns are visible in children only from the age of 12.
With the development of modern tools for gait analysis, it is possible to obtain a precise evaluation of the kinematic and kinetic for different segments of the human body. While many of these tools have been developed for lower limbs analysis, various authors have demonstrated their accuracy for trunk dynamic analysis[8-10]. Many studies have described the evolution of spinal curvatures with radiological or other methods[11,12]. Using these tools, it has been shown that thoracic kyphosis and lumbar lordosis increase with age.
To our knowledge, there is no evidence in literature about this development using gait analysis tools. Moreover, gait analysis provides dynamic data such as constraints applied to spinal joints, these parameters having never been discussed in literature before. The hypothesis of this work was that spinal motion changes all along growth. The aim of this study was to describe, using gait analysis, the development of spinal motion in the growing child.
To obtain a homogenous pediatric cohort, only healthy volunteers were included in this prospective study after informed consent. Inclusion criteria were children aged from 3 to 16 years old, without known disease and volunteers to participate to the study. Exclusion criteria were every history of orthopedic or neurologic disorders, major orthopedic trauma or allergy to the components used for gait analysis.
For each participant, the following anthropometric data were collected for gait analysis: Age, weight, height, lower limb length and knee and ankle diameters.
All measurements were obtained using an optoelectronic system (Vicon, Oxford, United Kingdom) with six high-resolution cameras with infrared light and a sampling frequency of 100 Hz which recorded the position of passive retroreflective markers and two force platforms (AMTI, United States). This protocol included all the markers necessary to obtain parameters of a standing posture and to calculate the force of external efforts in the different intersegmental centers, as described by Blondel et al[13], according to the International Society of Biomechanics[14,15].
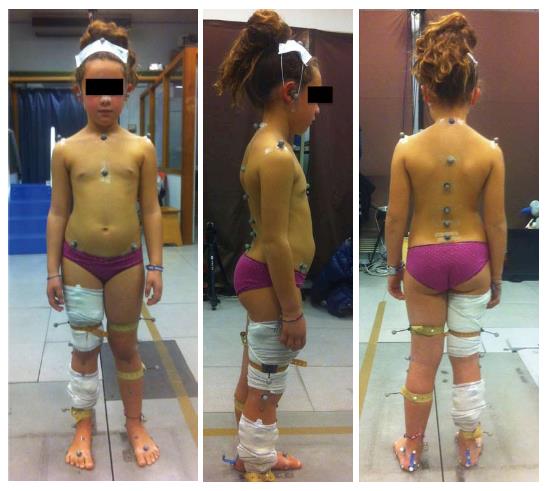
Subjects were equipped with a set of 28 retroflective markers as described in Table 1 and Figure 1. These markers allowed an analysis of different body segments such as head and neck, the scapular girdle, the thorax and thoracic spine, the abdomen and lumbar spine, the pelvis and the lower limbs.
| Parameters | |
| Head | Vertex: 1 |
| Nasion: 1 | |
| Tragus: 2 | |
| Trunk - thorax | Acromion: 2 |
| Manubrium: 1 | |
| Xiphoid: 1 | |
| C7: 1 | |
| T6: 1 | |
| T9: 1 | |
| Trunk - abdomen | T12: 1 |
| L3: 1 | |
| S1: 1 | |
| Pelvis | ASIS: 2 |
| Lower limbs - thighs | Femoral shaft: 2 |
| Lateral femoral condyle: 2 | |
| Lower limbs - legs | Tibial shaft: 2 |
| Lateral malleolus: 2 | |
| Lower limb - feet | Calcaneus: 2 |
| 2nd metatarsal head: 2 |
Before the beginning of gait analysis, a short trial was performed to check the good positioning of the markers according to the analysis of knee valgus/varus[16].
For gait analysis, subjects were asked to walk at a self-selected speed, barefoot, on a flat and straight 9 m-walkway. A minimum of seven trials was recorded to collect kinematic and kinetic data.
The data collected by the 6 high-resolution cameras were converted into a 3D model using NEXUS software (Vicon Motion Systems, Oxford, United Kingdom) for the lower limbs and data were integrated to MATLAB software for trunk analysis.
The characteristic moments of the beginning and the end of the double stance phase were used to compare subjects.
For kinetic analysis, calculations were made from anthropometric reference tables[17].
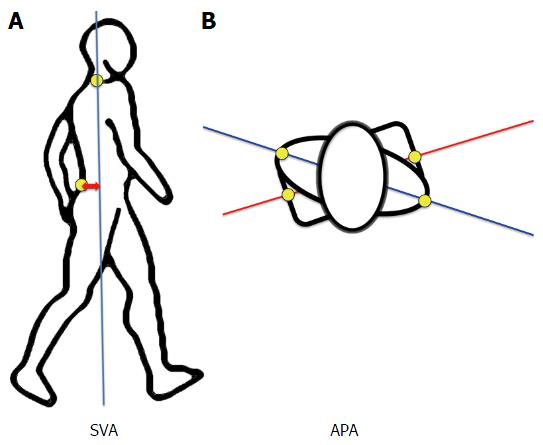
Kinematic parameters during gait are described hereafter and summarized in Table 2 and Figure 2: (1) Sagittal Vertical Axis Adimensioned (SVA Ad): distance between the marker “S1” and the vertical line passing by the marker “C7”. This value was weighted by the height of the subject to be comparable between subjects, regardless to age and height (SVA Ad=SVA/Height). This parameter reflects trunk position during gait: A great value of SVA indicates that the trunk is leaning forward; (2) angle pelvis-acromion (APA): Angle defined in the transverse plane between the line joining the 2 “Acromion” markers and the line joining the 2 “anterosuperior iliac spine” markers. The APA-rom (range of motion) was calculated as the difference between the maximum and the minimum values of the APA during a gait cycle[18]; (3) thoracic angle (TA): Angle between the “C7”-“T7” line and the “T9”-“T12” line; and (4) lumbar Angle (LA): Angle between the “T12”-“L3” line and the “L3”-“S1” line.
| Frontal | Sagittal | Transversal | |
| Overall balance | SVA Ad | ||
| Shoulders | APA | ||
| Thoracic spine | TA | ||
| Lumbar spine | LA | ||
| Pelvis | Pelvic version | ||
| Lower limbs | Knee Varus/valgus | Hip flex/ext | |
| Knee flex/ext |
Kinetic parameters are detailed in Table 3. In frontal plane, moments applied to the spine are relative to lateral bending movements, in sagittal plane they are flexion-extension movements and in transversal plane, they were consecutive to torsional movements. These data were dimensioned (i.e., divided by the weight) to be comparable between individuals, independently from their body mass.
| Frontal moments | Sagittal moments | Transversal moments | |
| Thoracolumbar junction | Lateral bending | Flexion-extension | Torsion |
| Lumbosacral junction | Lateral bending | Flexion-extension | Torsion |
Gait data were analyzed to compare subjects in a continuous analysis according to age. A Pearson Product Moment Correlation Coefficient (r) was used to determine differences between subjects according to age. Level of significance was set at 5% for every statistical analysis.
From October 2012 to October 2013, 36 subjects were included in this study. Mean age of the population was 8.8 years old (3.3 to 15.6 years old). Demographic and anthropometric data are shown in Table 4.
| Subject No. | Sex | Age (yr) | Height (cm) | Weight (kg) | Lower limb length (cm) | Knee diameter (cm) | Ankle diameter (cm) | |||
| Right | Left | Right | Left | Right | Left | |||||
| 1 | F | 3.3 | 880 | 11 | 420 | 420 | 55 | 55 | 45 | 45 |
| 2 | F | 3.4 | 1060 | 17 | 510 | 510 | 80 | 80 | 60 | 60 |
| 3 | M | 3.9 | 935 | 14 | 500 | 500 | 70 | 70 | 44 | 44 |
| 4 | F | 3.9 | 1050 | 19 | 520 | 520 | 80 | 80 | 60 | 60 |
| 5 | M | 4.1 | 1080 | 18 | 550 | 550 | 70 | 70 | 50 | 50 |
| 6 | F | 4.6 | 1090 | 16 | 650 | 650 | 50 | 50 | 45 | 45 |
| 7 | F | 5.8 | 1135 | 19 | 570 | 570 | 70 | 70 | 50 | 50 |
| 8 | M | 6.1 | 1150 | 19 | 575 | 575 | 80 | 80 | 60 | 60 |
| 9 | F | 7.0 | 1345 | 27 | 670 | 670 | 90 | 90 | 65 | 65 |
| 10 | F | 7.2 | 1200 | 21 | 570 | 570 | 70 | 70 | 50 | 50 |
| 11 | F | 7.4 | 1160 | 21 | 585 | 585 | 80 | 80 | 60 | 60 |
| 12 | M | 7.7 | 1370 | 34 | 730 | 730 | 110 | 110 | 70 | 70 |
| 13 | F | 7.7 | 1300 | 31 | 680 | 680 | 95 | 95 | 70 | 70 |
| 14 | F | 7.8 | 1280 | 26 | 650 | 650 | 90 | 90 | 70 | 70 |
| 15 | M | 8.0 | 1340 | 27 | 680 | 680 | 90 | 90 | 70 | 70 |
| 16 | M | 8.1 | 1330 | 28 | 685 | 685 | 95 | 95 | 65 | 65 |
| 17 | M | 8.5 | 1360 | 33 | 710 | 710 | 90 | 90 | 55 | 55 |
| 18 | M | 8.8 | 1400 | 40 | 720 | 720 | 110 | 110 | 70 | 70 |
| 19 | F | 8.9 | 1380 | 37 | 720 | 720 | 100 | 100 | 65 | 65 |
| 20 | M | 9.1 | 1320 | 24 | 680 | 680 | 80 | 80 | 60 | 60 |
| 21 | M | 9.2 | 1420 | 26 | 750 | 760 | 55 | 55 | 50 | 50 |
| 22 | F | 9.3 | 1524 | 38 | 820 | 820 | 100 | 100 | 65 | 65 |
| 23 | M | 9.5 | 1395 | 36 | 750 | 750 | 110 | 105 | 65 | 65 |
| 24 | F | 10.0 | 1360 | 29 | 710 | 710 | 70 | 70 | 55 | 55 |
| 25 | F | 10.6 | 1370 | 39 | 740 | 740 | 95 | 95 | 60 | 60 |
| 26 | F | 10.8 | 1425 | 32 | 750 | 750 | 90 | 90 | 65 | 65 |
| 27 | F | 11.0 | 1530 | 41 | 810 | 810 | 105 | 105 | 70 | 70 |
| 28 | M | 11.1 | 1520 | 51 | 850 | 850 | 100 | 100 | 70 | 70 |
| 29 | F | 11.1 | 1463 | 47 | 740 | 740 | 105 | 105 | 70 | 70 |
| 30 | F | 11.3 | 1610 | 46 | 840 | 840 | 105 | 105 | 70 | 80 |
| 31 | M | 11.9 | 1390 | 34 | 700 | 700 | 85 | 85 | 60 | 60 |
| 32 | F | 12.5 | 1470 | 35 | 740 | 740 | 100 | 100 | 70 | 70 |
| 33 | F | 12.7 | 1570 | 54 | 900 | 900 | 115 | 110 | 75 | 70 |
| 34 | F | 13.9 | 1690 | 47 | 925 | 925 | 100 | 100 | 70 | 70 |
| 35 | M | 15.5 | 1650 | 48 | 830 | 830 | 85 | 85 | 65 | 65 |
| 36 | M | 15.6 | 1770 | 87 | 930 | 930 | 100 | 100 | 70 | 70 |
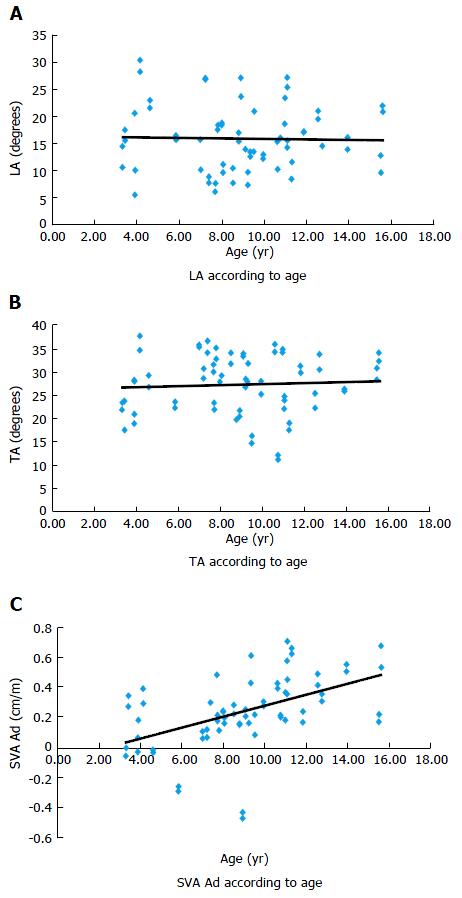
Sagittal plane: TA and LA curves were not statistically different (respectively, r = 0.06 and r = 0.023, P = 0.32 and P = 0.41, Figure 3).
SVA Ad was significantly correlated to the age (r = 0.488, P < 0.001), revealing a progressive anterior increase of the projection of the C7 marker with regards to the S1 marker (Figure 4).
Transversal plane: There was a non-significant negative correlation between APA-rom and age (r = -0.063, P = 0.71).
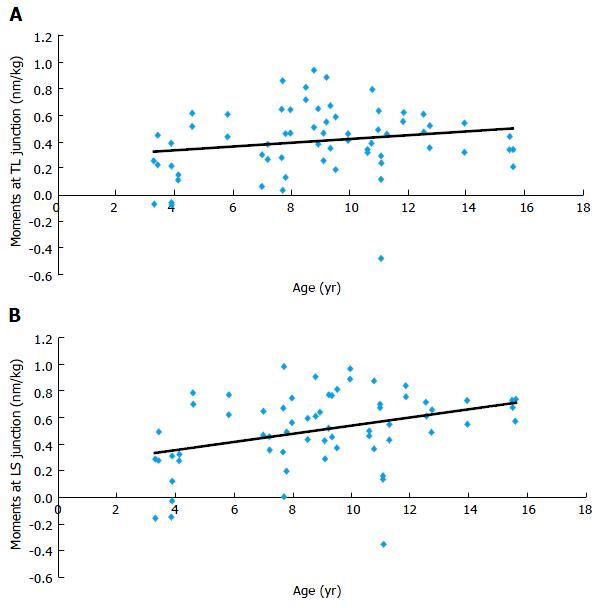
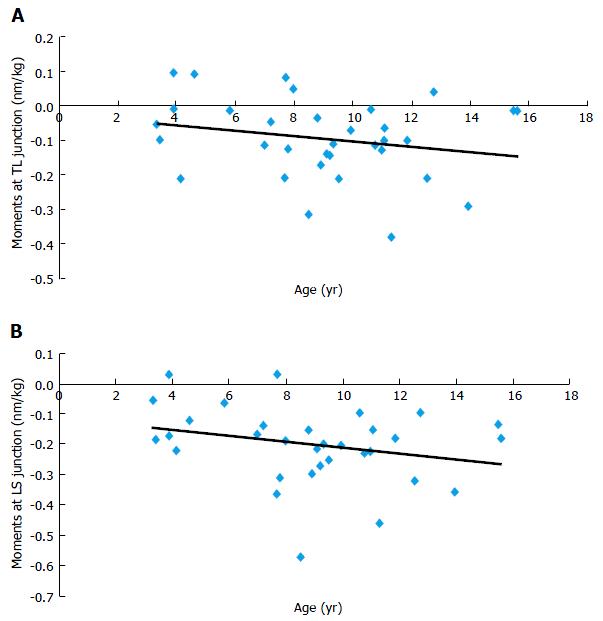
Sagittal plane: Results showed that flexion-extension moments at the lumbosacral junction were statistically correlated to age (r = 0.356, P = 0.003). In other words, mechanical sagittal constraints at the lumbosacral junction increase during growth. At the thoracolumbar junction, sagittal constraints were not significantly correlated to age (r = 0.189, P = 0.13, Figure 5).
Transversal plane: Results demonstrated that torsion moments at thoracolumbar and lumbosacral junctions were statistically correlated to age (r = -0.613 and r = -0.563, P = 0.0002 and P = 0.0006). In other words, transversal mechanical constraints at thoracolumbar and lumbosacral junctions decrease with age (Figure 6).
This study is the first to analyze spinal motion in children via gait analysis tool. Changes occur in spine motion in children with the acquisition of a mature gait even if dynamic parameters of the spine during growth seem to be established before the age of 3.
So far, only few studies have studied dynamic development of the spine according to age via gait analysis[19]. The studies from Wagner et al[20] and Farfan[21] showed that the presence of a lumbar spinal curvature concave toward the back is a necessary biomechanical condition for a stable erect posture, enabling an economic muscular functioning despite the posterior position of the spine. Lumbar lordosis thus appears as being a fundamental prerequisite to bipedalism, explaining its early appearance during childhood. Parameters determining bipedalism are acquired very early during growth[21,22]. However, some skeletal parameters which are not involved in the acquisition of bipedalism are variable and change until the end of growth. Some of these parameters are even found to be genetically predetermined during fetal life. This is, for example, the case of the morphology of the femoral trochlea[23] or the lumbar lordosis[24], which are genetically predetermined in humans. Their early kinematic setting is an element explaining the ability to bipedalism.
The spine appears to be of fundamental importance in the adaptation of the skeleton to bipedalism and we can define a real “spinal motor of bipedalism”; the spine being the first skeletal element to adjust its posture and functioning to bipedalism as the main element of locomotion[25]. The lower limbs adapt secondarily, around the age of 7, with a progressive pelvic anteversion, a progressive extension of the hips and the knees, lately mature.
Some radiographic and morphologic studies have evaluated the development of spinal curvatures during growth[11,12]. These studies revealed that from the age of 3 years until skeletal maturity, there is a linear enhancement of the thoracic kyphosis and lumbar lordosis. According to us, these changes do not reflect the adaptation of the skeleton to bipedalism, but an adaptation to the major constraints applied to the trunk during growth. In other words, formation of overlying sagittal curvatures to the lumbar lordosis with the appearance of thoracic kyphosis and cervical lordosis is related to biomechanical adaptation to an increase of load on the spine.
Most of the parameters used in this study for kinematic analysis, such as SVA, were chosen according to previous works[18]. These parameters seemed to be good descriptors because they are the dynamic equivalent of radiographic parameters. Thoracic angle and lumbar angle were meant to be the equivalent of thoracic kyphosis and lumbar lordosis, which are 2 radiographic parameters used in clinical practice.
Results from this study suggest that the sagittal efforts applied on the spine increase significantly with age leading to increased flexion-extension constraints at the lumbosacral junction. This phenomenon can be explained by the accentuation of spinal curvatures with age as a response to the increased load on the spine, deporting the lumbar spine forward and thereby increasing the lever arm and the moment applied to the underlying lumbosacral junction.
With regards to the kinetic parameters in the transverse plane, our results showed a significant reduction in torsional constraints at the thoracolumbar and lumbosacral junctions during growth. Although lumbar lordosis is acquired from fetal life, the central maturation processes coordinating the acquisition of a mature gait for the lower limbs appear only around the age of 7. Before this turning point, the lower limbs do not have a mature kinematics allowing balance and stability for satisfactory and stable erect posture. These results are in line with the posturographic study from Peterson et al[7] who have shown that sensory systems ensuring a satisfactory balance for maintaining erect station were efficient only from the age of 12. Thus, the spine undergoes greater constraints to compensate this permanent balance research. Large constraints applied to the spine and their reduction with age are a sign of the compensation by the trunk of a lack of stability due to lower limbs and sensory system immaturity. Prior to the acquisition of a definitive and mature bipedalism, the trunk is fundamental for the possibility of early bipedalism.
Furthermore, the significant increase of SVA during growth could be related to the same conclusion. The low value of SVA in young children reflects the need to keep the shoulders over the pelvis to stabilize the erect posture. With maturation and the acquisition of a final biped balance, the subject is projected more forward, then changing the direction of the constraints on the spine from the transverse plane to the sagittal plane.
These findings allow a better comprehension of the importance of constraints in the lumbar spine and can be a source of explanation for specific degenerative disorders of this anatomical region.
The small number of subject in each age group may be at the origin of a lack of statistical power and may explain the lack of significant difference. However, in similar series, changes in lower limb parameters are clearly established, these parameters being definitively acquired after the age of 7[26-31]. The protocol used for trunk assessment has been validated before in the study by Blondel et al[13]. This protocol is designed for clinical use and a low number of markers is a clear advantage in that case. The authors have demonstrated that 6 markers were sufficient to assess trunk kinematics and kinetics precisely. Moreover, there was a wide amount of variability. Including a greater number of subjects may increase statistical power and allow to highlight differences in sagittal kinematic parameters.
The biomechanical model developed by Blondel et al[13] in adults has enabled us to achieve the first dynamic study of spine development with age. The comparison of age groups and continuous analysis did not highlight major kinematic evolution of spinal curvatures during skeletal maturation. The acquisition of the lumbar lordosis and thoracic kyphosis is a morphological characteristic that probably appears very early in children, before the age of 3.
The kinetic analysis revealed a progressive decrease in torsional constraints applied on the spine while the constraints in flexion-extension increase with age. These changes allow stabilization of erect posture despite the immaturity of the lower limbs. With the acquisition of mature gait, the spine will mainly undergo constraints in the sagittal plane. These changes point out the major role of the trunk during the acquisition of bipedalism.
Although gait acquisition is apparently complete by the age of 3, adaptation to erect posture continues until the end of growth. Many studies have described the evolution of spinal curvatures with radiological methods. Using gait analysis tools, it is possible to obtain a precise analysis of the evolution of spinal alignment with age.
Even if the sample size is quite limited, this study provides interesting information about evolution of spinal dynamics with growth. This study may help to understand changes in gait in spinal disorders.
Results from this study confirm the technical feasibility of the protocol in young children. Using this methodology, it was possible to evaluate net moments applied to spinal junctions. To the authors’ knowledge, this the first study to provide dynamic data of the spine of healthy children.
By providing normative data, this study may help to understand the changes that occur in children with spinal disorders. It could also help to evaluate the behavior of the spine in children after spinal surgery.
Although the sample size is relatively small, this is an interesting study.
Manuscript source: Invited manuscript
Specialty type: Orthopedics
Country of origin: France
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Peng BG, Teli MGA S- Editor: Kong JX L- Editor: A E- Editor: Li D
| 1. | Tardieu C, Bonneau N, Hecquet J, Boulay C, Marty C, Legaye J, Duval-Beaupère G. How is sagittal balance acquired during bipedal gait acquisition? Comparison of neonatal and adult pelves in three dimensions. Evolutionary implications. J Hum Evol. 2013;65:209-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 2. | Van Emmerik RE, McDermott WJ, Haddad JM, Van Wegen EE. Age-related changes in upper body adaptation to walking speed in human locomotion. Gait Posture. 2005;22:233-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Whitcome KK, Shapiro LJ, Lieberman DE. Fetal load and the evolution of lumbar lordosis in bipedal hominins. Nature. 2007;450:1075-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 176] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 4. | Been E, Gómez-Olivencia A, Kramer PA. Lumbar lordosis of extinct hominins. Am J Phys Anthropol. 2012;147:64-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Been E, Gómez-Olivencia A, Kramer PA. Brief communication: Lumbar lordosis in extinct hominins: implications of the pelvic incidence. Am J Phys Anthropol. 2014;154:307-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Lapègue F, Jirari M, Sethoum S, Faruch M, Barcelo C, Moskovitch G, Ponsot A, Rabat MC, Labarre D, Vial J. [Evolution of the pelvis and hip throughout history: from primates to modern man]. J Radiol. 2011;92:543-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Peterson ML, Christou E, Rosengren KS. Children achieve adult-like sensory integration during stance at 12-years-old. Gait Posture. 2006;23:455-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 147] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 8. | Chung CY, Park MS, Lee SH, Kong SJ, Lee KM. Kinematic aspects of trunk motion and gender effect in normal adults. J Neuroeng Rehabil. 2010;7:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Konz RJ, Fatone S, Stine RL, Ganju A, Gard SA, Ondra SL. A kinematic model to assess spinal motion during walking. Spine (Phila Pa 1976). 2006;31:E898-E906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Ranavolo A, Don R, Draicchio F, Bartolo M, Serrao M, Padua L, Cipolla G, Pierelli F, Iavicoli S, Sandrini G. Modelling the spine as a deformable body: Feasibility of reconstruction using an optoelectronic system. Appl Ergon. 2013;44:192-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Cil A, Yazici M, Uzumcugil A, Kandemir U, Alanay A, Alanay Y, Acaroglu RE, Surat A. The evolution of sagittal segmental alignment of the spine during childhood. Spine (Phila Pa 1976). 2005;30:93-100. [PubMed] |
| 12. | Giglio CA, Volpon JB. Development and evaluation of thoracic kyphosis and lumbar lordosis during growth. J Child Orthop. 2007;1:187-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Blondel B, Pomero V, Moal B, Lafage V, Jouve JL, Tropiano P, Bollini G, Dumas R, Viehweger E. Sagittal spine posture assessment: feasibility of a protocol based on intersegmental moments. Orthop Traumatol Surg Res. 2012;98:109-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Dumas R, Chèze L, Verriest JP. Adjustments to McConville et al. and Young et al. body segment inertial parameters. J Biomech. 2007;40:543-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 276] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 15. | Wu G, Siegler S, Allard P, Kirtley C, Leardini A, Rosenbaum D, Whittle M, D’Lima DD, Cristofolini L, Witte H. ISB recommendation on definitions of joint coordinate system of various joints for the reporting of human joint motion--part I: ankle, hip, and spine. International Society of Biomechanics. J Biomech. 2002;35:543-548. [PubMed] |
| 16. | Rivest LP. A correction for axis misalignment in the joint angle curves representing knee movement in gait analysis. J Biomech. 2005;38:1604-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | McDowell MA, Fryar CD, Ogden CL. Anthropometric reference data for children and adults: United States, 1988-1994. Vital Health Stat 11. 2009;1-68. [PubMed] |
| 18. | Lenke LG, Engsberg JR, Ross SA, Reitenbach A, Blanke K, Bridwell KH. Prospective dynamic functional evaluation of gait and spinal balance following spinal fusion in adolescent idiopathic scoliosis. Spine (Phila Pa 1976). 2001;26:E330-E337. [PubMed] |
| 19. | Thummerer Y, von Kries R, Marton MA, Beyerlein A. Is age or speed the predominant factor in the development of trunk movement in normally developing children? Gait Posture. 2012;35:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Wagner H, Liebetrau A, Schinowski D, Wulf T, de Lussanet MH. Spinal lordosis optimizes the requirements for a stable erect posture. Theor Biol Med Model. 2012;9:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Farfan HF. Form and function of the musculoskeletal system as revealed by mathematical analysis of the lumbar spine. An essay. Spine (Phila Pa 1976). 1995;20:1462-1474. [PubMed] |
| 22. | Glard Y, Jouve JL, Garron E, Adalian P, Tardieu C, Bollini G. Anatomic study of femoral patellar groove in fetus. J Pediatr Orthop. 2005;25:305-308. [PubMed] |
| 23. | Glard Y, Jouve JL, Panuel M, Adalian P, Tardieu C, Bollini G. An anatomical and biometrical study of the femoral trochlear groove in the human fetus. J Anat. 2005;206:411-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Choufani E, Jouve JL, Pomero V, Adalian P, Chaumoitre K, Panuel M. Lumbosacral lordosis in fetal spine: genetic or mechanic parameter. Eur Spine J. 2009;18:1342-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Gracovetsky SA, Iacono S. Energy transfers in the spinal engine. J Biomed Eng. 1987;9:99-114. [PubMed] |
| 26. | Chester VL, Tingley M, Biden EN. A comparison of kinetic gait parameters for 3-13 year olds. Clin Biomech (Bristol, Avon). 2006;21:726-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Chester VL, Wrigley AT. The identification of age-related differences in kinetic gait parameters using principal component analysis. Clin Biomech (Bristol, Avon). 2008;23:212-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Cupp T, Oeffinger D, Tylkowski C, Augsburger S. Age-related kinetic changes in normal pediatrics. J Pediatr Orthop. 1999;19:475-478. [PubMed] |
| 29. | Hausdorff JM, Zemany L, Peng C, Goldberger AL. Maturation of gait dynamics: stride-to-stride variability and its temporal organization in children. J Appl Physiol (1985). 1999;86:1040-1047. [PubMed] |
| 30. | Samson W, Van Hamme A, Desroches G, Dohin B, Dumas R, Chèze L. Biomechanical maturation of joint dynamics during early childhood: updated conclusions. J Biomech. 2013;46:2258-2263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Sutherland DH, Olshen R, Cooper L, Woo SL. The development of mature gait. J Bone Joint Surg Am. 1980;62:336-353. [PubMed] |