Published online Oct 18, 2013. doi: 10.5312/wjo.v4.i4.207
Revised: May 21, 2013
Accepted: June 19, 2013
Published online: October 18, 2013
Processing time: 294 Days and 16.8 Hours
Osteoporosis is a common bone disease characterized by reduced bone and increased risk of fracture. In postmenopausal women, osteoporosis results from bone loss attributable to estrogen deficiency. Osteoclast differentiation and activation is mediated by receptor activator of nuclear factor-κB ligand (RANKL), its receptor receptor activator of nuclear factor-κB (RANK), and a decoy receptor for RANKL, osteoprotegerin (OPG). The OPG/RANKL/RANK system plays a pivotal role in osteoclast biology. Currently, a fully human anti-RANKL monoclonal antibody named denosumab is being clinically used for the treatment of osteoporosis and cancer-related bone disorders. This review describes recent advances in RANKL-related research, a story from bench to bedside. First, the discovery of the key factors, OPG/RANKL/RANK, revealed the molecular mechanism of osteoclastogenesis. Second, we established three animal models: (1) a novel and rapid bone loss model by administration of glutathione-S transferase-RANKL fusion protein to mice; (2) a novel mouse model of hypercalcemia with anorexia by overexpression of soluble RANKL using an adenovirus vector; and (3) a novel mouse model of osteopetrosis by administration of a denosumab-like anti-mouse RANKL neutralizing monoclonal antibody. Lastly, anti-human RANKL monoclonal antibody has been successfully applied to the treatment of osteoporosis and cancer-related bone disorders in many countries. This is a real example of applying basic science to clinical practice.
Core tip: This review describes a success story from discovery of osteoprotegerin/receptor activator of nuclear factor-κB ligand (RANKL)/receptor activator of nuclear factor-κB (RANK) to clinical application of a fully human anti-RANKL monoclonal antibody to the treatment of osteoporosis and cancer-related bone disorders. RANKL is a key molecule for osteoclast differentiation and activation. Inhibition of RANKL activity with anti-RANKL antibody reduces osteoclastogenesis, resulting in inhibition of bone resorption. Three animal disease models of osteoporosis, hypercalcemia, and osteopetrosis by treating normal mice with soluble RANKL (sRANKL), adenovirus expressing sRANKL, and anti-mouse RANKL neutralizing antibody, respectively, can be established in 2-14 d and the establishment of these animal models could help accelerate research on bone metabolism.
- Citation: Yasuda H. RANKL, a necessary chance for clinical application to osteoporosis and cancer-related bone diseases. World J Orthop 2013; 4(4): 207-217
- URL: https://www.wjgnet.com/2218-5836/full/v4/i4/207.htm
- DOI: https://dx.doi.org/10.5312/wjo.v4.i4.207
Morphogenesis and remodeling of bone depends on the integrated activity of osteoblasts which form bone and osteoclasts which resorb bone. There have been many attempts to develop pharmaceuticals to treat osteoporosis and other metabolic bone disorders. The major difficulty in the development of such drugs is the lack of clarification of the mechanisms regulating differentiation of the bone cells including osteoblasts and osteoclasts. In the late-1990s, dramatic findings of the key factors of osteoclast differentiation opened a new era in research of osteoclast biology and the development of anti-resorptive pharmaceuticals for osteoporosis[1-11].
We previously identified and cloned an osteoclastogenesis inhibitory factor named OCIF[2,3]. We used human fetal lung fibroblasts, IMR-90 cells, as the cell source and purified OCIF using the in vitro osteoclastogenesis assay established by Takahashi et al[12] and Udagawa et al[13]. Since fibroblasts are present ubiquitously in the body, it was surprising to find that these cells produce a novel osteoclastogenesis inhibitory factor. IMR-90 cells produce a number of cytokine and growth factors including hepatocyte growth factor (HGF)[14]. Suda et al[15] proposed a working hypothesis that osteoclasts are generated by cell-to-cell interaction with a hypothetical membrane-bound factor called osteoclast differentiation factor (ODF) on osteoblasts. To explore this hypothesis we attempted to find a novel osteoclastogenesis inhibitory factor that could be an inhibitor of the hypothetical factor, ODF[2,3]. Simonet et al[1] independently found the identical factor through the rat EST project and named it osteoprotegerin (OPG). They found a novel tumor necrosis factor (TNF) receptor family member in the comprehensive genomic sequencing project and identified it as an osteoclastogenesis inhibitor by overexpression of its cDNA in transgenic mice. Notably, two independent groups identified the same factor at almost the same time by different strategies.
Thereafter, Yasuda et al [4] and Lacey et al[5] independently identified a ligand of OCIF/OPG with expression cloning and named it ODF and OPG ligand (OPGL), respectively. ODF/OPGL was found to be identical to TNF-related activation-induced cytokine (TRANCE)[16] and RANKL[17], which were cloned as factors regulating T-cell and dendritic cell functions. We confirmed that ODF was the long-sought after ligand regulating osteoclast differentiation and activation[4]. As standard nomenclature of the same molecule, OPG and RANKL were proposed by the ASBMR President’s Committee on Nomenclature, respectively[18].
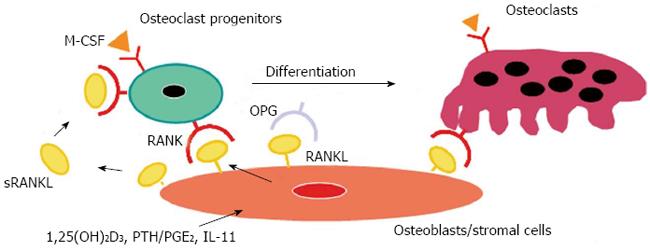
We further identified RANK as a receptor for RANKL on osteoclasts[6]. Although RANK was known to be a receptor for RANKL in the T-cell and dendritic cell interaction[17], the receptor responsible for the RANKL-mediated osteoclastogenesis had not been identified. Some ligands of the TNF family bind to several receptors of the TNF receptor family. It was suspected that RANKL might bind to another member of the TNF receptor family, but not to RANK. We molecularly cloned the RANKL receptor from mouse osteoclast progenitors by panning and identified it as RANK[6]. A polyclonal antibody against soluble RANK (sRANK) mimicked the RANKL function by clustering of RANK. In contrast, sRANK and Fab fragment of anti-RANK polyclonal antibody completely inhibited RANKL-mediated osteoclastogenesis by binding to RANKL and RANK, respectively. Hsu et al[7] also led to the same conclusion using transgenic mice overexpressing soluble RANK. The importance of OPG/RANKL/RANK was demonstrated in vivo with gene-deficient mice[19-23]. The summary of these results is illustrated in the model of osteoclast differentiation (Figure 1). The details of OPG/RANKL/RANK are described elsewhere[8-11].
To investigate the effects and functions of RANKL in vivo, transgenic mice overexpressing mouse soluble RANKL (sRANKL-TG mice)[24] and RANKL-deficient mice[21] were generated. They are useful animal models but it takes several months to interbreed them with other TG mice or gene-deficient mice. Alternatively, we attempted to establish three animal disease models by treating normal mice with either sRANKL, adenovirus vector harboring mouse sRANKL cDNA (Ad-sRANKL), or anti-mouse RANKL neutralizing monoclonal antibody. It takes two to 14 d to make these animal models using normal mice. Thus, the establishment of these quick animal models could help accelerate research on bone metabolism.
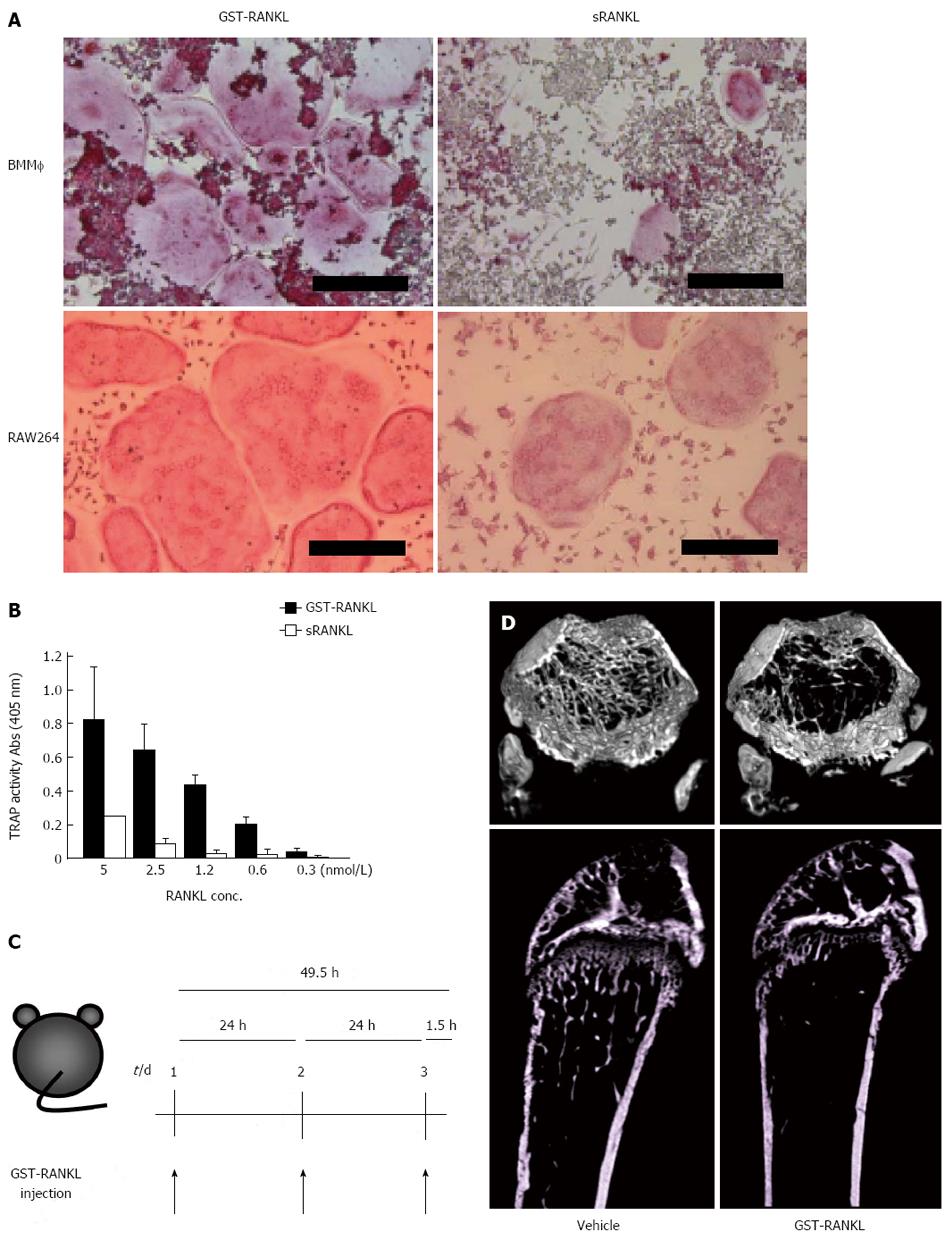
Osteoporosis remains a major public health problem through its associated fragility fractures. Several animal models for the study of osteoporotic bone loss, such as ovariectomy (OVX) and denervation, require surgical skills and several weeks to establish[25-31]. We tried to establish a novel and rapid bone loss model by the administration of glutathione-S transferase (GST)-RANKL to mice[32,33] (Figure 2). GST-RANKL is a fusion protein of GST and the extracellular domain of human RANKL (aa 140-317). GST-RANKL showed stronger activity in osteoclastogenesis using mouse bone marrow macrophages (BMM) and the mouse macrophage cell line, RAW264 cells, respectively, compared with a commercially available soluble RANKL (sRANKL) (Figure 2A, B). Mice were injected intraperitoneally with GST-RANKL and used to evaluate existing anti-osteoporosis drugs. GST-RANKL decreased bone mineral density (BMD) within 50 h in a dose-dependent manner. The marked decrease in femoral trabecular BMD demonstrated by pQCT and the 3D images obtained by micro computer tomography (CT) were indistinguishable from those observed in the OVX model. Histomorphometry revealed significant increase in osteoclastic activity in the GST-RANKL-injected mice. In addition, serum biochemical markers of bone turnover such as calcium, C-terminal cross-linked telopeptides of collagen I (CTx), and tartarate-resistant acid phosphatase-5b (TRAP-5b) were also significantly increased in the GST-RANKL-injected mice in a dose-dependent manner. One of the gold standard models for osteoporosis is OVX which mimics osteoporosis in postmenopausal women. Moreover, the GST-RANKL-induced bone loss model was successfully applied to C57/B6 male and female mice, ICR mice, and Fisher rats. Very recently we successfully shortened the experimental period from 50 to 24 h and established a 1-d bone loss model with one injection of GST-RANKL in mice and rats (Tomimori et al, unpublished).
To apply this bone loss model in the evaluation of pharmaceuticals for osteoporosis, we tested bisphosphonates (BPs), PTH and a selective estrogen receptor modulator (SERM), which are commonly used for the treatment of osteoporosis. We successfully evaluated BPs and PTH within 4 d and 2 wk, respectively, and a combination of GST-RANKL injections and OVX allowed evaluation of a SERM in 18 d. As major pharmaceuticals for osteoporosis have been evaluated using the GST-RANKL-induced bone loss model, it could also be used to evaluate novel drug candidates. In fact, using the bone loss model we evaluated an inhibitor of Btk/Tec tyrosine kinases that are essential for signal transduction through RANK in osteoclast differentiation in approximately 50 h[32].
We also evaluated a denosumab-like anti-human RANKL neutralizing monoclonal antibody as a new osteoporosis therapeutic drug candidate in 10 d[33]. We made anti-human RANKL monoclonal antibodies and selected a neutralizing antibody using the in vitro osteoclastogenesis assay with RAW264 cells. This antibody bound to and neutralized the activity of human but not mouse RANKL. The anti-human RANKL neutralizing antibody (100 g/mouse) or PBS was injected subcutaneously into mice 7 and 4 d before the GST-RANKL injection. Antibody treatment of the model mice completely inhibited the decrease in femoral trabecular BMD. In contrast, BMD in PBS-injected control mice was unaffected by the antibody treatment.
Notably, two or three injections of GST-RANKL induced a weak coupling, whereas longer treatments induced strong coupling[33]. Because OVX-induced bone loss is accompanied with a high turnover of bone remodeling, the GST-RANKL model is similar in mechanism to that of OVX-induced bone loss. Bahtiar et al[34] also used GST-RANKL to develop a high bone turnover model. Other models for high-turnover bone disease with sRANKL have been reported, using continuous infusion[35] and subcutaneous injections of sRANKL[36]. Some of the mice exhibited hypercalcemia. The infusion model requires a large amount of sRANKL and insertion of osmotic pumps subcutaneously into rats, while the injection model requires twice-daily subcutaneous injections into mice for 10 d. As a local bone loss model GST-RANKL was injected into mouse calvaria several times to induce osteoclastogenesis and bone loss near the injection sites within several days[37,38].
A summary of the characteristics of the GST-RANKL-induced bone loss model in comparison to the OVX model is shown in Table 1. First of all, the GST-RANKL-induced bone loss model is rapid, being established within 24-50 h. Second, it is easy, as two or three intraperitoneal injections of sRANKL are sufficient to induce osteoporotic bone loss. Third, it is simple. The mechanism of bone loss in the model is simply due to a stimulation of osteoclast differentiation and activation with endogenous sRANKL. Lastly, it is useful for evaluation of major pharmaceuticals and/or candidates for osteoporosis. A Btk/Tec tyrosine kinase inhibitor, BPs, anti-human RANKL neutralizing monoclonal antibody, PTH and a SERM were evaluated within 50 h, 3 d, 10 d, 2 wk, and 18 d, respectively. Overall, the GST-RANKL model is the simplest, fastest, and easiest osteoporosis model and could be a gold standard for the evaluation of novel drug candidates of osteoporosis as well as OVX[32,33].
| Technique | OVX model | GST-RANKL bone loss model |
| Technique | OVX | Intraperitoneal injections |
| Term for establishment | > 4 wk | 24-50 h |
| Term for evaluation of BP | > 4 wk | 3 d |
| Term for evaluation of PTH | > 4 wk | 14 d |
| Term for evaluation of SERM | > 4 wk | 18 d |
| Term for evaluation of anti-human RANKL | No | 9 d |
| Term for evaluation of Tec tyrosine kinase inhibitor | ||
| NA | 50 h | |
| Evaluation of male animals | No | Yes |
| Term for pharmacological experiments | Several mon | Several wk/d |
| Advantages | ||
| Human disease model | Rapid, easy, simple, and inducible model |
Hypercalcemia is a significant complication in human malignancies, including squamous cell, renal cell, and breast carcinomas. Humoral hypercalcemia of malignancy (HHM) is caused by the overproduction of parathyroid hormone related protein (PTHrP) by tumors[39,40]. PTHrP mobilizes calcium from bone by inducing the expression of RANKL[41]. RANKL is overexpressed in the marrow microenvironment in myeloma patients; the RANKL to OPG ratio is markedly increased compared to that in healthy controls[42].
Symptoms of hypercalcemia manifest as a reflection of the extent and rate of increase of serum ionized calcium. Mild to moderate hypercalcemia is usually asymptomatic[43], whereas moderate to severe hypercalcemia is usually symptomatic[44] and includes anorexia, constipation, vomiting, nausea, weakness and mental confusion.
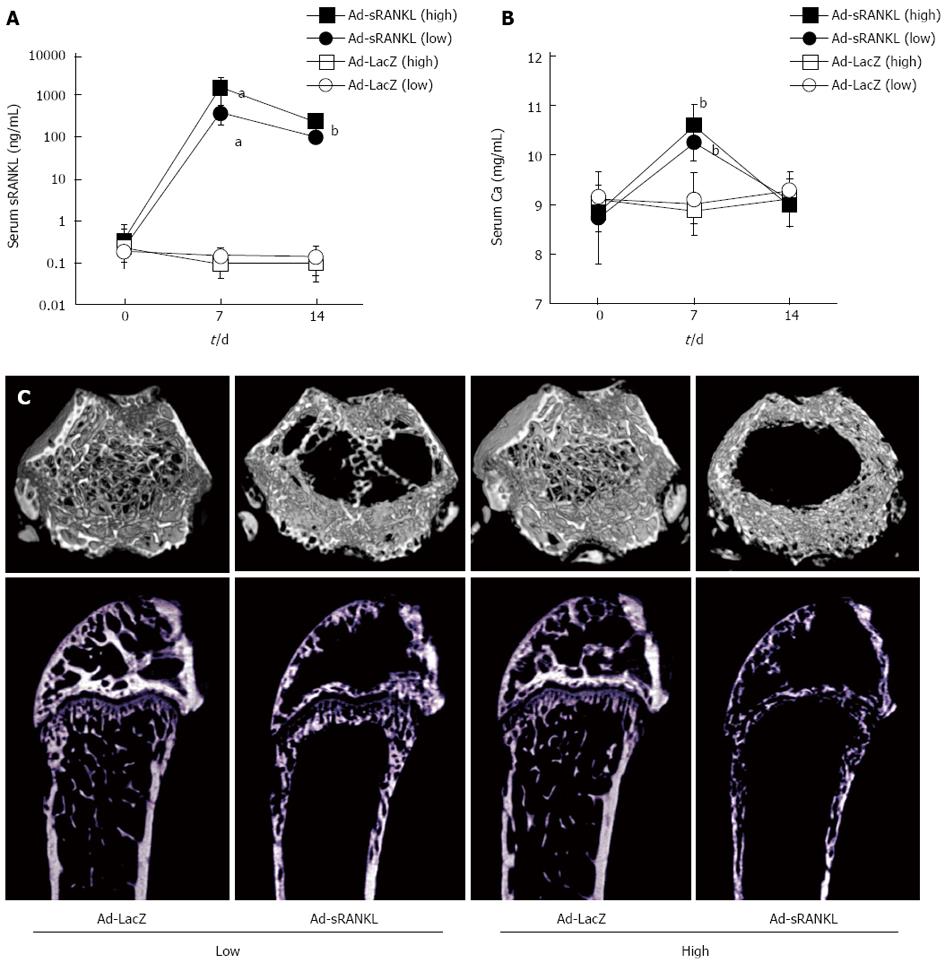
In a previous study, we generated sRANKL-TG mice[24]. The sRANKL-TG mice exhibited severe osteoporosis accompanied with enhanced osteoclastogenesis, but no hypercalcemia. To analyze the relationship between the serum concentration of sRANKL and hypercalcemia and generate a simple and quick hypercalcemia model, Ad-sRANKL was injected intraperitoneally into male C57BL/6 mice[45]. Table 2 summarizes the results of serum sRANKL and calcium levels. Serum sRANKL increased markedly on day 7, while serum calcium increased with a peak on day 7 and returned to the baseline level on day 14. Food intake and body weight significantly declined on day 7. Taken together, the mice appeared to have anorexia as a symptom of hypercalcemia.
| Mouse model | sRANKL (ng/mL) | Phenotype |
| Ad-sRANKL injection (High) | 1500 | Severe osteoporosis/hypercalcemia |
| Ad-sRANKL injection (Low) | 233 | Severe osteoporosis/hypercalcemia |
| sRANKL-Tg mice | 30 | Severe osteoporosis |
| GST-RANKL injection | 3.51 | Osteoporosis |
| Wild mice | 0.1 | Normal |
In addition, increases in markers for bone resorption (TRAP-5b) and formation (ALP, alkaline phosphatase) with a marked decrease in BMD measured by dual-energy X-ray absorptiometry were observed on day 14. The severe bone loss was confirmed by microCT (Figure 3). These results reflect accelerated bone formation following activation of osteoclasts, indicating coupling between bone formation and resorption.
Serum sRANKL level in the Ad-sRANKL group on day 7 was 15000 times higher than those in wild type mice. In sRANKL-TG mice, serum sRANKL was within normal range around 30 ng/mL[24]. Serum sRANKL level in the Ad-sRANKL group was about 50 times higher than that in the transgenic mice, which do not exhibit hypercalcemia even though they have severe osteoporosis and enhanced osteoclastogenesis. These observations suggest that 30 ng/mL sRANKL in serum is insufficient to induce hypercalcemia and that severe osteoporosis with enhanced osteoclastogenesis does not always accompany hypercalcemia. There may be a threshold sRANKL concentration for induction of hypercalcemia with anorexia.
Several experimental animal models of hypercalcemia have been described: a model with vitamin D treatment, a tumor transplant model and an infusion model using PTHrP[46-49]. These models have not shown a clear relationship between body weight loss and anorexia. Sato et al[49] showed that the recovery from hypercalcemia is accompanied by an improvement in body weight using a model of HHM, but the association of body weight loss with a decrease in food intake was not clearly shown in this model.
In summary, we established a novel model of hypercalcemia in normal mice injected intraperitoneally with Ad-sRANKL[45]. Overexpression of sRANKL activated osteoclasts to resorb bone, resulting in an increase in serum calcium. Hypercalcemic mice exhibited typical symptoms such as anorexia and weakness. The Ad-sRANKL-injected hypercalcemia model is the first one in which overexpressed sRANKL directly activates osteoclasts to increase serum calcium level. This simple and rapid model mimics HHM in terms of exhibiting anorexia and weakness and could be useful for investigating coupling between bone formation and resorption in high-turnover bone diseases, as well as for examining hypercalcemia with anorexia[45].
A fully human anti-RANKL monoclonal antibody (denosumab) is clinically used for the treatment of osteoporosis and cancer-related bone disorders[50-54]. It is a strong inhibitor of RANKL and is very stable in the blood stream for several months after single subcutaneous injection. Since denosumab does not cross react with rodent RANKL, its evaluation in vivo can be done only with non-human primates[55,56] or human RANKL-knock-in mice (HuRANKL mice)[57]. After replacing the exon 5 in mouse RANKL with that in human RANKL, denosumab can bind to and neutralize the chimeric mouse e/human RANKL in the HuRANKL mice. Cynomolgus monkeys have been used for the preclinical animal experiments of denosumab[55,56]. To investigate the effect of RANKL inhibition in normal mice, we prepared anti-mouse RANKL neutralizing monoclonal antibody (OYC1) and established a novel mouse model of osteopetrosis by administration of the anti-mouse RANKL antibody to normal mice[58].
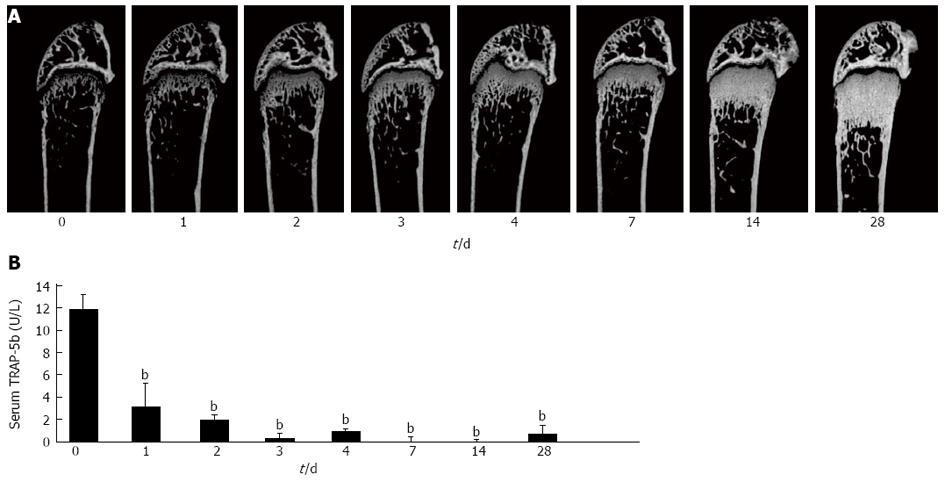
Single subcutaneous injection of the antibody markedly increased bone mass in a time-dependent manner for 4 wk (Figure 4A). Histomorphometry showed remarkable decreases in osteoclast surface and number, as well as decreases in osteoblast surface, mineral apposition rate, and bone formation rate after 2 wk. These results are consistent with the previous report on HuRANKL mice treated with denosumab[57], which showed a negative coupling between bone resorption and formation. Decreases in bone resorption marker (TRAP-5b) and formation marker (ALP) were also observed in anti-RANKL-antibody-treated mice. There was almost no serum TRAP-5b activity for 4 wk, and anti-RANKL antibody was detected in serum of the treated mice even 4 wk after the injection (Figure 4B).
Histological and microCT analyses showed that the anti-RANKL antibody-treated mice exhibit an osteopetrotic phenotype, similar to the observation in OPG-treated mice[1,3]. The osteopetrotic phenotype was evident 2 d after a single injection in normal mice. The effect of a single injection (5 mg/kg body weight) of anti-RANKL antibody on bone mass is roughly equivalent to that of three daily injections (24 mg/kg body weight) of OPG, indicating that the efficacy and stability of anti-RANKL antibody in vivo was much higher than those of OPG[3,58].
Osteopetrosis is generally caused by failure of osteoclast-mediated resorption of skeleton. There are numerous mouse models of osteopetrosis without osteoclasts, including c-fos-deficient mice[59], op/op mice[60], RANKL-deficient mice[21] and RANK-deficient mice[22,23]. The anti-RANKL antibody-treated mouse is an inducible osteopetrosis model. It is possible to investigate the difference between BPs and anti-RANKL antibody in normal mice. It is also possible to test the effects of switching pharmaceuticals, e.g., BP to denosumab and PTH to denosumab, and to test the effects of combinations of pharmaceuticals, e.g., PTH and denosumab. We have demonstrated that the combination of a denosumab-like anti-mouse RANKL monoclonal antibody and PTH synergistically increases bone mass in normal mice. We also showed that PTH increases bone formation in osteoclast-deficient mice treated with the anti-RANKL antibody, suggesting that PTH requires no osteoclasts for its bone anabolic activity. The anti-mouse RANKL neutralizing antibody (OYC1) is a surrogate antibody for denosumab and is useful for investigating unidentified functions of RANKL in mice[58]. In fact, several important functions of RANKL were identified in other tissues in addition to bones. They include fever control in the brain[61], proliferation of mammary gland epithelial cells[62] and stem cells[63,64], proliferation of mammary cancer cells[65,66], hematopoiesis in bone marrow[67], development of epithelial cells in the thymic medulla[68], lymphogenesis[69], proliferation of regulatory T cells via activation of dendritic cells[70], and development of Microfold cells in intestinal epithelium[71].
These inducible models of osteoporosis and osteopetrosis using normal mice exhibit exactly mirror images in terms of the change in bone mass and are useful to advance research on osteoclast biology as well as bone metabolism in vivo.
The discovery of the OPG/RANKL/RANK system guided us to the mechanisms of osteoclast differentiation and activation[8-11]. Inhibition of the RANKL/RANK signal in bone can increase bone mass and is useful for treatment of osteoporosis. OPG and soluble RANK have been developed as pharmaceutical candidates, and anti-human RANKL neutralizing antibody (denosumab) has been clinically used for osteoporosis and cancer-related bone disorders[50-54]. The past decade has witnessed significant progress in the development of the anti-human RANKL neutralizing antibody as a pharmaceutical agent. This is an outstanding story starting from the discovery of RANKL and advancing to the clinical application of anti-RANKL antibody[72].
At present denosumab is clinically used for the treatment of osteoporosis and cancer-related bone diseases in Japan, Europe, United States and many other countries. A phase II clinical trial for rheumatoid arthritis is ongoing in Japan. The future treatment option of rheumatoid arthritis thus looks promising.
I thank all collaborators, especially Yoshiya Tomimori, Tetsuro Enomoto, and Yuriko Furuya, for their help in the preparation of the manuscript.
P- Reviewers Canavese F, Lykissas MG, Stefan J S- Editor Zhai HH L- Editor A E- Editor Liu XM
| 1. | Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3723] [Cited by in RCA: 3566] [Article Influence: 127.4] [Reference Citation Analysis (0)] |
| 2. | Tsuda E, Goto M, Mochizuki S, Yano K, Kobayashi F, Morinaga T, Higashio K. Isolation of a novel cytokine from human fibroblasts that specifically inhibits osteoclastogenesis. Biochem Biophys Res Commun. 1997;234:137-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 569] [Cited by in RCA: 514] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 3. | Yasuda H, Shima N, Nakagawa N, Mochizuki SI, Yano K, Fujise N, Sato Y, Goto M, Yamaguchi K, Kuriyama M. Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): a mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology. 1998;139:1329-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 214] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 4. | Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 1998;95:3597-3602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3136] [Cited by in RCA: 3052] [Article Influence: 113.0] [Reference Citation Analysis (0)] |
| 5. | Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165-176. [PubMed] |
| 6. | Nakagawa N, Kinosaki M, Yamaguchi K, Shima N, Yasuda H, Yano K, Morinaga T, Higashio K. RANK is the essential signaling receptor for osteoclast differentiation factor in osteoclastogenesis. Biochem Biophys Res Commun. 1998;253:395-400. [PubMed] |
| 7. | Hsu H, Lacey DL, Dunstan CR, Solovyev I, Colombero A, Timms E, Tan HL, Elliott G, Kelley MJ, Sarosi I. Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc Natl Acad Sci USA. 1999;96:3540-3545. [PubMed] |
| 8. | Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Goto M, Mochizuki SI, Tsuda E, Morinaga T, Udagawa N. A novel molecular mechanism modulating osteoclast differentiation and function. Bone. 1999;25:109-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 197] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 9. | Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev. 1999;20:345-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 433] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 10. | Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5206] [Cited by in RCA: 4831] [Article Influence: 219.6] [Reference Citation Analysis (0)] |
| 11. | Yasuda H, Higashio K, Suda T. Vitamin D and osteoclastigenesis. Vitamin D. 2nd ed. New York: Elsevier/Academic Press 2005; 665–685. |
| 12. | Takahashi N, Yamana H, Yoshiki S, Roodman GD, Mundy GR, Jones SJ, Boyde A, Suda T. Osteoclast-like cell formation and its regulation by osteotropic hormones in mouse bone marrow cultures. Endocrinology. 1988;122:1373-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 532] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 13. | Udagawa N, Takahashi N, Akatsu T, Sasaki T, Yamaguchi A, Kodama H, Martin TJ, Suda T. The bone marrow-derived stromal cell lines MC3T3-G2/PA6 and ST2 support osteoclast-like cell differentiation in cocultures with mouse spleen cells. Endocrinology. 1989;125:1805-1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 365] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 14. | Higashio K, Shima N, Goto M, Itagaki Y, Nagao M, Yasuda H, Morinaga T. Identity of a tumor cytotoxic factor from human fibroblasts and hepatocyte growth factor. Biochem Biophys Res Commun. 1990;170:397-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 146] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Suda T, Takahashi N, Martin TJ. Modulation of osteoclast differentiation. Endocr Rev. 1992;13:66-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 280] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 16. | Wong BR, Rho J, Arron J, Robinson E, Orlinick J, Chao M, Kalachikov S, Cayani E, Bartlett FS, Frankel WN. TRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-Jun N-terminal kinase in T cells. J Biol Chem. 1997;272:25190-25194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 801] [Cited by in RCA: 777] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 17. | Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, Teepe MC, DuBose RF, Cosman D, Galibert L. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390:175-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1712] [Cited by in RCA: 1647] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 18. | Proposed standard nomenclature for new tumor necrosis factor family members involved in the regulation of bone resorption. The American Society for Bone and Mineral Research President's Committee on Nomenclature. J Bone Miner Res. 2000;15:2293-2296. [PubMed] |
| 19. | Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL. osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260-1268. [PubMed] |
| 20. | Mizuno A, Amizuka N, Irie K, Murakami A, Fujise N, Kanno T, Sato Y, Nakagawa N, Yasuda H, Mochizuki S. Severe osteoporosis in mice lacking osteoclastogenesis inhibitory factor/osteoprotegerin. Biochem Biophys Res Commun. 1998;247:610-615. [PubMed] |
| 21. | Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2595] [Cited by in RCA: 2512] [Article Influence: 96.6] [Reference Citation Analysis (0)] |
| 22. | Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, Daro E, Smith J, Tometsko ME, Maliszewski CR. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999;13:2412-2424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1092] [Cited by in RCA: 1081] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 23. | Li J, Sarosi I, Yan XQ, Morony S, Capparelli C, Tan HL, McCabe S, Elliott R, Scully S, Van G. RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proc Natl Acad Sci USA. 2000;97:1566-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 830] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 24. | Mizuno A, Kanno T, Hoshi M, Shibata O, Yano K, Fujise N, Kinosaki M, Yamaguchi K, Tsuda E, Murakami A. Transgenic mice overexpressing soluble osteoclast differentiation factor (sODF) exhibit severe osteoporosis. J Bone Miner Metab. 2002;20:337-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 93] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Kalu DN, Liu CC, Hardin RR, Hollis BW. The aged rat model of ovarian hormone deficiency bone loss. Endocrinology. 1989;124:7-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 255] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 26. | Anderson JJ, Garner SC, Mar MH, Boass A, Toverud SU, Parikh I. The ovariectomized, lactating rat as an experimental model for osteopenia: calcium metabolism and bone changes. Bone Miner. 1990;11:43-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Sessions ND, Halloran BP, Bikle DD, Wronski TJ, Cone CM, Morey-Holton E. Bone response to normal weight bearing after a period of skeletal unloading. Am J Physiol. 1989;257:E606-E610. [PubMed] |
| 28. | Dehority W, Halloran BP, Bikle DD, Curren T, Kostenuik PJ, Wronski TJ, Shen Y, Rabkin B, Bouraoui A, Morey-Holton E. Bone and hormonal changes induced by skeletal unloading in the mature male rat. Am J Physiol. 1999;276:E62-E69. [PubMed] |
| 29. | Wakley GK, Baum BL, Hannon KS, Turner RT. The effects of tamoxifen on the osteopenia induced by sciatic neurotomy in the rat: a histomorphometric study. Calcif Tissue Int. 1988;43:383-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Broulik PD. Tamoxifen prevents bone loss in ovariectomized mice. Endocr Regul. 1991;25:217-219. [PubMed] |
| 31. | Turner RT, Vandersteenhoven JJ, Bell NH. The effects of ovariectomy and 17 beta-estradiol on cortical bone histomorphometry in growing rats. J Bone Miner Res. 1987;2:115-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 231] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 32. | Shinohara M, Koga T, Okamoto K, Sakaguchi S, Arai K, Yasuda H, Takai T, Kodama T, Morio T, Geha RS. Tyrosine kinases Btk and Tec regulate osteoclast differentiation by linking RANK and ITAM signals. Cell. 2008;132:794-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 247] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 33. | Tomimori Y, Mori K, Koide M, Nakamichi Y, Ninomiya T, Udagawa N, Yasuda H. Evaluation of pharmaceuticals with a novel 50-hour animal model of bone loss. J Bone Miner Res. 2009;24:1194-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 34. | Bahtiar A, Matsumoto T, Nakamura T, Akiyama M, Yogo K, Ishida-Kitagawa N, Ogawa T, Takeya T. Identification of a novel L-serine analog that suppresses osteoclastogenesis in vitro and bone turnover in vivo. J Biol Chem. 2009;284:34157-34166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 35. | Yuan YY, Kostenuik PJ, Ominsky MS, Morony S, Adamu S, Simionescu DT, Basalyga DM, Asuncion FJ, Bateman TA. Skeletal deterioration induced by RANKL infusion: a model for high-turnover bone disease. Osteoporos Int. 2008;19:625-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | Lloyd SA, Yuan YY, Kostenuik PJ, Ominsky MS, Lau AG, Morony S, Stolina M, Asuncion FJ, Bateman TA. Soluble RANKL induces high bone turnover and decreases bone volume, density, and strength in mice. Calcif Tissue Int. 2008;82:361-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 37. | Soysa NS, Alles N, Weih D, Lovas A, Mian AH, Shimokawa H, Yasuda H, Weih F, Jimi E, Ohya K. The pivotal role of the alternative NF-kappaB pathway in maintenance of basal bone homeostasis and osteoclastogenesis. J Bone Miner Res. 2010;25:809-818. [PubMed] |
| 38. | Kinugawa S, Koide M, Kobayashi Y, Mizoguchi T, Ninomiya T, Muto A, Kawahara I, Nakamura M, Yasuda H, Takahashi N. Tetracyclines convert the osteoclastic-differentiation pathway of progenitor cells to produce dendritic cell-like cells. J Immunol. 2012;188:1772-1781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 39. | Grill V, Ho P, Body JJ, Johanson N, Lee SC, Kukreja SC, Moseley JM, Martin TJ. Parathyroid hormone-related protein: elevated levels in both humoral hypercalcemia of malignancy and hypercalcemia complicating metastatic breast cancer. J Clin Endocrinol Metab. 1991;73:1309-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 174] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 40. | Martin TJ, Suva LJ. Parathyroid hormone-related protein in hypercalcaemia of malignancy. Clin Endocrinol (Oxf). 1989;31:631-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 41. | Thomas RJ, Guise TA, Yin JJ, Elliott J, Horwood NJ, Martin TJ, Gillespie MT. Breast cancer cells interact with osteoblasts to support osteoclast formation. Endocrinology. 1999;140:4451-4458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 84] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 42. | Roux S, Mariette X. The high rate of bone resorption in multiple myeloma is due to RANK (receptor activator of nuclear factor-kappaB) and RANK Ligand expression. Leuk Lymphoma. 2004;45:1111-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 43. | Shepard MM, Smith JW. Hypercalcemia. Am J Med Sci. 2007;334:381-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Pecherstorfer M, Brenner K, Zojer N. Current management strategies for hypercalcemia. Treat Endocrinol. 2003;2:273-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 45. | Enomoto T, Furuya Y, Tomimori Y, Mori K, Miyazaki J, Yasuda H. Establishment of a new murine model of hypercalcemia with anorexia by overexpression of soluble receptor activator of NF-κB ligand using an adenovirus vector. J Bone Miner Metab. 2011;29:414-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 46. | Rizzoli R, Caverzasio J, Chapuy MC, Martin TJ, Bonjour JP. Role of bone and kidney in parathyroid hormone-related peptide-induced hypercalcemia in rats. J Bone Miner Res. 1989;4:759-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 32] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 47. | Strassmann G, Jacob CO, Fong M, Bertolini DR. Mechanisms of paraneoplastic syndromes of colon-26: involvement of interleukin 6 in hypercalcemia. Cytokine. 1993;5:463-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 48. | Takahashi K, Shirahata A, Fukushima S, Kokubo S, Teramura K, Usuda S. Effects of YM175, a new-generation bisphosphonate, on hypercalcemia induced by tumor-derived bone resorbing factors in rats. Jpn J Pharmacol. 1998;76:155-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 49. | Sato K, Onuma E, Yocum RC, Ogata E. Treatment of malignancy-associated hypercalcemia and cachexia with humanized anti-parathyroid hormone-related protein antibody. Semin Oncol. 2003;30:167-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 50. | Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2264] [Cited by in RCA: 2278] [Article Influence: 142.4] [Reference Citation Analysis (0)] |
| 51. | Smith MR, Egerdie B, Hernández Toriz N, Feldman R, Tammela TL, Saad F, Heracek J, Szwedowski M, Ke C, Kupic A. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;361:745-755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 865] [Cited by in RCA: 786] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 52. | Stopeck AT, Lipton A, Body JJ, Steger GG, Tonkin K, de Boer RH, Lichinitser M, Fujiwara Y, Yardley DA, Viniegra M. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28:5132-5139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1096] [Cited by in RCA: 1070] [Article Influence: 71.3] [Reference Citation Analysis (0)] |
| 53. | Fizazi K, Carducci M, Smith M, Damião R, Brown J, Karsh L, Milecki P, Shore N, Rader M, Wang H. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377:813-822. [PubMed] |
| 54. | Henry DH, Costa L, Goldwasser F, Hirsh V, Hungria V, Prausova J, Scagliotti GV, Sleeboom H, Spencer A, Vadhan-Raj S. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol. 2011;29:1125-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 846] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 55. | Kostenuik PJ, Smith SY, Jolette J, Schroeder J, Pyrah I, Ominsky MS. Decreased bone remodeling and porosity are associated with improved bone strength in ovariectomized cynomolgus monkeys treated with denosumab, a fully human RANKL antibody. Bone. 2011;49:151-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 56. | Ominsky MS, Stouch B, Schroeder J, Pyrah I, Stolina M, Smith SY, Kostenuik PJ. Denosumab, a fully human RANKL antibody, reduced bone turnover markers and increased trabecular and cortical bone mass, density, and strength in ovariectomized cynomolgus monkeys. Bone. 2011;49:162-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 57. | Kostenuik PJ, Nguyen HQ, McCabe J, Warmington KS, Kurahara C, Sun N, Chen C, Li L, Cattley RC, Van G. Denosumab, a fully human monoclonal antibody to RANKL, inhibits bone resorption and increases BMD in knock-in mice that express chimeric (murine/human) RANKL. J Bone Miner Res. 2009;24:182-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 320] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 58. | Furuya Y, Mori K, Ninomiya T, Tomimori Y, Tanaka S, Takahashi N, Udagawa N, Uchida K, Yasuda H. Increased bone mass in mice after single injection of anti-receptor activator of nuclear factor-kappaB ligand-neutralizing antibody: evidence for bone anabolic effect of parathyroid hormone in mice with few osteoclasts. J Biol Chem. 2011;286:37023-37031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 59. | Wang ZQ, Ovitt C, Grigoriadis AE, Möhle-Steinlein U, Rüther U, Wagner EF. Bone and haematopoietic defects in mice lacking c-fos. Nature. 1992;360:741-745. [PubMed] |
| 60. | Yoshida H, Hayashi S, Kunisada T, Ogawa M, Nishikawa S, Okamura H, Sudo T, Shultz LD, Nishikawa S. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990;345:442-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1320] [Cited by in RCA: 1228] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 61. | Hanada R, Leibbrandt A, Hanada T, Kitaoka S, Furuyashiki T, Fujihara H, Trichereau J, Paolino M, Qadri F, Plehm R. Central control of fever and female body temperature by RANKL/RANK. Nature. 2009;462:505-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 195] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 62. | McGowan B, Harrold DR. Epididymitis in rams: studies on vaccine efficacy. Cornell Vet. 1979;69:73-76. [PubMed] |
| 63. | Asselin-Labat ML, Vaillant F, Sheridan JM, Pal B, Wu D, Simpson ER, Yasuda H, Smyth GK, Martin TJ, Lindeman GJ. Control of mammary stem cell function by steroid hormone signalling. Nature. 2010;465:798-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 541] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 64. | Joshi PA, Jackson HW, Beristain AG, Di Grappa MA, Mote PA, Clarke CL, Stingl J, Waterhouse PD, Khokha R. Progesterone induces adult mammary stem cell expansion. Nature. 2010;465:803-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 580] [Cited by in RCA: 541] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 65. | Schramek D, Leibbrandt A, Sigl V, Kenner L, Pospisilik JA, Lee HJ, Hanada R, Joshi PA, Aliprantis A, Glimcher L. Osteoclast differentiation factor RANKL controls development of progestin-driven mammary cancer. Nature. 2010;468:98-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 479] [Cited by in RCA: 458] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 66. | Gonzalez-Suarez E, Jacob AP, Jones J, Miller R, Roudier-Meyer MP, Erwert R, Pinkas J, Branstetter D, Dougall WC. RANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature. 2010;468:103-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 461] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 67. | Miyamoto K, Yoshida S, Kawasumi M, Hashimoto K, Kimura T, Sato Y, Kobayashi T, Miyauchi Y, Hoshi H, Iwasaki R. Osteoclasts are dispensable for hematopoietic stem cell maintenance and mobilization. J Exp Med. 2011;208:2175-2181. [PubMed] |
| 68. | Akiyama T, Shimo Y, Yanai H, Qin J, Ohshima D, Maruyama Y, Asaumi Y, Kitazawa J, Takayanagi H, Penninger JM. The tumor necrosis factor family receptors RANK and CD40 cooperatively establish the thymic medullary microenvironment and self-tolerance. Immunity. 2008;29:423-437. [PubMed] |
| 69. | Mebius RE. Organogenesis of lymphoid tissues. Nat Rev Immunol. 2003;3:292-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 553] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 70. | Loser K, Mehling A, Loeser S, Apelt J, Kuhn A, Grabbe S, Schwarz T, Penninger JM, Beissert S. Epidermal RANKL controls regulatory T-cell numbers via activation of dendritic cells. Nat Med. 2006;12:1372-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 309] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 71. | Knoop KA, Kumar N, Butler BR, Sakthivel SK, Taylor RT, Nochi T, Akiba H, Yagita H, Kiyono H, Williams IR. RANKL is necessary and sufficient to initiate development of antigen-sampling M cells in the intestinal epithelium. J Immunol. 2009;183:5738-5747. [PubMed] |
| 72. | Lacey DL, Boyle WJ, Simonet WS, Kostenuik PJ, Dougall WC, Sullivan JK, San Martin J, Dansey R. Bench to bedside: elucidation of the OPG-RANK-RANKL pathway and the development of denosumab. Nat Rev Drug Discov. 2012;11:401-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 487] [Article Influence: 37.5] [Reference Citation Analysis (0)] |