INTRODUCTION
Fracture nonunion is a common complication encountered during fracture repair and bone defect treatment[1]. Currently, there is no universally agreed-upon definition for bone nonunion. The United States Food and Drug Administration defines fracture nonunion as incomplete healing of a fracture within 9 months after its occurrence, accompanied by the absence of progressive healing signs on X-rays for three consecutive months[2]. Nonunion of bone is most commonly observed in clinical practice in long bones such as forearm, humerus, tibia, clavicle, and femur[3,4]. Fracture nonunion can result in long-term pain, functional impairment, and psychological distress, significantly affecting patients’ lives[5].
Types of nonunion fractures include hypertrophic, oligotrophic, and atrophic forms[6]. The treatment approach typically depends on the specific type of nonunion and its underlying causes. Conservative treatments, such as physical therapy and ultrasound therapy, can be employed to stimulate bone healing[7]. However, in cases of complex or persistent nonunion, surgical intervention becomes necessary[8]. Surgical options include internal fixation to stabilize the fracture and bone grafting to fill the gap at the nonunion site[9-11]. Nevertheless, surgery has its own set of drawbacks, including infection, delayed union, and complications related to anesthesia and donor site morbidity[12-14]. Furthermore, many patients with nonunion also suffer from metabolic bone diseases such as osteoporosis, which make them unsuitable candidates for autograft procedures[15]. Patient reluctance towards surgical procedures is also a significant factor, as many individuals prefer to avoid the associated risks and choose alternative treatments. This emphasizes the necessity for effective non-surgical therapies that can address bone nonunion and yield satisfactory outcomes.
Teriparatide, also known as recombinant human parathyroid hormone (PTH) 1-34, is a synthetic form of PTH approved for the treatment of osteoporosis[16,17]. It acts as a synthetic anabolic agent, enhancing callus formation, increasing bone mineral density, and accelerating the healing process by stimulating osteoblast activity and promoting bone remodeling[18]. In recent years, numerous animal models and clinical studies have explored the efficacy of teriparatide in promoting fracture healing and addressing nonunion[19-21]. To date, no previous cases have documented the use of teriparatide for the treatment of a fracture nonunion lasting as long as four years. After such an extended period of nonunion, the patient was successfully treated with teriparatide, resulting in complete healing.
CASE PRESENTATION
Chief complaints
A four-year nonunion following open reduction and internal fixation of a right humeral fracture caused by a car accident.
History of present illness
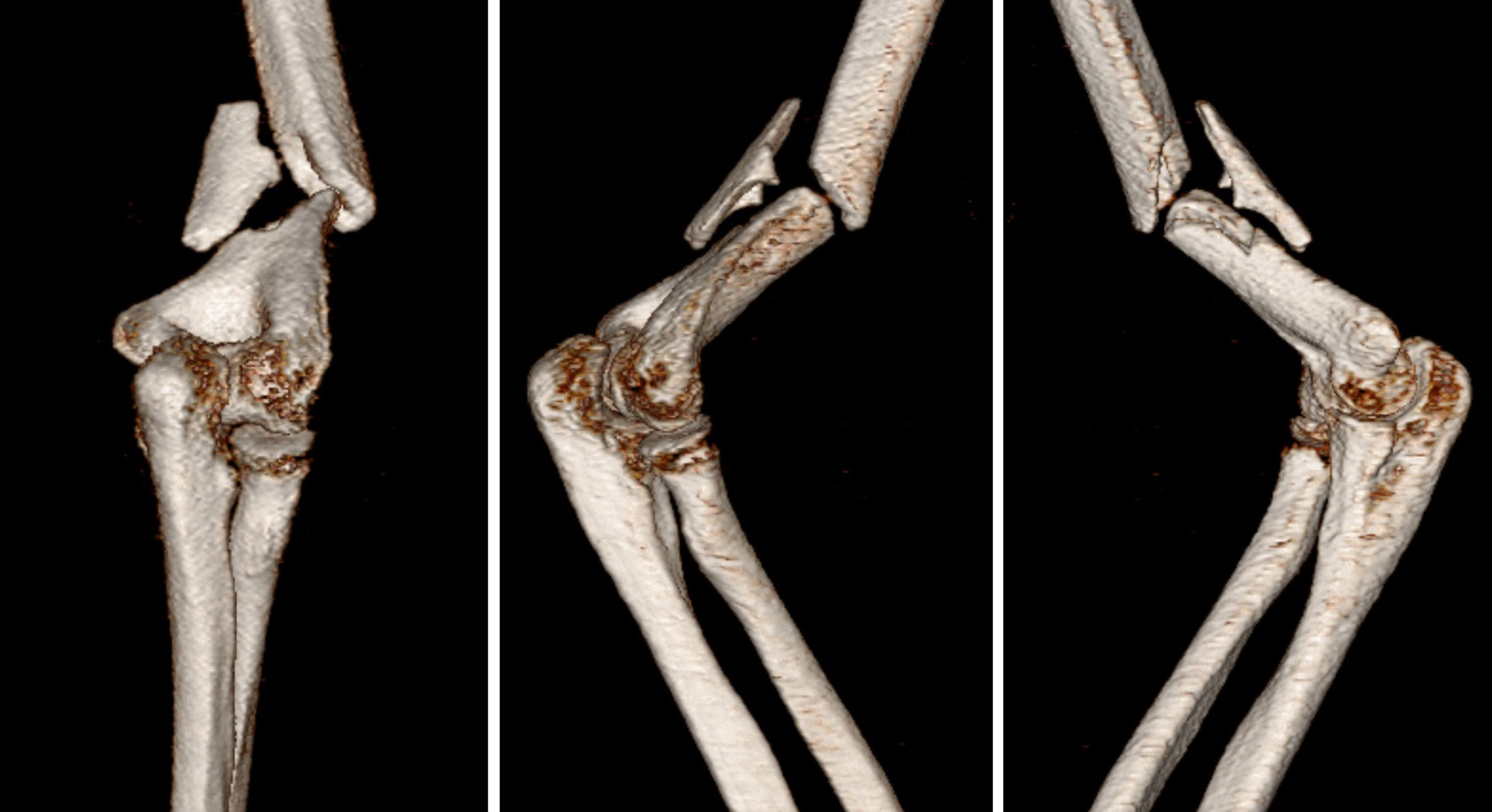
Four years ago, the patient sustained a right humerus fracture in a traffic accident. A routine computed tomography (CT) scan of the right elbow joint, along with three-dimensional reconstruction, were performed, revealing a right humerus fracture classified as 12B2.3 according to the AO classification system (Figure 1). The patient underwent an open reduction surgical procedure, followed by stabilization of the fracture using plate-screw fixation. At the 4-month follow-up after surgery, X-ray imaging revealed a delayed union of the fracture, along with a broken screw at the site of the plate (Figure 2A and B). Due to the absence of significant pain or discomfort and acceptable upper limb function, the patient chose a conservative approach, which included rehabilitation therapy, calcium supplementation, and vitamin D intake, rather than undergoing another surgical procedure. At the 9-month postoperative mark, X-ray imaging revealed poor healing with an absence of callus formation, leading to a diagnosis of atrophic nonunion (Figure 2C and D). Despite this, the patient continued to be reluctant about undergoing another surgical procedure. At the 4-year mark, the fracture still exhibited unsatisfactory healing, but the patient experienced only mild pain without significant discomfort.
Figure 1 Preoperative three-dimensional computed tomography imaging conducted upon hospital admission.
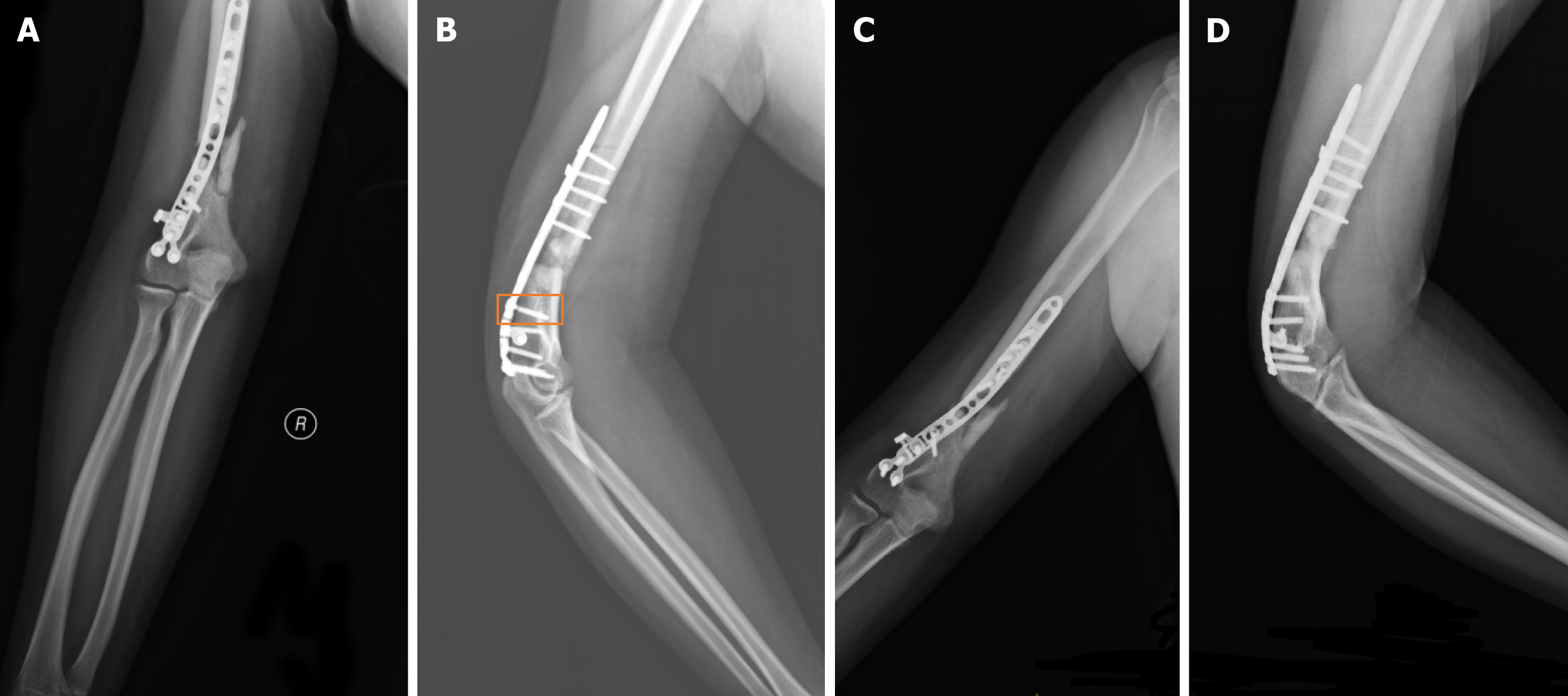
Figure 2 X-ray images.
A: X-ray images taken four months after surgery anterior-posterior view; B: X-ray images taken four months after surgery lateral view. The orange box highlights the location of the broken screw; C: X-ray images taken nine months after surgery anterior-posterior view; D: X-ray images taken nine months after surgery lateral view.
History of past illness
The patient had no history of chronic diseases, significant prior injuries, or surgeries, and was otherwise healthy.
Personal and family history
The patient reported no family history of genetic disorders, significant medical conditions, or similar fractures, and no history of major diseases.
Physical examination upon admission
The patient's right upper limb exhibited no significant tenderness or swelling. Its functionality was intact, and the range of motion in the elbow joint was normal.
Laboratory examinations
Nothing abnormal was detected.
Imaging examinations
Radiographic imaging performed 9 months postoperatively showed no signs of healing at the fracture sites (Figure 2C and D).
FINAL DIAGNOSIS
The final diagnosis of the presented case was right humeral atrophic nonunion.
TREATMENT
Four years after undergoing open reduction and internal fixation for a right humeral fracture, the patient agreed to try teriparatide treatment after being informed of its potential benefits and risks. After signing the informed consent, the patient began daily subcutaneous injections of teriparatide (Xinfutai®, Shenzhen Salubris Pharmaceuticals Co., Ltd.) at a dosage of 20 μg per day. After 3 months of teriparatide treatment initiation CT scans revealed initial stages of callus formation at the fracture site, along with partial closure of the fracture gap. Given this positive response, the treatment was extended for another 3 months, resulting in a total treatment duration of 6 months. Teriparatide was discontinued at the 7-month mark, but the patient continued to show progressive bone formation. Laboratory tests were conducted, including a complete blood count, liver and kidney function tests, glucose and electrolyte levels, markers of bone metabolism (b-C-terminal telopeptide of type I collagen, procollagen type 1 N-terminal propeptide, and osteocalcin), erythrocyte sedimentation rate, C-reactive protein, and tumor markers [alpha-fetoprotein, carcinoembryonic antigen (CA) 199, CA 125, total prostate-specific antigen, and ferritin]. All results were within normal limits.
OUTCOME AND FOLLOW-UP
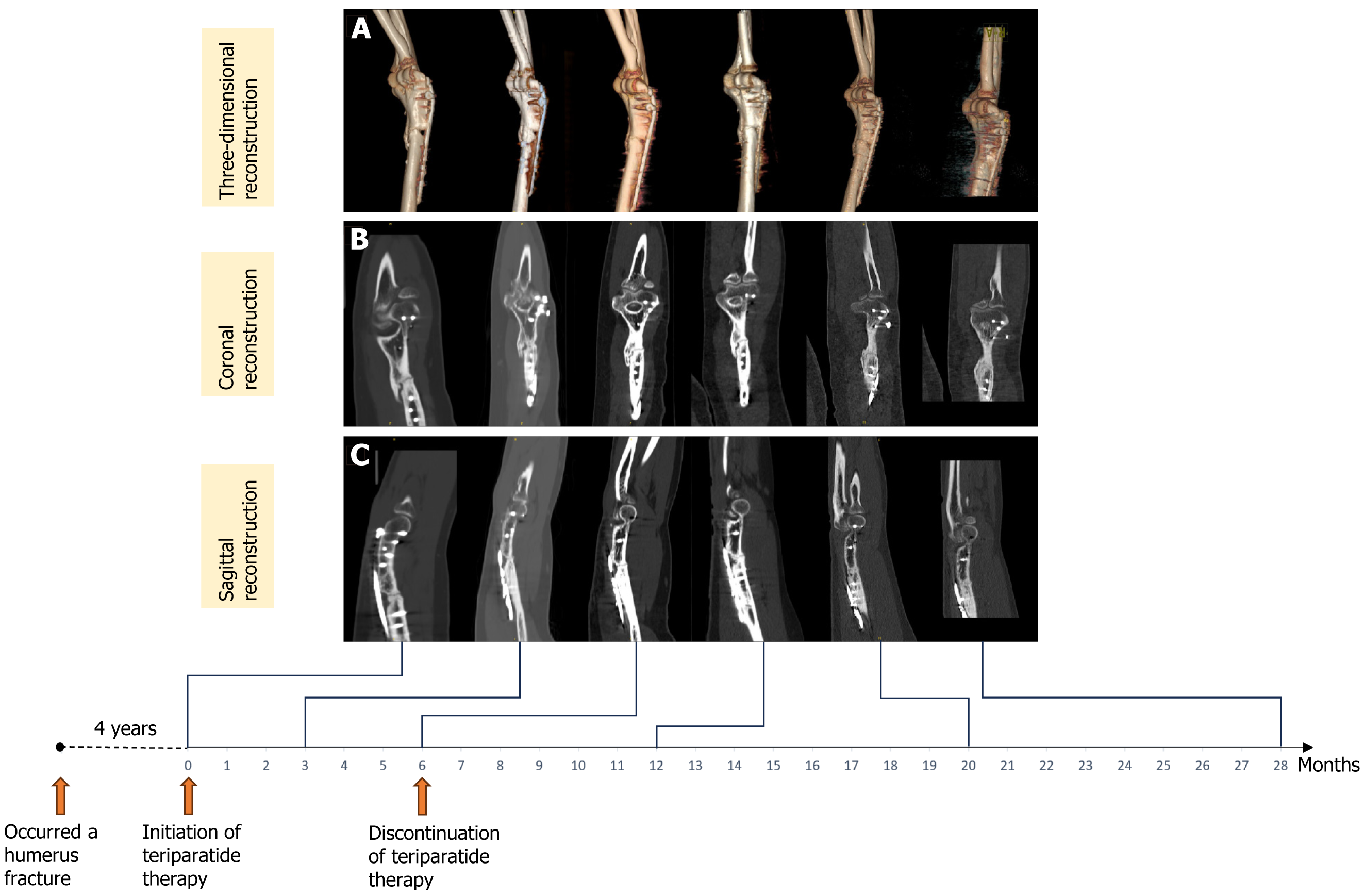
At the 28-month follow-up, the CT scan and three-dimensional reconstruction of the right elbow joint demonstrated complete fusion around the plate and bone graft, with adequate formation of callus at the site of nonunion (Figure 3). The prolonged humerus fracture nonunion had fully healed, the patient's mild pain had resolved, and full functionality of the upper limb was restored. The patient did not report any medication-related adverse effects. Dual-energy X-ray absorptiometry performed using a Hologic dual-energy X-ray bone density instrument (Discovery Wi, Hologic Inc., United States) indicated normal bone mineral density.
Figure 3 Computed tomography scan images showing the progression of fracture healing before and after teriparatide treatment.
A: Three-dimensional reconstruction; B: Coronal two-dimensional reconstruction; C: Sagittal two-dimensional reconstruction. From left to right: Prior to teriparatide treatment (four years after the initial fracture surgery), 3 months, 6 months, 12 months, 20 months and 28 months post-teriparatide treatment initiation. Note: The teriparatide treatment duration was 6 months.
DISCUSSION
We present a case of nonunion in a patient following open reduction and internal fixation of a humerus fracture. After 4 years of follow-up, during which symptoms persisted and radiological union did not progress, we initiated systemic treatment with teriparatide for the patient over a period of 6 months. At the completion of the treatment, the fracture exhibited stable healing, and the pain completely resolved. The prolonged duration of nonunion posed a unique challenge for orthopedic surgeons in the conservative treatment of this patient. However, long-term follow-up results demonstrated satisfactory outcomes with teriparatide therapy. Our case report suggests that teriparatide may be a valuable therapeutic option for treating established prolonged humerus fracture nonunion.
Fracture healing involves several stages: (1) Hematoma and inflammation; (2) Formation of granulation tissue; (3) Membranous and endochondral ossification; and (4) Remodeling[22,23]. At each stage, interference can impair or interrupt bone healing. Insufficient stability at the fracture site, lack of blood supply, infection, as well as conditions such as diabetes, smoking, malnutrition, and certain medications (such as corticosteroids), can all have a negative impact on the body's ability to heal fractures[24]. When there is decreased bone formation activity at the fracture site resulting in little or no callus formation, it is referred to as atrophic nonunion[25]. The surgical principles include curing infection, performing debridement if necessary, correcting deformity, providing stability, and adding biological stimulus[26]. In recent years, biological stimulation primarily involves autogenous bone grafting and allograft bone grafting. Autogenous bone grafting is currently considered the gold standard for treating atrophic nonunion. However, drawbacks of autografts include donor site morbidity, limited availability, and the risk of infection[27]. Allografts eliminate donor site morbidity but may induce immunological sensitization[28].
For over a decade, PTH has been used for fracture prevention in osteoporosis. A wealth of fundamental research has shown the beneficial effects of PTH in promoting bone formation in addressing bone defects and persistent nonunions[29,30]. In addition, several studies on animals have also verified the effectiveness of teriparatide in the treatment of nonunion. In 2010, Mognetti et al[31] conducted a study using a murine model of closed tibial fracture to test different doses of teriparatide. They reported that 40 μg/kg per day of teriparatide increased callus tissue mineralization in the initial phases of bone recovery, thereby stimulating bone synthesis metabolism and ensuring a more stable early-stage repair. In a related prospective clinical trial, teriparatide was administered to 32 patients suffering from nonunion, with 30 patients showing bone consolidation within no more than six months of starting treatment. This study suggests that teriparatide contributes to the complete healing of aseptic nonunion[20]. Our findings align with recent literature documenting teriparatide's efficacy in similar complex fracture healing scenarios. For instance, Wang et al[32] reported on the use of teriparatide after zoledronic acid in a severe case of osteoporotic vertebral compression fracture. This approach noting marked improvements in bone density and structural integrity over a five-year period. Similarly, Lai et al[33] described the successful treatment of a nonunion associated with loosening of internal fixations in a Garden IV femoral neck fracture, where teriparatide facilitated complete bone healing and functional recovery. These cases further support the notion that teriparatide can be a valuable therapeutic option for treating established nonunions, especially when other conventional treatments have failed. The ability of teriparatide to enhance osteoblastic activity and promote bone remodeling, as demonstrated in these reports, provides a compelling rationale for its use in clinical practice. Our case involved a patient who opted for teriparatide treatment after experiencing nonunion for 4 years. The treatment led to complete consolidation of the fracture. The patient reported satisfaction with daily activities and physical exercise. Our study differs from previous investigations in its longer time span, highlighting the potential effectiveness of teriparatide even in long-standing nonunion cases.
The mechanism underlying the therapeutic effect of teriparatide in promoting fracture healing, particularly in cases of nonunion, involves its ability to enhance bone formation and remodeling processes. Teriparatide, a recombinant form of PTH, primarily exerts its actions through the activation of the PTH/PTH-related protein receptor on osteoblasts and osteocytes[34]. Teriparatide promotes fracture healing, particularly in cases of nonunion, through several mechanisms. Firstly, it stimulates osteoblastic activity, leading to increased bone formation and enhanced callus formation at the fracture site[35]. Secondly, teriparatide promotes the differentiation of osteoprogenitor cells, accelerating the recruitment of mesenchymal stem cells to the fracture site and facilitating bone regeneration[36,37]. Additionally, teriparatide modulates bone remodeling processes by promoting the coupling of bone formation and resorption, thereby optimizing the balance between osteoblast and osteoclast activity for efficient bone repair[38]. Furthermore, teriparatide enhances angiogenesis, supporting the vascularization of newly formed bone tissue and providing essential nutrients and oxygen for optimal fracture healing[39].
Despite the successful outcome observed in our patient, it is crucial to acknowledge the limitations of our study. As this is a case report involving only one patient, the observed efficacy of teriparatide may be influenced by factors specific to the individual, such as the patient's young age and strong potential for bone regeneration. Consequently, the conclusions drawn from this case may not be generalizable to the broader population. Further studies are needed to more reliably validate the efficacy of teriparatide. Larger-scale clinical trials, ideally with control groups, are essential for conducting a more extensive investigation and establishing generalizable findings about the treatment’s effectiveness. Additionally, the optimal dosage and duration of teriparatide treatment for nonunion have yet to be determined. Future research should focus on rigorous clinical validation to potentially expand the indications for teriparatide use in fracture nonunion scenarios.
CONCLUSION
We present a patient who experienced nonunion following open reduction and internal fixation of a humerus fracture. After the nonunion persisted for four years, we initiated treatment with teriparatide, resulting in complete healing of the fracture and resolution of pain. Our findings suggest that teriparatide may be a valuable therapeutic option for treating established prolonged humerus fracture nonunion, even in cases of prolonged duration. However, further clinical studies are needed to validate the efficacy and establish the optimal treatment protocols for teriparatide in fracture nonunion.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade A, Grade B, Grade B, Grade C, Grade C
Novelty: Grade A, Grade A, Grade B, Grade B, Grade B
Creativity or Innovation: Grade A, Grade B, Grade B, Grade B, Grade B
Scientific Significance: Grade A, Grade A, Grade B, Grade B, Grade B
P-Reviewer: Li YF; Liang YZ; Xia M S-Editor: Luo ML L-Editor: A P-Editor: Zhang YL