Published online Jan 18, 2025. doi: 10.5312/wjo.v16.i1.101424
Revised: November 3, 2024
Accepted: December 19, 2024
Published online: January 18, 2025
Processing time: 121 Days and 17 Hours
Tuberculosis is among the most devastating infectious diseases worldwide. Spinal tuberculosis is not easy to detect at an early stage, which without effective treat
To establish a rabbit model of spinal tuberculosis and examine the effect on the model’s efficacy using different concentrations of Mycobacterium tuberculosis (M. tuberculosis) inoculum.
New Zealand rabbits were randomly divided into experimental, control and blank groups. The experimental and control animals were sensitized with complete Freund′s adjuvant, a hole was drilled beneath the upper endplate of the L6 vertebral body and filled with gelfoam sponge. The experimental group was divided into three subgroups (experimental 1, experimental 2, experimental 3) and infused with M. tuberculosis suspension at various concentrations. The control group was inoculated with saline and the blank group received no treatment. The 12-week post-operative survival rates were 100%, 80% and 30% in the experimental groups inoculated with concentrations of 106, 107 and 108 CFU/mL bacteria, respectively.
The survival rate of the control and blank groups was 100%. Vertebral body destruction at 8 weeks in the three experimental groups as determined by X-ray analysis was 33.3%, 62.5% and 66.7%, and by computed tomography (CT) and 3-dimensional CT 44.4%, 75% and 100%, respectively. At 12 weeks, the figures were 44.4%, 75% and 100% by X-ray analysis and 44.4%, 100% and 100% by CT and 3-dimensional CT, respectively. All surviving rabbits of the experimental groups had vertebral destruction. The positive bacterial culture rates were 22.2%, 75% and 66.7%, respectively, in the experimental groups. After being sensitized with complete Freund's adjuvant, large differences were observed in the extent of spinal tuberculosis after inoculation of the rabbits with different concentrations of H37RV standard M. tuberculosis.
The experimental 1 had a low success rate at establishing an infection. The experimental 3 resulted in high mortality and complication rates. The experimental 2 was optimum for establishing a spinal tuberculosis model based on the high level of symptoms observed and the low rabbit mortality.
Core Tip: One study reported having established osteoarticular tuberculosis using non-sensitized animals; however, the result of the experiment was unsatisfactory because of the high mortality rate. In recent studies, Liu and Geng successfully established a spinal tuberculosis model in New Zealand rabbits after sensitizing the rabbits with complete Freund’s adjuvant. However, they established the model without making a comparative study of the effect of using different bacterial concentrations. In this study, we established a model using three concentrations of bacteria and carried out a comprehensive evaluation of the model by imaging, general observations, and histopathological and bacteriological studies.
- Citation: Qiao YJ, Song XY, Zhang LD, Li F, Zhang HQ, Zhou SH. Comparative study of a rabbit model of spinal tuberculosis using different concentrations of Mycobacterium tuberculosis. World J Orthop 2025; 16(1): 101424
- URL: https://www.wjgnet.com/2218-5836/full/v16/i1/101424.htm
- DOI: https://dx.doi.org/10.5312/wjo.v16.i1.101424
Tuberculosis is among the most devastating infectious diseases worldwide. Tuberculosis still accounts for the highest mortality from any infectious diseases worldwide, even surpassing human immunodeficiency virus/acquired immu
Choosing a suitable animal model to simulate the development of human disease is extremely important, needing to be suitable for studies involving inter alia pathogenesis, pathology, immune mechanisms and drug therapy. Local injection of tuberculosis bacteria without using anti-tuberculosis drugs often causes a fatal bacteremia. However, the stringent laboratory conditions required when working with Mycobacterium tuberculosis (M. tuberculosis) greatly hinder progress.
One study reported having established osteoarticular tuberculosis using non-sensitized animals; however, the result of the experiment was unsatisfactory because of the high mortality rate. In recent studies, Liu et al[9] and Geng et al[11] successfully established a spinal tuberculosis model in New Zealand rabbits after sensitizing the rabbits with complete Freund’s adjuvant. However, they established the model without making a comparative study of the effect of using di
The study fulfilled the requirements of the 309th Hospital of Chinese PLA (Army Tuberculosis Prevention and Control Key Laboratory, Beijing Key Laboratory of New Techniques or Tuberculosis Diagnosis and Treatment, Institute for Tu
M. tuberculosis strain H37RV was obtained from the Army Tuberculosis Prevention and Control Key Laboratory of the 309th Hospital of Chinese PLA. Instruments utilized included a Computed Radiography Digital Imaging System (Philips, Eindhoven, Netherlands) and a 16-slice computed tomography (CT) scanner (United Imaging, Shanghai, China). The main reagents included complete Freund’s adjuvant (Sigma-Aldrich, St. Louis, MO), Lowenstein-Jensen (LJ) medium and Petri dishes (Baso, Zhuhai, China).
Forty-two healthy New Zealand white rabbits (aged 11-12 weeks, of mixed sex, with body weights of 2.25 ± 0.15 kg) were obtained from the Center for Animal Experiment of the 309th Hospital of Chinese PLA. The license number for medical experimental animal production was SYXK(Army)2012-0016. The rabbits were randomly divided into five groups: Three experimental groups of 10 each The experimental group was divided into three subgroups (experimental 1, experimental 2, experimental 3, inoculated with concentrations of 106, 107 and 108 CFU/mL bacteria, respectively), 10 in the control group and two in the blank group. During the experiments, all the rabbits were raised separately in individual cages with a standardized living environment and feeding pattern. At the end of the experiment, the rabbits were killed (pen
H37RV standard M. tuberculosis was cultured for 4 weeks in LJ medium. Well-established colonies were selected and triturated in 0.9% saline. Bacterial suspensions were collected and diluted with saline to obtain suspensions of 106, 107 and 108 CFU/mL as determined by the colony counting method. These were maintained in a 4 °C refrigerator (none for more than 24 hours) and shaken well before application.
Sensitization with complete Freund’s adjuvant: The experimental and control rabbits received 0.1 mL of complete Freund’s adjuvant (a mixture of lanolin and liquid paraffin containing 4.5 mg/mL bovine M. tuberculosis) through an intradermal injection in the nape of the lower neck. Two animals developed anorexia, fever, spirits are drooping, ac
Anesthesia: Pentobarbital sodium was injected into the auricular vein at a dose of 30-40 mg/Kg. None of the animals showed obvious discomfort during surgery.
Operation: The rabbits were given small amounts of water and fasted for 12 hours pre-operatively. After the anesthesia had taken effect, the rabbit was placed on an operating table and the right lower back was shaved, disinfected twice with Povidone-iodine and covered with aseptic towel. A longitudinal incision of 5-6 cm was made along the end of the left 12th rib inferior to the iliac crest, the subcutaneous fascia and muscle were separated carefully to avoid injury to vessels and nerves, and the intervertebral disc of L5/6 and adjacent vertebral bodies was completely exposed using the method of Geng et al[11]. An electric drill was used to bore a hole (3-mm diameter, 6-mm depth) from the right front to the left rear of the L6 vertebral body at a 30° angle to the transverse plane. After hemostasis, the cavity was filled with gelfoam sponge, then 0.1-mL aliquots at 106, 107 or 108 CFU/mL were slowly infused into the gelfoam sponge, using 10 rabbits for each of the three bacterial concentrations. The control group each received 0.1 mL 0.9% saline, while no treatment was performed on the blank group. Finally, the incisions were sutured layer by layer, and sterile drape was fixed over the incision.
After surgery, the rabbits were housed separately following the principles of aseptic technique, safety and animal ethics.
General observations: The animals were observed twice daily for state of health, eating habits, mental state, activity and wound healing.
Imaging examinations: X-ray analysis, CT and 3-dimensional CT (3DCT) were performed at 8 and 12 weeks post-operatively (po) to observe the extent of destruction of the intervertebral disk and vertebral body and the formation of sequestra and abscesses. All these imaging examinations were performed under anesthesia.
Histopathological observation: Rabbits were sacrificed 12 weeks po after imaging examinations had been completed. We dissected out lesion tissue (including the intervertebral disk of L5/6, the upper and lower endplates, the lesions' vertebral body and soft tissue, and abscesses) for routine hematoxylin and eosin (HE) staining to detect any histopathological changes. Soft tissue and bone tissue were fixed in 10% buffered formalin for 3-5 days before routine processing. After the fixation had been completed, bone tissue decalcification was performed with 10% nitric acid, followed by dehydration, embedding, sectioning, staining with HE and microscopic observation.
Culturing of M. tuberculosis: Diseased tissue (0.5 g of granulous tissue or paravertebral abscess) was removed from the operation site of all surviving rabbits in the experimental and the control group, cut into pieces and put into 1 mL 0.9% saline. After homogenization, 1 mL of 6% sodium hydroxide (NaOH) was added to the mixture and allowed to stand at room temperature for 30 minutes to kill the non-tuberculous mycobacteria. Finally, a further 1 mL 0.9% saline was added to the mixture, from which 0.1 mL was collected and cultured on LJ medium at 37 °C for 4-8 weeks. The results were analyzed according to the standards for tuberculosis diagnosis and bacteriological testing published in 1995 by the Chinese Tuberculosis Prevention and Treatment Association[12].
All results were expressed as the mean ± SEM. The Fisher exact probability method was used for the 12-week post-operative animal survival rate.
Experimental 1: Of the 10 rabbits inoculated, one rabbit suddenly appeared opisthotonus and died of cardiopulmonary arrest when we drilled the hole, possibly due to spinal cord or nerve injury. The other rabbits underwent surgical pro
Experimental 2: Of the 10 rabbits, one became paraplegic the next day and spirits are drooping, activities were sig
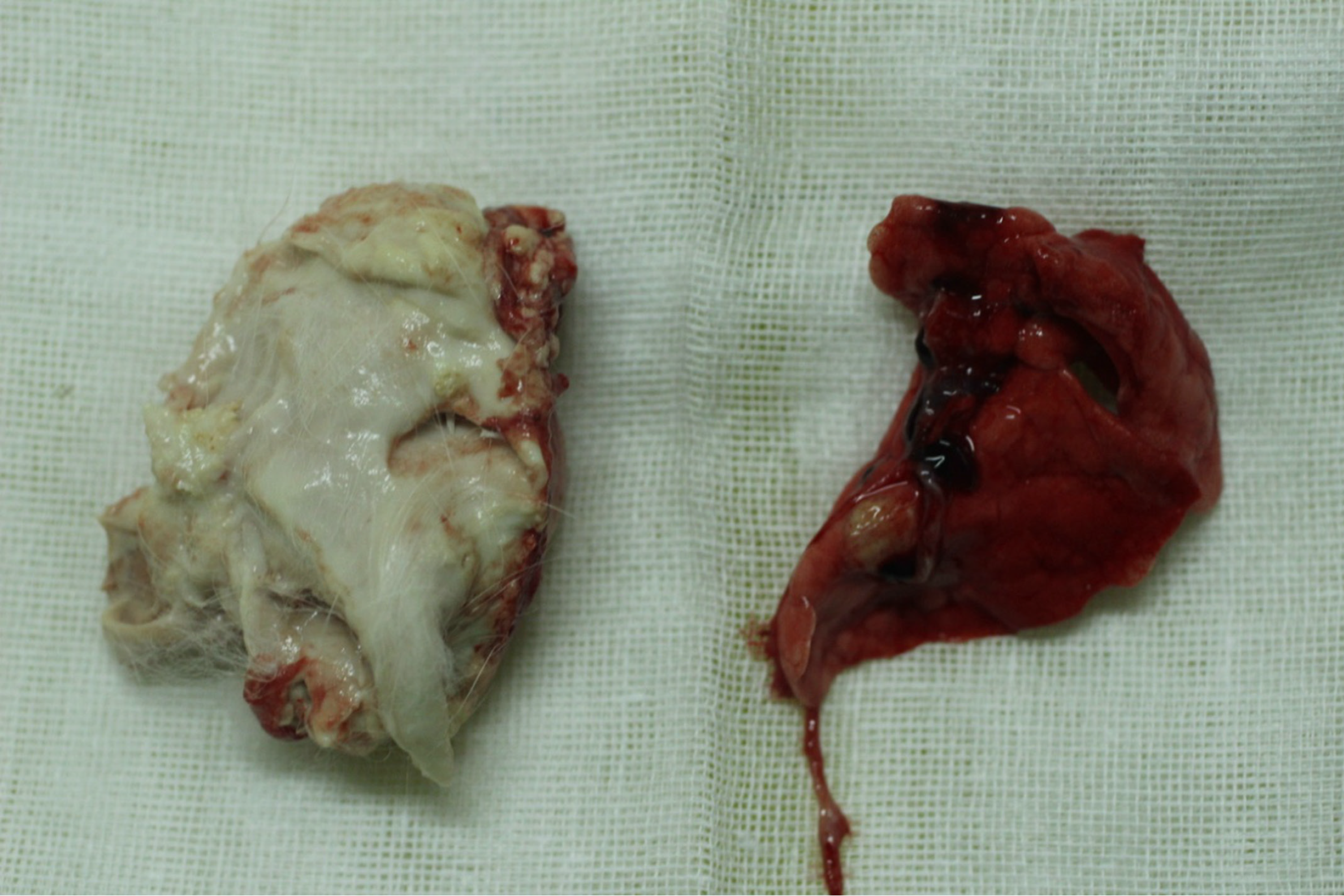
Experimental 3: Of the 10 rabbits, seven experienced mental fatigue and ate poorly, four of them having nonunions and pus at the incision site and dying 12-20 days po. Dissection of the dead bodies yielded huge amounts of pus in the surgical site and right lung with caseous necrosis (Figure 1). Three rabbits each lost about 1 kg of weight after about 1 month and died 38-51 days po (one of which were euthanized with air embolism after anesthesia, the others died a natural death). Dissection of the rabbits yielded serious swelling at the surgical site, right lumbar region and the right thigh. Off-white pus from the lumbar region was cultured on LJ medium at 37 °C for 4 weeks and good colony growth was observed. The cause of death was ascribed to disseminated M. tuberculosis. Finally, three rabbits survived to the end of the experiment, all three showing weight loss, anorexia and reduced activity.
Control group: Of the 10 rabbits, one died because of an anesthetic accident, while nine survived to the end of the experiment, none of them showing weight loss, anorexia or reduced activity.
Blank group: The animals maintained a good appetite with normal activity and zero mortalities. Animal survival rates are shown in Table 1.
| Groups (n) | Day of model establishment | 4 weeks post model establishment | 8 weeks post model establishment | 12 weeks post model establishment | Survival rate (%) |
| Experimental 1 (n = 10) | 9 | 9 | 9 | 9 | 100 |
| experimental 2 (n = 10) | 10 | 9 | 8 | 8 | 80 |
| experimental 3 (n = 10) | 10 | 6 | 3 | 3 | 30 |
| Control group (n = 10) | 9 | 9 | 9 | 9 | 100 |
| Blank group (n = 2) | 2 | 2 | 2 | 2 | 100 |
The Fisher exact probability method was used for the 12-week post-operative animal survival rate. The experimental groups (1 and 2) compared with the control group: P > 0.05. The experimental group 3 compared with the control group: P < 0.05. The experimental group 1 compared with the experimental group 2: P > 0.05. The experimental group 1 compared with the experimental group 3: P < 0.05. The experimental group 2 compared with the experimental group 3: P < 0.05.
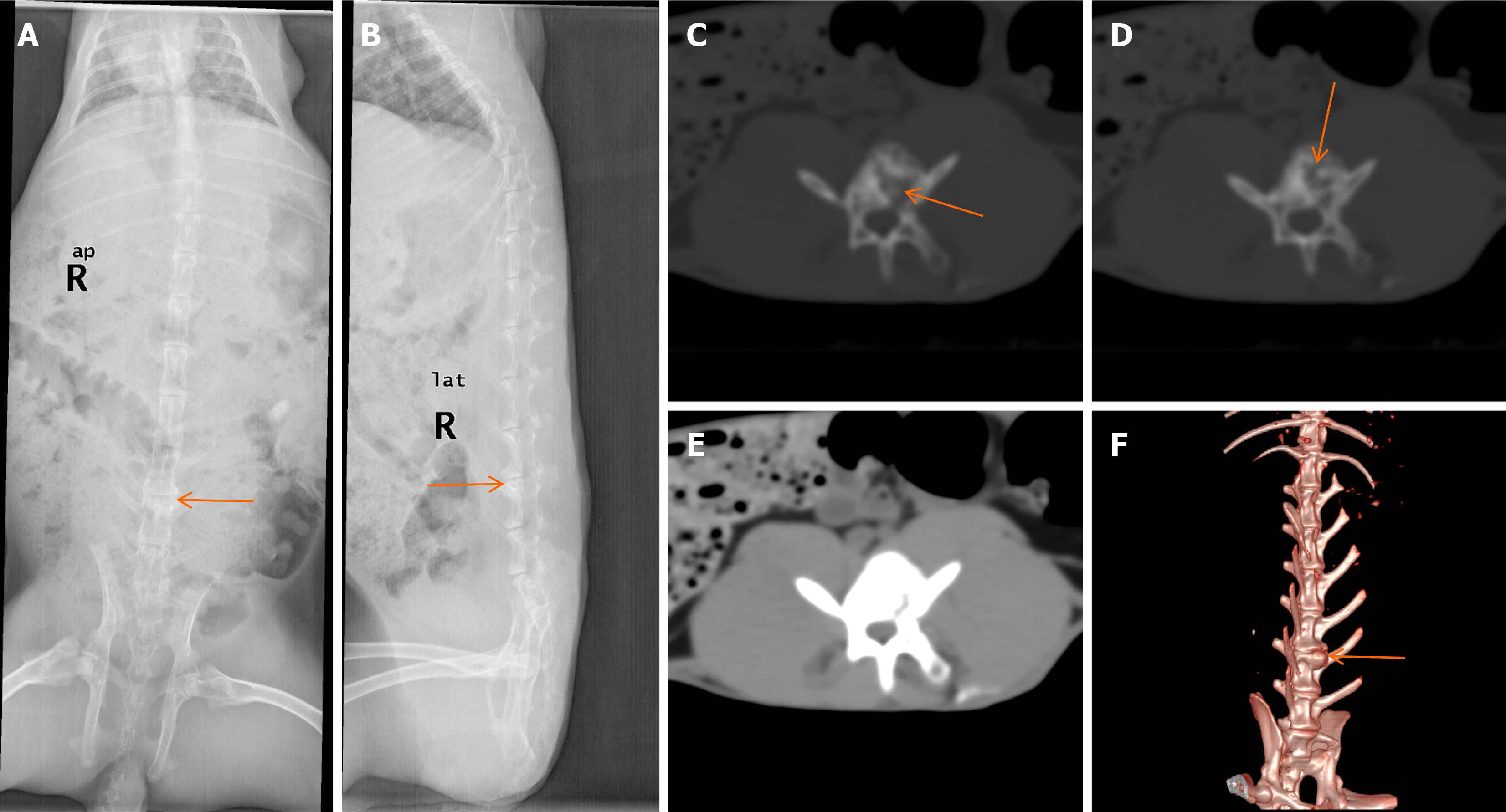
Imaging results: Typical results of X-ray analysis, CT and 3DCT scans performed at 12 weeks are shown in Figure 2. The number of animals with bone destruction is recorded in Table 2. X-ray examinations revealed that, in the experimental groups, the intervertebral spaces appeared blurred and narrowed, while the adjacent endplates appeared to have increased bone density shadows (Figure 2A and B). CT scans showed that the bone mineral densities (BMD) in the vertebral bodies were not homogeneous and that some areas had irregularly low BMDs. Dotted sequestra and increased BMD shadows were also visible in the vertebral bodies, while the local vertebral bone cortices were nonunion. The soft tissue did not show obvious swelling around the vertebral bodies (Figure 2C-E). 3DCT showed that the intervertebral spaces had narrowed with surrounding osteophyte formation (Figure 2F). X-ray analysis of the control group showed no post-operative changes in the intervertebral spaces and no damage to the vertebral bodies at either 8 or 12 weeks. CT scans revealed that the vertebral body edges were slightly hardened with no evidence of bone destruction; the intervertebral spaces had not changed and there were no abscesses or soft tissue calcification shadows around the vertebral bodies. There was no abnormal imaging observed in the blank group.
| Results | Experimental 1 | Experimental 2 | Experimental 3 | |||
| 8 weeks | 12 weeks | 8 weeks | 12 weeks | 8 weeks | 12 weeks | |
| X-ray | ||||||
| Intervertebral space changes | 3 | 4 | 5 | 6 | 2 | 3 |
| Osteophyte | 1 | 2 | 4 | 4 | 2 | 3 |
| CT | ||||||
| Osteophyte | 2 | 3 | 5 | 8 | 3 | 3 |
| Destruction of the vertebral body | 4 | 4 | 6 | 8 | 3 | 3 |
| Abscess | 0 | 0 | 0 | 0 | 0 | 0 |
| 3-dimensional CT | ||||||
| Intervertebral space changes | 4 | 4 | 6 | 8 | 3 | 3 |
| Osteophyte | 2 | 3 | 5 | 8 | 3 | 3 |
General observations: We dissected all animals 12 weeks po after completing the imaging examinations.
Experimental 1: The incisions of all the animals had healed well, with no fistulas or discharge phenomena being ob
Experimental 2: One rabbit’s incision had not healed well, showing a little light yellow pus. Obvious bone destruction had occurred on the inferior border of the L5 vertebra or the superior border of the L6 vertebra in all animals; the intervertebral spaces had become narrowed with obvious destruction of the intervertebral discs. Two rabbits were found with sequestra within the vertebral bodies, with the vertebral sides having scattered small nodules with caseous necrotic material overflowing after piercing. No obvious psoas abscesses occurred in these rabbits.
Experimental 3: The incisions of two of the rabbits had not healed well and fistulas with diameters of about 0.2 cm were found near the incision, caseous necrotic material emerging when squeezed. Serious vertebral body destruction had occurred and the intervertebral spaces had narrowed; the intervertebral discs were extensively damaged and had become dark. Scattered small nodules with caseous necrotic material that overflowed on piercing were present on both sides of the badly damaged paravertebral muscle. No obvious psoas abscesses occurred in these rabbits. Two rabbits had slightly damaged vertebral bodies and intervertebral discs among the five animals with negative imaging results. No other organs with obvious abnormalities were observed in any of the animals. No obvious destruction of the intervertebral disks or vertebral bodies was detected and no abscesses were found in either the control group of rabbits, the blank group or those rabbits with negative imaging results.
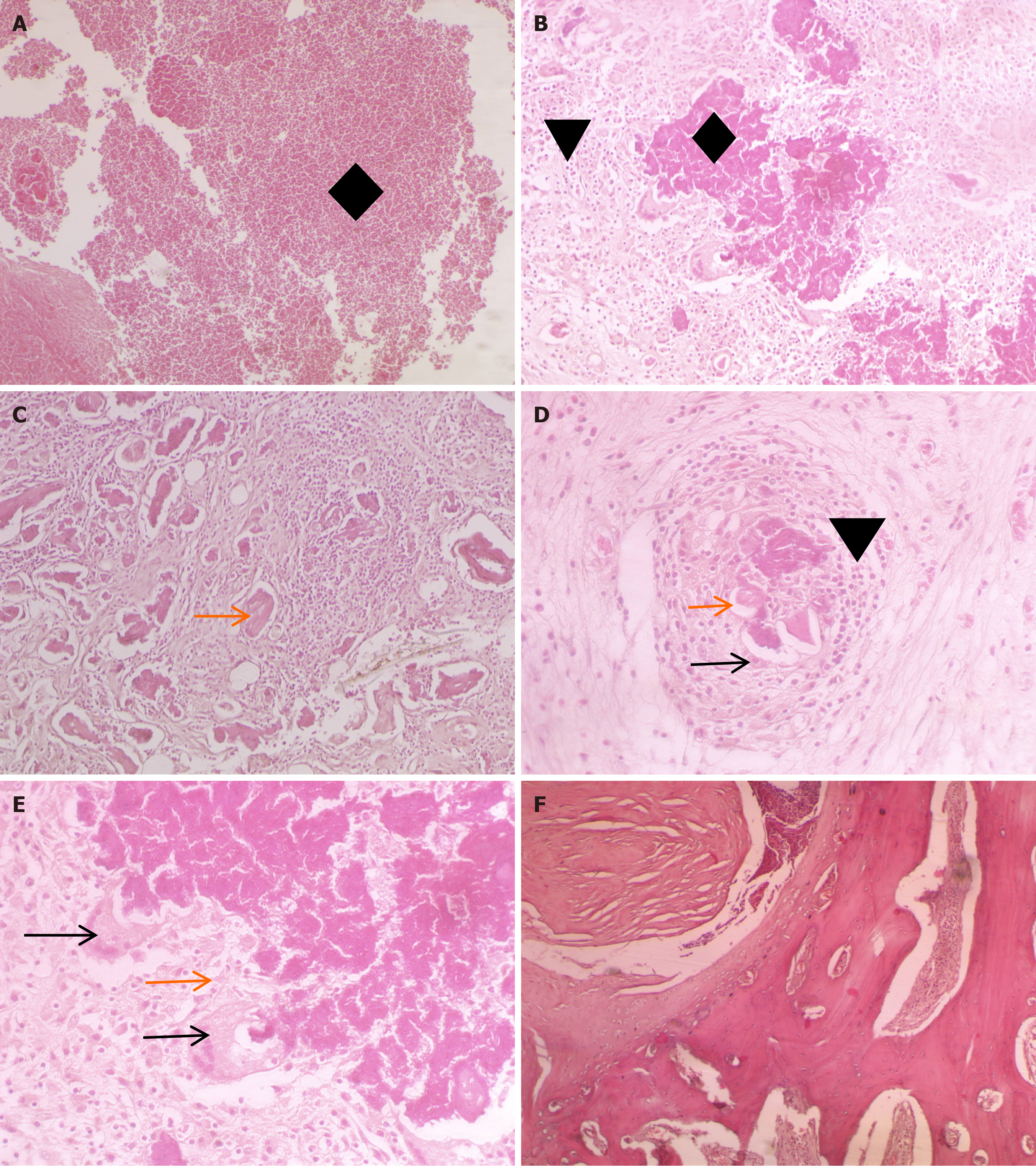
Histopathological observations: After the experiment is completed , air embolism was applied after anesthesia in the rabbits to minimize pain and distress .Histopathological studies of the rabbits with positive imaging results showed pa
M. tuberculosis culture: After the experiment is completed , air embolism was applied after anesthesia in the rabbits to minimize pain and distress. Paravertebral pus, soft tissue and necrotic tissue were taken from all experimental animals for culture of M. tuberculosis. After culturing for 4 weeks, 10 of the 20 rabbits in the experimental groups had positive results with the appearance of pale yellow colonies (two of the experimental 1, six of the experimental 2 and two of the experimental 3). Yellow colonies were evenly and firmly attached to the medium (Figure 4). No mycobacterial growth was found in the other 10 specimens after 8 weeks of culture. The rates of positive culture were 22.2%, 75% and 66.7% for the above three groups, respectively. No mycobacterial growth was observed with either the control or blank groups.
Choosing a suitable animal is critical to the success of establishing a model for spinal tuberculosis. Many animals have been tried for establishing a model, including mice, guinea pigs, rabbits, monkeys, cows, sheep and fish[13-15]. As early as 1928, Lurie successfully established the first pulmonary tuberculosis model[16]. Rabbits are sensitive to M. tuberculosis and can form typical tuberculous granulomas after infection[17] and are commonly used to establish models for pu
Local direct inoculation with M. tuberculosis results in different pathological outcomes post-infection. If the bacterial concentration in the inoculum is too low, the success rate of the model, and of obtaining positive images, is low and the bone tissue mainly presents as hyperplasia. However, large concentrations of bacteria rapidly cause blood bacteremia and caseous pneumonia, which lead to early mortality and a low success rate. Therefore, the appropriate quantity of bacteria, the necessary prior interventions and the correct infection pathway are the keys to establishing a successful model.
H37RV standard M. tuberculosis is widely used in tuberculosis research[19-21]. We kept the bacteria at 4 °C in a re
Complete Freund’s adjuvant is an oily emulsion enriched with bovine heat-killed M. tuberculosis. The slow release and continuous stimulation by immunogenic substances enhances the systemic resistance of the host to M. tuberculosis. Moreover, local lesions on the vertebral structure are aggravated by type IV immune hypersensitivity reactions, which enhance bone destruction. Some researchers have used unsensitized animals to establish models of osteoarticular tuberculosis, but because the local and systemic responses were more severe, rapid mortality of the experimental animals occurred. Wu and Duan[22] and Li[23] sensitized rabbits using complete Freund’s adjuvant to establish a model of knee tuberculosis and got satisfactory experimental results because of the lighter systemic and local reactions. Likewise, when we established the model for osteoarticular tuberculosis, the use of complete Freund’s adjuvant significantly increased the animals’ survival and the experimental success rate, making it worthy of becoming a generalized procedure.
As early as 1928, John Fraser had tried local, venous and arterial implanting of M. tuberculosis to establish a model of osteoarticular tuberculosis, but the survival of, and success rate with, the animals was very low. Subsequently, many scholars have used the method of directly implanting M. tuberculosis in the nutrient foramen. A hole was drilled in the long bone for local implanting in a sensitized animal, which procedure successfully established osteoarticular tuberculosis models, but the success rate was not very satisfactory. Wu et al[22] successfully established a rabbit model of knee joint tuberculosis for the first time in China by injection of 0.5 mg bovine mycobacterium in 0.1 mL. Li[23] es
An early diagnosis of spinal tuberculosis is often difficult because of the lack of specific clinical manifestations. Imaging examination is an important method for evaluating the spinal tuberculosis model and has made earlier diagnosis possible. In our study, X-ray presentations were blurring and narrowing of the intervertebral space, with uneven vertebral density and new bone formation at the margin of the vertebral body; however, we had few positive results and most appeared relatively late. CT imaging is superior to plain radiography for demonstrating the presence of paraspinal abscesses. Uneven bone density, irregular areas of decreased bone density, dotted sequestral shadows, increased bone density within the vertebral body and uneven partial vertebral bone cortices were observed. 3DCT is a superior method for detecting narrowing of the intervertebral spaces and osteophytes.
Histopathological examination showed vast areas of caseous necrosis and inflammatory cell infiltration in the vertebral bodies. There were numerous sequestra within the vertebral bodies and the structure of the trabecular bone was di
While imaging and histopathological examination are highly suited for the detection of spinal tuberculosis, mycobacterial culture remains the diagnostic “gold standard”. However, mycobacterial culture cannot be used to obtain an accurate diagnosis in a short-term study, due to the slow growth rate of the bacteria and the low frequency of detection. In our study, paravertebral pus, soft tissue and necrotic tissue were used as a source of bacteria for culture. The rate of positive culture was 22.2%, 75% and 66.7%, respectively, from the groups inoculated with 106, 107 and 108 bacteria per mL.
We used the method of implanting the mycobacterial suspension by infusion into a gelfoam sponge that had been inserted into the vertebral body, using three concentrations of M. tuberculosis in separate experimental groups. Finally, we concluded that, of the three concentrations used, injection of 0.1 ml at 107 CFU/mL was superior for establishing a spinal tuberculosis model, because of the high rate of disease signs accompanied by low rabbit mortality. In conclusion, by inoculating local vertebral bodies of New Zealand white rabbits with an appropriate dose of strain H37RV, we es
The limitations of our study included the small sample size for each group (laboratory facilities were limited) and the large M. tuberculosis concentration range employed; thus, we cannot say with certainty that 0.1 mL of bacteria at 107 CFU/mL was the most suitable for establishing the spinal tuberculosis model. Therefore, further studies should be carried out by improving the experimental conditions, employing larger numbers of test animals, and doing a comparative study with a smaller concentration gradient of bacteria, to determine an accurate figure for the optimum bacterial dose for the establishment of a rabbit model of spinal tuberculosis.
The experimental 1 had a low success rate at establishing an infection. The experimental 3 resulted in high mortality and complication rates. The experimental 2 was optimum for establishing a spinal tuberculosis model based on the high level of symptoms observed and the low rabbit mortality.
| 1. | Williams V, Onwuchekwa C, Vos AG, Grobbee DE, Otwombe K, Klipstein-Grobusch K. Tuberculosis treatment and resulting abnormal blood glucose: a scoping review of studies from 1981 - 2021. Glob Health Action. 2022;15:2114146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 2. | Harding E. WHO global progress report on tuberculosis elimination. Lancet Respir Med. 2020;8:19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 268] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 3. | Yadla M, Sriramnaveen P, Kishore CK, Sivakumar V, Reddy YS, Sridhar AV, Vijayalakshmi B, Lakshmi AY. Backache in patients on maintenance hemodialysis: Beware of spinal tuberculosis. Saudi J Kidney Dis Transpl. 2015;26:1015-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Jain AK, Rajasekaran S, Jaggi KR, Myneedu VP. Tuberculosis of the Spine. J Bone Joint Surg Am. 2020;102:617-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 5. | Singh S, Dawar H, Das K, Mohapatra B, Prasad S. Functional and Radiological Outcomes of Anterior Decompression and Posterior Stabilization via Posterior Transpedicular Approach in Thoracic and Thoracolumbar Pott's Disease: A Retrospective Study. Asian Spine J. 2017;11:618-626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Barwar N, Sharma A, Sharma PP, Elhence A. Evaluation of Web-Based Information on Spine Tuberculosis. Cureus. 2022;14:e28321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 7. | Sampath S, Basumani P, Kothandaraman A, Ramakrishnan R. Detection of Spinal Tuberculosis by F-18 FDG PET/CT as a Cause of Unusual Referred Pain in the Right Upper Quadrant of Abdomen. World J Nucl Med. 2022;21:69-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 8. | Spaggiari D, Desfontaine V, Cruchon S, Guinchard S, Vocat A, Blattes E, Pitteloud J, Ciullini L, Bardinet C, Ivanyuk A, Makarov V, Ryabova O, Buclin T, Cole ST, Decosterd LA. Development and validation of a multiplex UHPLC-MS/MS method for the determination of the investigational antibiotic against multi-resistant tuberculosis macozinone (PBTZ169) and five active metabolites in human plasma. PLoS One. 2019;14:e0217139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Liu X, Jia W, Wang H, Wang Y, Ma J, Wang H, Zhou X, Li G. Establishment of a rabbit model of spinal tuberculosis using Mycobacterium tuberculosis strain H37Rv. Jpn J Infect Dis. 2015;68:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Yue X, Zhu X, Wu L, Shi J. A comparative study of a rabbit spinal tuberculosis model constructed by local direct infection via the posterior lateral approach. Sci Rep. 2022;12:12853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 11. | Geng G, Wang Q, Shi J, Yan J, Niu N, Wang Z. Establishment of a New Zealand rabbit model of spinal tuberculosis. J Spinal Disord Tech. 2015;28:E140-E145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Chinese Anti-tuberculosis Association. [The laboratory science procedure of diagnostic bacteriology in tuberculosis]. Zhongguo Fanglao Zazhi. 1996;18. |
| 13. | Calderon VE, Valbuena G, Goez Y, Judy BM, Huante MB, Sutjita P, Johnston RK, Estes DM, Hunter RL, Actor JK, Cirillo JD, Endsley JJ. A humanized mouse model of tuberculosis. PLoS One. 2013;8:e63331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 14. | Bekara Mel A, Courcoul A, Bénet JJ, Durand B. Modeling tuberculosis dynamics, detection and control in cattle herds. PLoS One. 2014;9:e108584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Dharmadhikari AS, Nardell EA. What animal models teach humans about tuberculosis. Am J Respir Cell Mol Biol. 2008;39:503-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Lurie MB. The Fate of Human and Bovine Tubercle Bacilli in Various Organs of the Rabbit. J Exp Med. 1928;48:155-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Manabe YC, Dannenberg AM Jr, Tyagi SK, Hatem CL, Yoder M, Woolwine SC, Zook BC, Pitt ML, Bishai WR. Different strains of Mycobacterium tuberculosis cause various spectrums of disease in the rabbit model of tuberculosis. Infect Immun. 2003;71:6004-6011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Subbian S, Bandyopadhyay N, Tsenova L, O'Brien P, Khetani V, Kushner NL, Peixoto B, Soteropoulos P, Bader JS, Karakousis PC, Fallows D, Kaplan G. Early innate immunity determines outcome of Mycobacterium tuberculosis pulmonary infection in rabbits. Cell Commun Signal. 2013;11:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 19. | Gautam US, Asrican R, Sempowski GD. Targeted dose delivery of Mycobacterium tuberculosis in mice using silicon antifoaming agent via aerosol exposure system. PLoS One. 2022;17:e0276130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Villarreal-Ramos B, Berg S, Whelan A, Holbert S, Carreras F, Salguero FJ, Khatri BL, Malone K, Rue-Albrecht K, Shaughnessy R, Smyth A, Ameni G, Aseffa A, Sarradin P, Winter N, Vordermeier M, Gordon SV. Experimental infection of cattle with Mycobacterium tuberculosis isolates shows the attenuation of the human tubercle bacillus for cattle. Sci Rep. 2018;8:894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 21. | Gong WP, Liang Y, Ling YB, Zhang JX, Yang YR, Wang L, Wang J, Shi YC, Wu XQ. Effects of Mycobacterium vaccae vaccine in a mouse model of tuberculosis: protective action and differentially expressed genes. Mil Med Res. 2020;7:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Wu QQ, Duan LS. [A Model for Tuberculosis of the Knee Joint in Rabbit and its Application]. Jiehebing Yu Xiongbuzhongliu. 2022;3:174-176. |
| 23. | Li WL. [Experimental study of isoniazid microspheres for the treatment of synovial membrane tuberculosis in the knee joint of rabbit]. M.D. Thesis, Sichuan University 2006. |
| 24. | Liu ZW, Hu XD, Fu MH. [Establishment of a rabbit model of spinal tuberculosis with mycobacterium tuberculosis H37Rv]. Zhonghua Shiyan Waike Zazhi. 2012;29:758-761. [DOI] [Full Text] |
| 25. | Shang B, Fang JF, Hou YP, Li QF, Wang XQ. [Effects of rifampin-chitosan-calcium alginate sustained release microspheres in spinal tuberculosis models in rabbits]. Zhonghua Shiyan Waike Zazhi. 2015;32:1541-1543. [DOI] [Full Text] |