Published online Aug 18, 2024. doi: 10.5312/wjo.v15.i8.813
Revised: July 17, 2024
Accepted: July 26, 2024
Published online: August 18, 2024
Processing time: 90 Days and 6.5 Hours
Hemophagocytic lymphohistiocytosis (HLH) is a rare, life-threatening disorder caused by abnormal histiocytes and T cell activation. In adults, it is predominantly associated with infections, cancers, and autoimmune diseases. Relapsing polych
A 74-year-old woman visited the emergency room with a fever of 38.6 °C. Blood tests, cultures, and imaging were performed to evaluate fever. Results showed increased fluorescent antinuclear antibody levels and mild cytopenia, with no other specific findings. Imaging revealed lymph node enlargement was observed; however, biopsy results were inconclusive. Upon re-evaluation of the physical exam, inflammatory signs suggestive of RP were observed in the ears and nose, prompting a tissue biopsy for confirmation. Simultaneously, persistent fever accompanied by cytopenia prompted a bone marrow examination, revealing he
Thorough physical examination enabled diagnosis and treatment of HLH trig
Core Tip: Hemophagocytic lymphohistiocytosis (HLH) is a rare, life-threatening condition typically triggered in adults by conditions such as cancer, infections, and autoimmune disorders, resulting in immune system over-activation. Relapsing polychondritis (RP), an uncommon disease, is diagnosed through physical examination. In contrast to primary HLH, which necessitates stem cell transplantation is the only definitive cure, acquired HLH can be managed with therapies such as chemotherapy and immunosuppressive therapy, tailored to the underlying cause. The patient received treatment for HLH using RP therapies and was successfully cured with methylprednisolone (1 mg/kg) and azathioprine.
- Citation: Han MR, Hwang JH, Cha S, Jeon SY, Jang KY, Kim N, Lee CH. Hemophagocytic lymphohistiocytosis triggered by relapsing polychondritis: A case report. World J Orthop 2024; 15(8): 813-819
- URL: https://www.wjgnet.com/2218-5836/full/v15/i8/813.htm
- DOI: https://dx.doi.org/10.5312/wjo.v15.i8.813
Hemophagocytic lymphohistiocytosis (HLH) is a rare disease that results from immune system dysregulation and is characterized by recurrent fever, cytopenia, reactive marrow, and hepatosplenomegaly. It is a response caused by overactivation of the cluster of differentiation (CD) 8 + T cells and antigen-presenting cells, inducing a cytokine storm[1,2]. Immune regulation leads to elevated levels of interferon-γ, tumor necrosis factor (TNF), and soluble CD8, resulting in fever, increased ferritin, hypertriglyceridemia, and hypofibrinogenemia based on HLH-2004 diagnostic criteria[1]. However, these criteria are based on genetic HLH rather than adult HLH, necessitating a comprehensive evaluation for diagnosis in adults[3]. Diagnostic and therapeutic approaches are challenging because of the need for prior confirmation of potential triggers such as infections, malignancies, and autoimmune conditions[3].
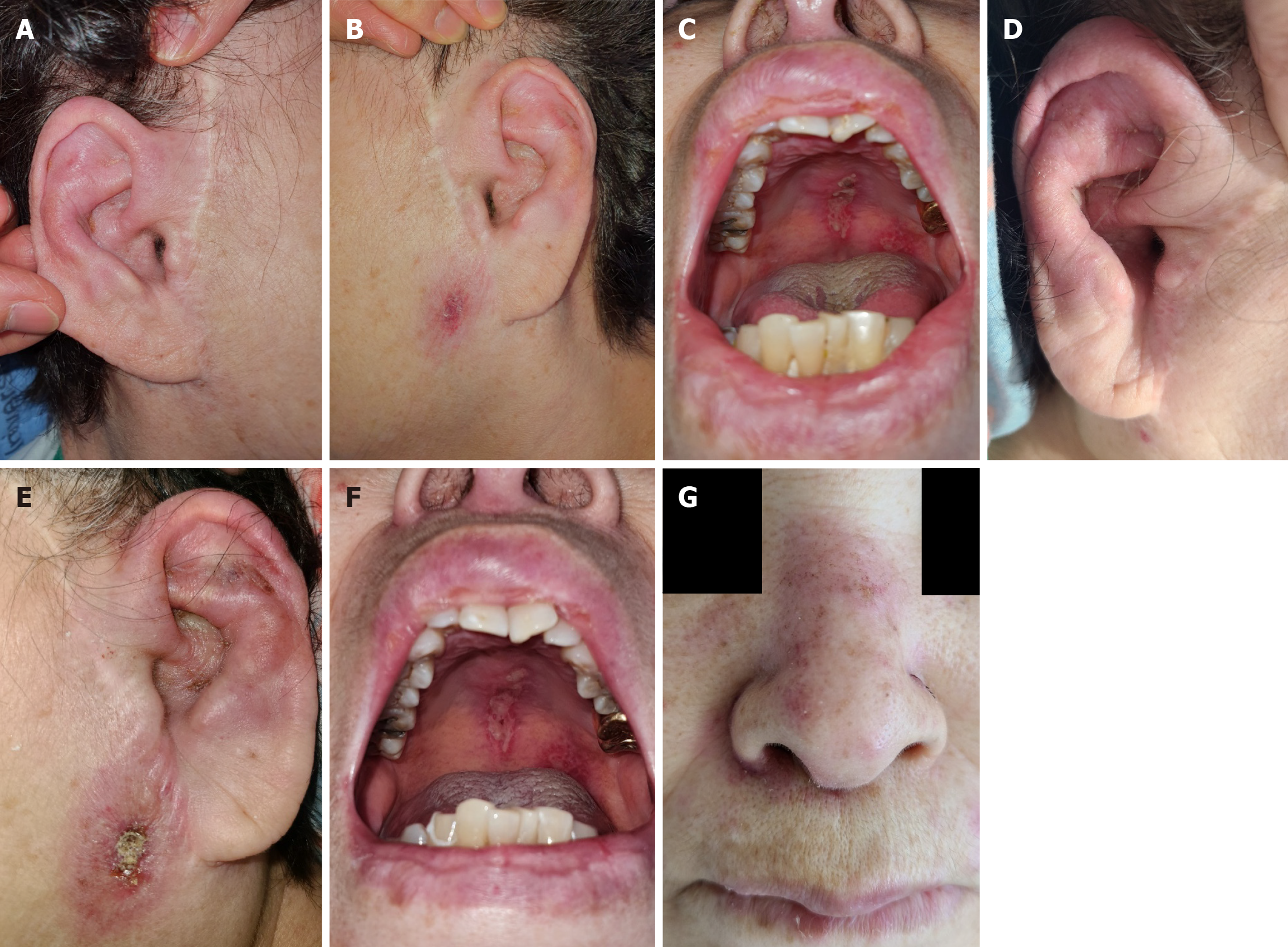
Relapsing polychondritis (RP) is characterized by the invasion of cartilaginous structures (ear, nose, larynx, tracheobronchial tree, and ribs) and other connective tissue organs. Although its prevalence varies by country, it is generally low, estimated to between 0.71 and 2 per million person years[4]. Inflammation around the ear is a typical symptom that aids in prompt diagnosis[4]. HLH induced by RP is rare, with only a few case reports documented. Here, we report the diagnosis and treatment course of this patient as a case study.
A 71-year-old woman with fever visited the emergency room.
The patient presented with sore throat and cough, alongside fever and altered consciousness. The fever started 10 d prior to presentation and recurred multiple times daily.
The patient had taken antidepressants for major depressive disorder.
The patient had no relevant family history.
Body temperature of the patient was 38.6 °C, and no other abnormal findings were detected during the initial physical examination.
Initial testing revealed leukopenia (white blood cell count: 3890 /μL, normal range: 4000-10000 /μL) and anemia [hemoglobin (Hb): 9.9 g/dL, normal range: 12-16 g/dL]. Peripheral blood smear revealed leukopenia with left-shifted maturation. Elevated levels of C-reactive protein (44.94 mg/L, normal range: 0-5.0 mg/L) and ferritin (1414 ng/mL, normal range: 13-150 ng/mL) were observed. The fluorescent antinuclear antibody titer was 1:1280, and the complement component 3 level was decreased (63.1 mg/dL, normal range: 90-180 mg/dL). Blood cultures showed no identifiable organisms, and viral markers for Epstein-Barr virus, cytomegalovirus and others did not yield significant results. The initial lesion around the ear resembled an infection caused by the herpes virus, so polymerase chain reaction was performed, but all results were negative.
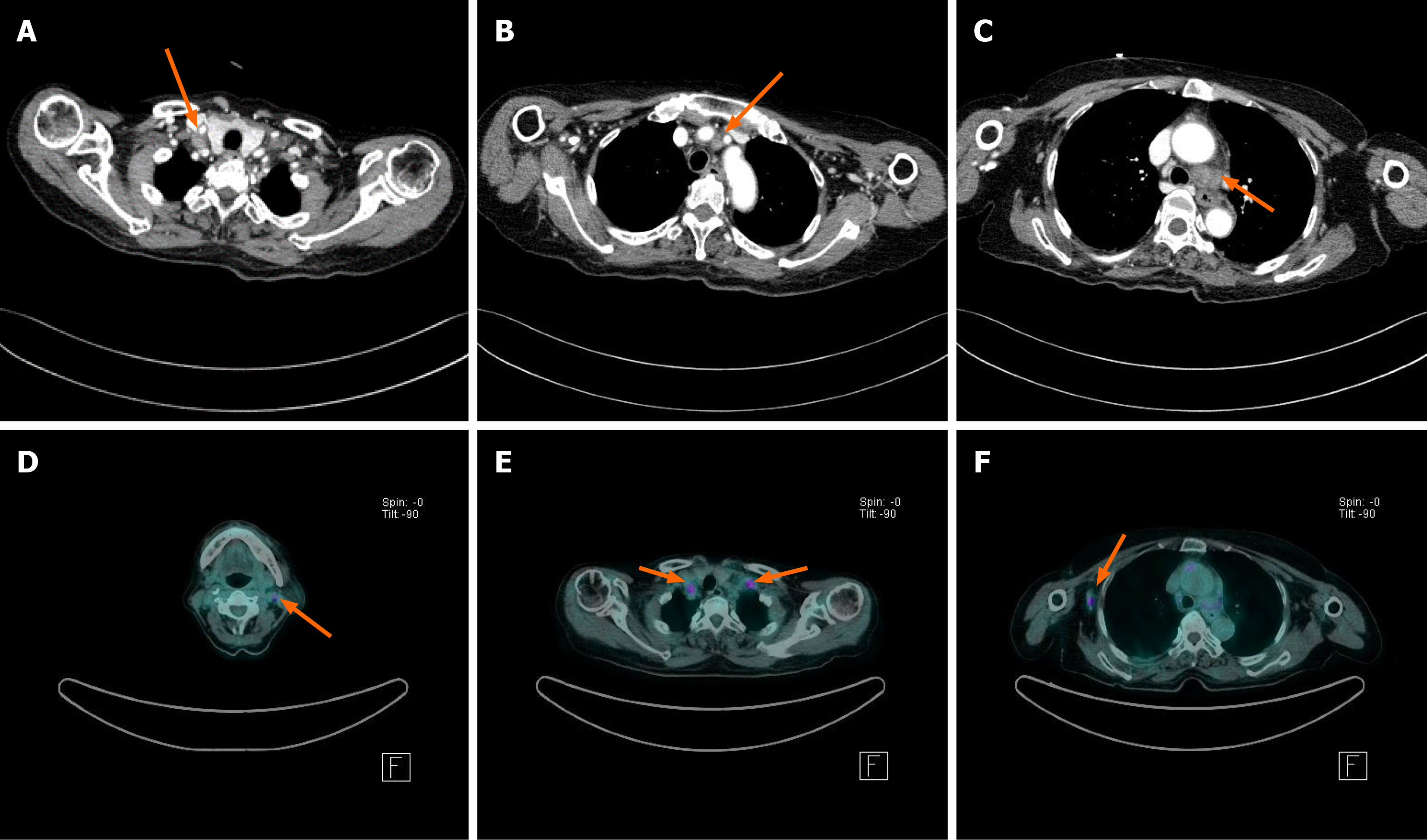
Computed tomography (CT) revealed multiple enlarged lymph nodes (LNs) in the supraclavicular area and mediastinum, with necrotic changes observed in the left lower paratracheal LN (Figure 1). Additionally, 18F-fluorodeoxyglucose (FDG) avid LNs in the left cervical, supraclavicular, and axillary areas were detected using FDG positron emission tomography/CT (PET/CT) (Figure 1).
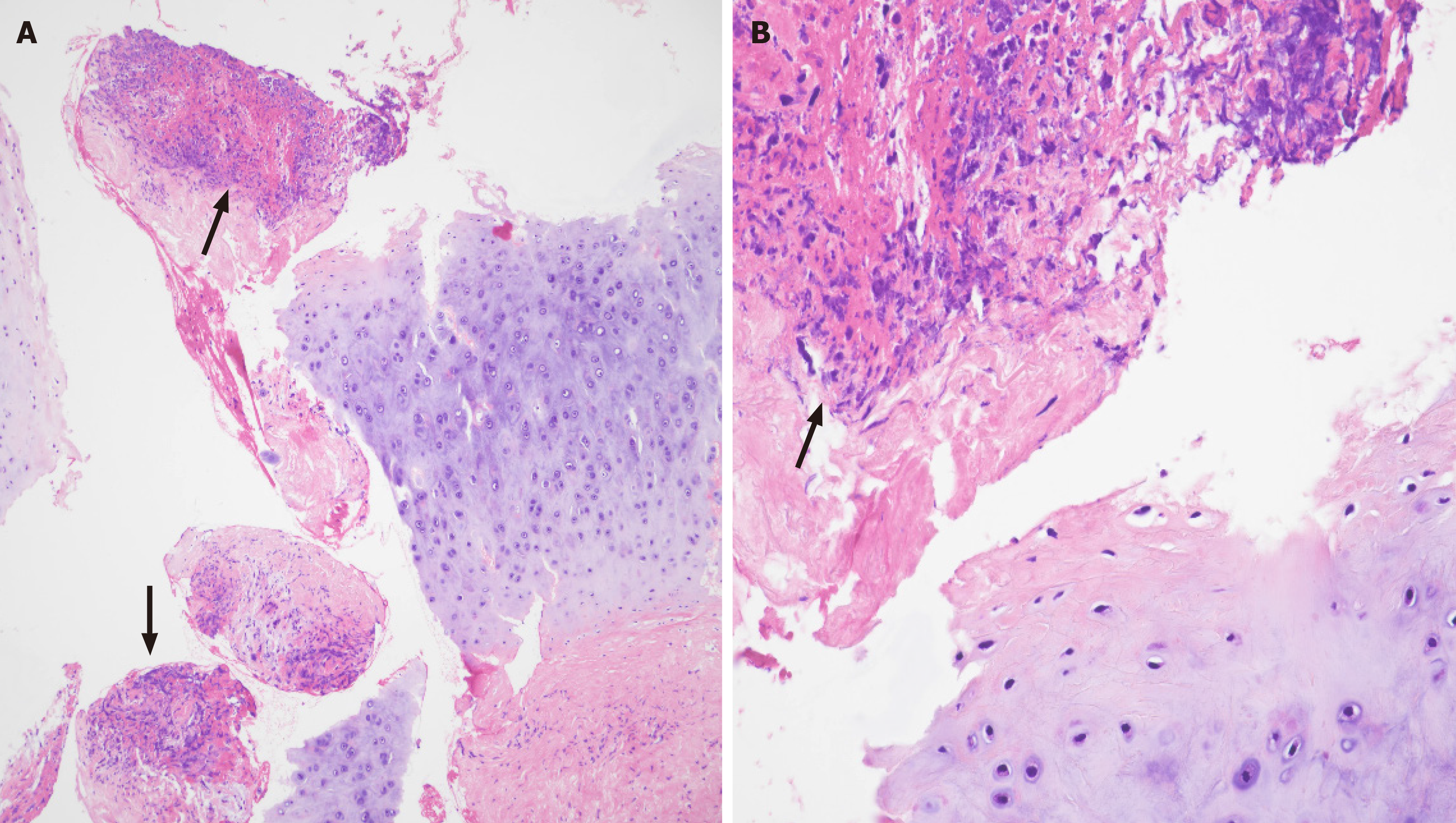
For diagnostic purposes, endobronchial ultrasound (EBUS) tissue examination was performed on the enlarged media
High-intensity treatment such as the HLH 2004 protocol was not feasible because of the patient’s poor general condition. Instead, high-dose steroids (1 mg/kg of methylprednisolone) were administered to control RP. By the second day of steroid administration, the fever resolved, and her consciousness recovered. Inflammation in both the auricles and nasal ridge gradually subsided. The patient received 1 mg/kg of methylprednisolone for 3 weeks. Thereafter, the dose was reduced to 0.5 mg/kg and gradually tapered over 4 months. Azathioprine (50 mg twice daily) was added upon steroid dose reduction.
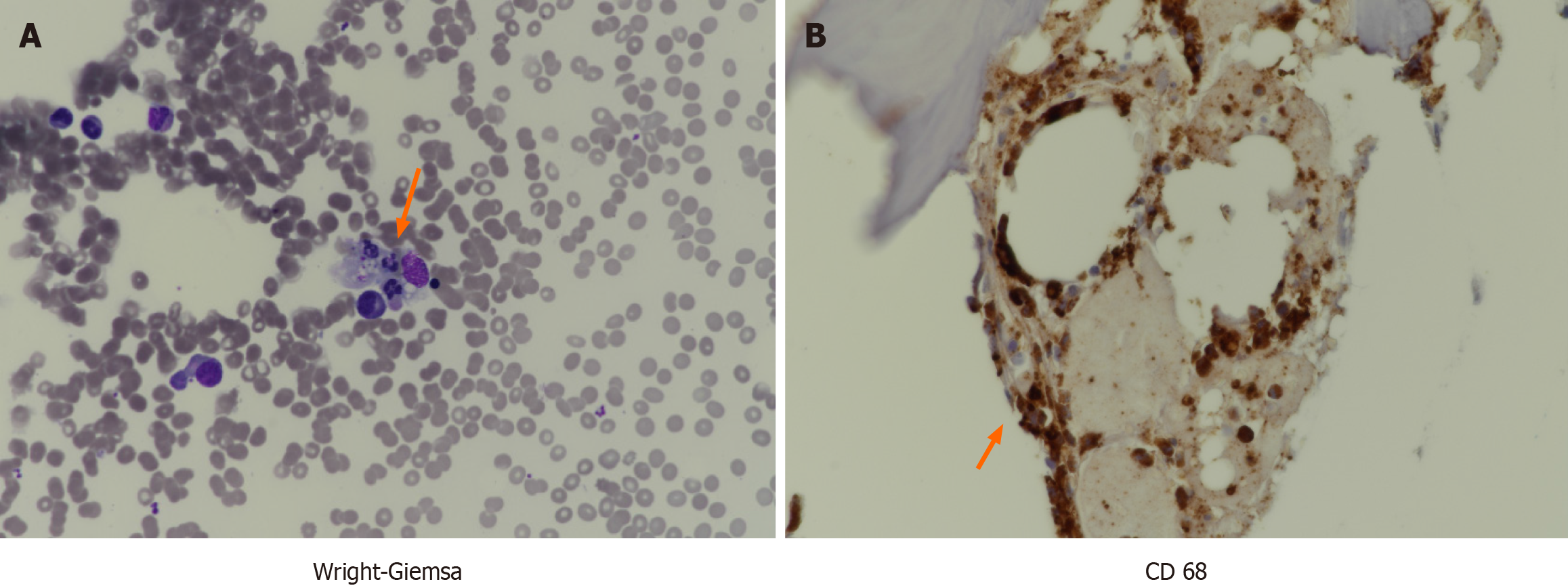
After 6 weeks of steroid treatment, a bone marrow examination exhibited resolution of hemophagocytosis, and pancytopenia and hyperferritinemia improved to normal. However, pneumonia developed while on azathioprine, leading to a reduction in dosage to 25 mg twice daily. Acute exacerbation of RP and HLH relapses did not occur; both conditions were well-controlled. Overall survival was 2 years and 10 months.
HLH is a rare, rapidly progressive, and life-threatening disease with a mortality rate of up to 40%. It is caused by immune reactions involving a severe inflammatory responses, cytokine overproduction, and hemophagocytosis, leading to fever, multiple organ dysfunction, shock, and even death. It can be classified into two types. Primary HLH is associated with genetic defects or abnormalities in immune function, primarily affection infants and young children; however, it can also be diagnosed in adults. Genetic defects in proteins responsible for cytotoxic function in T lymphocytes and NK cells can lead to dysregulated immune responses and excessive inflammation. Representative genes include PRF1, UNC13D, STX11, RAB27A[2,3]. Aggressive treatments such as hematopoietic stem cell transplantation are necessary, especially in cases with genetic predisposition. In contrast to primary HLH, where specific genetic mutations are the primary cause, secondary HLH typically arises from increased immune responses triggered by external factors such as underlying immune disorders, tumors, infections, or immunosuppressive agents. HLH frequently manifests in adults and is associated with various factors affecting immune function. Its pathogenesis involves genetic defects and abnormal activation or dysregulation of the immune system, leading to excessive activation of immune cells and tissue damage due to exaggerated inflammatory responses, cytokine storms, and other factors. In secondary HLH, a marked decrease in the cytotoxic function of lymphocytes and NK cells was observed, with excessive activation of the monocyte-phagocyte system being a characteristic feature[3]. Given the various triggering factors for HLH, recognizing presenting features and making a diagnosis can be challenging. The diagnostic criteria, including fever (≥ 38.5 °C), splenomegaly, cytopenia, ferritin level (> 500 ng/L), hypofibrinogenemia and/or hypertriglyceridemia, hemophagocytosis, low NK cell activity, and elevated soluble interleukin-2 receptor alpha level, lack sensitivity and specificity, especially in conditions such as lymphoma or sepsis[5,6]. Because the median survival period is < 2 months (1.8-2.2 months) without intervention, early treatment following accurate diagnosis is crucial. This patient was suspected to have secondary HLH owing to a confirmed preexisting disease at an advanced age. Genetic testing for accurate differential diagnosis was warranted, it is recommended to check genetic abnormalities, yet unavailable at our institution at the time.
RP, a rare autoimmune disease with an incidence rate of 3.5 cases per million per year, was identified as the trigger for HLH[7]. RP is characterized by inflammation of specific tissues and cartilage, affecting various areas including the ears, nose, airways, trachea, joints, and eyes, potentially spreading to involve the vasculitis, respiratory, and circulatory systems. It frequently occurs between 40 and 60 years old. The immune system attacks its own tissues, causing inflammation of the cartilage, primarily activated by T cells[7]. Excessive activation of CD4 + T cells, pivotal in immune regu
The treatment of HLH depends on symptom severity and the patient’s overall condition. Typically, it involves medications such as etoposide, cyclosporine, and dexamethasone. If treatment response is inadequate or relapse occurs, hematopoietic stem cell transplantation is considered. In cases where HLH is associated with autoimmune diseases, treatment is based on addressing the primary disease[8]. RP treatment typically involves the use of immunosuppressants or anti-inflammatory agents to manage symptoms. If symptoms are not severe, anti-inflammatory medications such as colchicine or dapsone may be used. However, in cases of severe symptoms such as airway involvement or hearing loss, high-dose steroid administration or immunosuppressive agents such as cyclophosphamide, cyclosporine, or azathioprine are necessary[9]. Tocilizumab (an IL-6 targeted agent), rituximab (targeting CD20-positive B cells), and infliximab are being explored as biologic agents for RP treatment, functioning as monoclonal antibodies and TNF-α inhibitors.
In summary, we reported the diagnosis and treatment of HLH caused by RP in a patient presenting with a fever of unknown origin. Treatment involved administering steroids for RP at a dose of 1 mg/kg, followed by azathioprine. After the initial treatment, RP was not aggravated, and HLH did not relapse. RP diagnosis, enabling treatment, was not based on diagnostic tests and rather on clinical suspicion, prioritizing the observation of suspected lesions. Physician-conducted physical examination linked the two rare conditions, facilitating prompt diagnosis and treatment.
| 1. | Kleynberg RL, Schiller GJ. Secondary hemophagocytic lymphohistiocytosis in adults: an update on diagnosis and therapy. Clin Adv Hematol Oncol. 2012;10:726-732. [PubMed] |
| 2. | Meeths M, Bryceson YT. Genetics and pathophysiology of haemophagocytic lymphohistiocytosis. Acta Paediatr. 2021;110:2903-2911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Oh EJ, Yoon JH, Park KH, Bae HJ, Yun SJ, Min GJ, Park SS, Park S, Lee SE, Cho BS, Eom KS, Kim YJ, Lee S, Kim HJ, Min CK, Cho SG, Han K, Lee JW. Natural-killer cell cytotoxicity as a diagnostic and prognostic marker for adult patients with secondary hemophagocytic lymphohistiocytosis: a prospective phase II observational study. Ther Adv Hematol. 2021;12:20406207211020544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Maciążek-Chyra B, Szmyrka M, Skoczyńska M, Sokolik R, Lasocka J, Wiland P. Relapsing polychondritis - analysis of symptoms and criteria. Reumatologia. 2019;57:8-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Shakoory B, Geerlinks A, Wilejto M, Kernan K, Hines M, Romano M, Piskin D, Ravelli A, Sinha R, Aletaha D, Allen C, Bassiri H, Behrens EM, Carcillo J, Carl L, Chatham W, Cohen JI, Cron RQ, Drewniak E, Grom AA, Henderson LA, Horne A, Jordan MB, Nichols KE, Schulert G, Vastert S, Demirkaya E, Goldbach-Mansky R, de Benedetti F, Marsh RA, Canna SW; HLH/MAS task force. The 2022 EULAR/ACR points to consider at the early stages of diagnosis and management of suspected haemophagocytic lymphohistiocytosis/macrophage activation syndrome (HLH/MAS). Ann Rheum Dis. 2023;82:1271-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 67] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 6. | Shah AR, Muzzafar T, Assi R, Schellingerhout D, Estrov Z, Tamamyan G, Kantarjian H, Daver N. Hemophagocytic lymphohistiocytosis in adults: An under recognized entity. BBA Clin. 2017;7:36-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Alsaid HM, Wahdan AAM, Tahboub IN, Almakadma NM. Hemophagocytic Lymphohistiocytosis and Relapsing Polychondritis with Acute Myelogenous Leukemia: Case Report and Review of the Literature. Am J Case Rep. 2020;21:e925287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 8. | Ali R, Mehannek R, Patel A, Paige A, Reddy S, Guma M, Guron G. Systemic Lupus Erythematosus With Hemophagocytic Lymphohistiocytosis: Is COVID-19 the Inciting Factor? Cureus. 2021;13:e19657. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |