Published online Mar 18, 2024. doi: 10.5312/wjo.v15.i3.204
Peer-review started: November 16, 2023
First decision: December 29, 2023
Revised: January 15, 2024
Accepted: February 1, 2024
Article in press: February 1, 2024
Published online: March 18, 2024
Processing time: 119 Days and 14.8 Hours
Bone regeneration is a critical area in regenerative medicine, particularly in orthopedics, demanding effective biomedical materials for treating bone defects. 45S5 bioactive glass (45S5 BG) is a promising material because of its osteoconductive and bioactive properties. As research in this field continues to advance, keeping up-to-date on the latest and most successful applications of this material is imperative. To achieve this, we conducted a comprehensive search on Pub
Core Tip: Regenerative medicine demands materials with effective osteoconductive and bioactive properties. Compared with other materials, 45S5 bioactive glass not only exhibits more biocompatibility but also enhances bone growth when combined with composites. Moreover, its antimicrobial properties offer many possibilities for future applications.
- Citation: Nogueira DMB, Rosso MPO, Buchaim DV, Zangrando MSR, Buchaim RL. Update on the use of 45S5 bioactive glass in the treatment of bone defects in regenerative medicine. World J Orthop 2024; 15(3): 204-214
- URL: https://www.wjgnet.com/2218-5836/full/v15/i3/204.htm
- DOI: https://dx.doi.org/10.5312/wjo.v15.i3.204
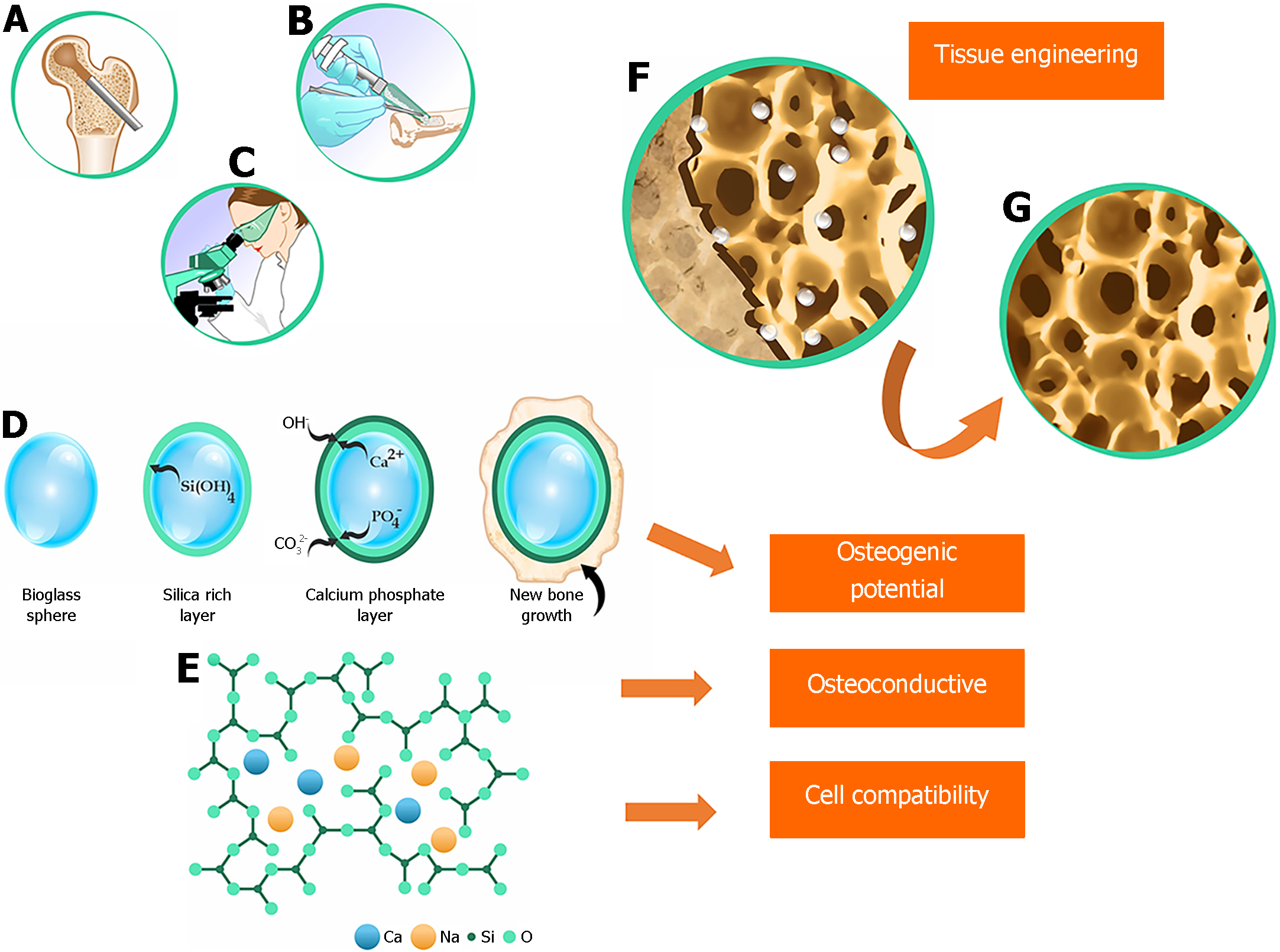
As human life expectancy increases, there is a corresponding rise in the prevalence of bone-related medical conditions, such as fractures, bone tumors, periodontal diseases, and degenerative cartilage disorders. These conditions can significantly affect individuals' daily activities, given the vital role bones play in providing mechanical support, facilitating hematopoiesis, and protecting internal organs. Bone regeneration is a complex biological process that involves a series of coordinated events to stimulate and regulate the formation of new bone. Considering the negative impact on the skeletal system, there is an increasing demand for tissue engineering approaches that specifically focus on promoting bone regeneration in humans[1-3].
Reconstructing critical bone defects resulting from trauma, accidents, and bone necrosis has historically posed a complex challenge for patients and surgeons worldwide. Although autologous bone grafts and allografts have shown potential in restoring lost structure and function, they face significant challenges such as size incompatibility, immunological rejection, donor shortage, extensive graft resorption, prolonged surgical time, and the risk of postoperative infection and pain. These challenges ultimately limit the application of autologous bone grafts and allografts. Accordingly, numerous studies have been conducted in recent decades to identify viable alternatives, resulting in the introduction of various substitute materials in the field of regenerative medicine. These materials are often made of metals such as aluminum, zirconium, and titanium, which are used in the manufacturing of prostheses, plates, pins, screws, and similar devices. However, these materials often lack the durability required for long-term human use, prompting the search for more enduring alternatives[4-7].
To overcome these challenges, the field of medical biomaterials has undergone substantial growth in recent years, offering innovative solutions to reduce fracture healing time and address other bone regeneration issues. Currently, biomaterials play a prominent role in promoting bone tissue regeneration in humans. Various synthetic materials have been developed, with bioactive glass (BG) ceramics emerging as a significant contributor. Categorized as second-generation biomaterials, BG interacts with the biological environment, enhancing tissue adhesion and progressively degrading as new tissue regenerates and heals, similar to hydroxyapatite[8].
We are currently in the era of third-generation biomaterials, which have the capability to trigger specific cellular responses at the molecular level. At the forefront of this field are bioactive glasses (BGs). These glasses consist of a group of calcium phosphate compounds that exhibit the remarkable capacity to rapidly form a strong bond with tissue, as exemplified by 45S5 bioactive glass (45S5 BG)[9-11].
In the late 1960s, researcher Larry L. Hench and his pioneering team at the University of Florida introduced 45S5 BG[12]. During their research, they made a remarkable discovery: this type of glass formed such a strong bond with bone that separation was impossible without causing a fracture. Subsequent in vivo studies showed that 45S5 BG exhibited osteoinductive and osteoconductive properties by forming carbonated hydroxyapatite (CHA) within the bone[13].
BGs are typically amorphous calcium-containing silicates that have osteoinductive capacity[14]. The most commonly used type of BG is 45S5 (Figure 1), composed of 45% SiO2, 24.5% CaO, 24.5% Na2O, and 6% P2O5[15,16]. This type of BG is osteoconductive, osteogenic, and biodegradable. Currently, BGs are produced using the sol-gel method, which uses a solvent at low temperatures. This method has the advantages of creating a porous and highly bioavailable structure and incorporating various additives to produce a range of glass-ceramics[15]. The unique combination of characteristics makes BG a potential substitute biomaterial because of its association with growth factors and biomolecules used in regenerative medicine[16,17].
BGs from various commercial brands have been successfully used, either alone or in combination with various metal ions, to reconstruct jawbone defects. BGs elicit a biocompatible response at the bone-tissue interface, thus enabling numerous medical applications[16]. Initially, the main goal of these materials was to enhance bone regeneration[14]. Applying a BG coating to a surface before it receives a metal prosthetic implant can provide stability by creating a bonding interface between the bioactive coating and the host tissue[18]. BG-coated surfaces can also protect the substrate (thus preventing corrosion) and even inhibit the release of potentially toxic metal ions[19].
More than 60 years after the discovery of BGs, the field of regenerative medicine continues to evolve, with many studies indicating vast opportunities for exploitation. BG shows remarkable efficacy in promoting bone regeneration, surpassing other bioactive ceramics. This particularity is related to BG's dissolution products, which act at the genetic level, stimulating the cells. This characteristic has fundamentally changed the way doctors, scientists, and regulatory agencies perceive the concept of bioactivity. 45S5 BG, a pioneer in this category, has only recently become widely used in orthopedics. To date, 45S5 BG has contributed to the bone regeneration of more than 1.5 million patients in the fields of orthopedics and dentistry[20,21].
45S5 BG was first used in medical practice to restore the bones of the middle ear and thus hearing. Subsequent research has advanced, presenting BG in the form of granules and modified compositions. This advancement enabled surgeons to manipulate it more precisely and customize it to meet the specific needs of each patient[20,22].
Under the name Perioglass®, 45S5 BG was initially used to treat jawbone defects. In 1999, 45S5 BG was launched as NovaBone® and used in clinical trials for the surgical treatment of adolescent idiopathic scoliosis. These trials demonstrated several advantages, including reduced infection and mechanical failure rates. Additionally, the use of 45S5 BG eliminates the need for a local donor. In 2015, the Food and Drug Administration (FDA) approved the term "osteostimulation" for 45S5 BG[23]. Another variation, BonAlive®, has received approval for use in orthopedic surgeries in more than 50 countries, being used for synthetic bone grafting in trauma, tumor removal, and the treatment of chronic osteomyelitis[20,24].
The application of 45S5 BG extends beyond orthopedics. Clinical and experimental tests of this biomaterial, molded with a borate glass structure, have successfully healed diabetic ulcers in humans that did not respond to conventional treatment[25]. Other potential applications of BG-containing composites include tissue engineering (e.g., heart, lung, and nerve tissues), intervertebral disc structures, antibacterial activity, and dressing materials. However, in vivo tests are required to confirm their effectiveness before they can be recommended for clinical trials[17].
Bone regeneration is a critical aspect of regenerative medicine, and research in this field continues to advance. Therefore, it is crucial to stay up-to-date on the latest developments in biomaterials. The applications of 45S5 BG in the treatment of bone defects have progressed significantly. Therefore, this editorial aims to provide a comprehensive and up-to-date analysis of its applications. It will serve as a valuable resource for professionals and researchers in the field of regenerative medicine, helping to guide their clinical decisions and identify areas for future research.
To develop this editorial and conduct a critical analysis, we searched the PubMed/MEDLINE database for articles published over the past 10 years using the search terms "bioglass 45S5 AND bone defect". The search returned 27 articles. We then analyzed the titles and abstracts to determine elegibility. Subsequently, we reviewed the articles to ascertain whether they met the eligibility criteria, which included application to both animals and humans, publication in English, full-text accessibility, and relevance to the topic. We included 15 articles as the basis for this editorial. Nine of these articles involved in vitro and in vivo experiments, five involved only in vivo experiments, and only one involved a human cohort study. Regarding the animal models used in the experiments, eight articles used rats, three used rabbits, two used mice, and one used sheep. Regarding the experimental region of interest, six articles used the calvaria, four used the femur, two used the tibia, and two involved graft implantation in subcutaneous pouches in the hind and forelimbs.
The articles included in this editorial are listed in Table 1[3,10,26-38], along with the elements categorized according to the PICO strategy (P: Patient or problem; I: Intervention; C: Control or comparison; O: Outcome). Table 1 also provides details on the reference, objective, study type, bioactive glass composition, methods, and outcome measures for each article.
| Ref. | Objective | Type of study | Composition | Methods | Outcome measures |
| Souza et al[26], 2020 | To compare the biocompatibility of a bioactive sodium calcium silicate glass containing 2.6 mol% Nb2O5 with that of the archetypal 45S5 BG | In vitro and in vivo | A variation of 45S5 BG in which 2.6 mol% of The glass was mixed with the precursor oxides SiO2 (99.5%), CaCO3 (99.95-100.5%), Na2CO3 (≥ 99.5%), | Biocompatibility and genotoxicity tests Bone regeneration: rat calvarial defect (5 mm). Seventy-two rats (sham group: no defect; control group: empty defect; 45S5 BG group: filled defect; BGPN2.6 group: filled defect), with 6 rats per group for 14, 28 and 56 d Qualitative and quantitative analysis of 3D micro-CT images | BGPN2.6 glass was not cytotoxic to BM-MSCs and had no mutagenic potential Micro-CT showed that BGPN2.6 almost completely regenerated a critical-sized calvarial defect within 8 wk, surpassing the performance of standard 45S5 BG. BGPN2.6 glass demonstrated more than 90% coverage compared to 66% for 45S5 BG |
| Souza et al[3], 2018 | To study the bioactive properties of Nb-substituted silicate glass derived from 45S5 B | In vitro and in vivo | Compositions (mol%): 45S5 BG (46.1 SiO2; 26.9 CaO; 24.4 Na2O; 2.6 BGPN2.6 (46.1 SiO2; 26.9 CaO; 24.4 Na2O; no BGPN1.3 (46.1 SiO2; 26.9 CaO; 24.4 Na2O; 1.3 High purity powders SiO2, Na2CO3, CaCO3, Glass particles between 38-53 µm in size | Compatibility and osteogenic differentiation of hESCs. Bone formation: rods composed of different glass types (BGPN1.3, BGPN2.6, and 45S5 BG) were implanted into bone defects (2 mm) in rat tibiae. Five animals per group were analyzed after 14 and 28 d | Nb-substituted BG is non-toxic to hESCs. There was a significant increase in osteogenic capacity and biocompatibility when up to 1.3 mol% Nb2O5 was added to 45S5 BG. The same increase in Nb2O5, replacing phosphorus, increased the osteostimulation of the BG |
| Thomas and Anbarasu[27], 2022 | To evaluate cell compatibility and regenerative potential of 45S5 BG graft in critical size defects (CSD) in rat calvaria | In vitro and in vivo | 45S5 BG: 45% SiO2; 24.5% Na2O; 24.5% CaO and 6% | In vitro cell viability assay of 45S5 BG using MTT assay with Novabone® and 10% DMSO as positive and negative controls, respectively, whereas cells alone served as the control Bone regeneration: 20 male rats with 6 mm diameter calvarial defects (control group: empty cavity) loaded with 2.5 mg of 45S5 BG (test group). Evaluation by CBCT after 4 and 8 wk | 45S5 BG achieved a cell viability rate of > 70%, confirming cell compatibility. CBCT analysis showed a significant increase in VGi and a reduction in ROI of CSD from the fourth to the eighth weeks, showing its potential for bone regeneration |
| Ma et al[28], 2017 | To evaluate a silicate-based composite bone cement (CSC) in a rabbit femur defect in terms of in vivo bone integration and biodegradability and compare the results with those of BG particulates and a calcium phosphate cement (CPC) | In vivo | CSC composition: tricalcium silicate (35%) and 45S5 BG (30%) with particles < 50 µm and 90-710 µm. The ratio of the two components was 1:2 (small:large); calcium sulfate (35%) | CSC cylinders molded with a 5 mm × 10 mm diameter, and CPC cylinders. Experiments conducted on 30 adult New Zealand white rabbits with femur defects. Control groups: BG particles and CPC. Analyses were conducted after 3, 6, and 12 months | The CSC underwent slower in vivo degradation compared with BG and CPC. The bone contact area at the interface between the surrounding bone and CSC gradually increased over time. CSC kept its structural integrity during in vivo implantation because of its acceptable mechanical strength |
| Esfahanizadeh et al[29], 2022 | To evaluate bone regeneration in critical defects of rabbit calvaria filled with magnesium- and strontium-doped BGs and compare it with standard 45S5 BG | In vivo | Standard 45S5 BG with particles of approximately 20-50 nm | Experiments on 12 male New Zealand rabbits allocated to 2 groups. Four lesions were created in each calvaria with a diameter of 8 mm spaced apart. Each lesion was filled with (1) strontium-doped BG, (2) magnesium-doped BG, (3) 45S5 BG (positive control), and (4) an empty lesion (negative control). Evaluation occurred at the end of 4 and 8 wk | At 4 wk, magnesium-doped BG showed the highest new bone formation with a mean of 11.66 ± 2.64, followed by strontium-doped BG with a mean of 11.10 ± 1.69 (P = 0.0001). At 8 wk, the highest amount of new bone was observed in the strontium-doped group with a mean of 28.22 ± 3.19, followed by the magnesium-doped group with a mean of 22.55 ± 3.43 |
| Lopes et al[10], 2020 | To evaluate the solubility, apatite-forming capacity, cytocompatibility, osteostimulation, and osteoinduction of Nb-containing bioactive glasses (BGNb) derived from the composition of 45S5 BG | In vitro and in vivo | Composition (mol%) of 45S5 BG and Nb-substituted 45S5 BG: 45S5 BG (46.1 SiO2; 26.9 CaO; 24.4 Na2O; 2.6 BGSN1 (45.1 SiO2; 26.9 CaO; 24.4 Na2O; 2.6 BGSN2.5 (43.6 SiO2; 26.9 CaO; 24.4 Na2O; 2.6 BGSN5 (41.1 SiO2; 26.9 CaO; 24.4 Na2O; 2.6 | In vitro: BMMSCs were isolated from the tibia and femur of adult Wistar rats. MTT assay was conducted for each of the BG compositions. Cells were cultured in complete DMEM (positive control), and cells were previously incubated in DMSO for 30 min (negative control) In vivo: glass rods (4 mm length × 2 mm diameter) composed of 45S5 BG (45S5 BG or BGSN1 groups were implanted into circular defects (2 mm diameter) in the tibia of rats (5 animals/group) Evaluated after 28 d | 45S5 BG and BGSN1 developed an apatite layer on their surfaces within 3 h. Glasses with higher concentrations of Nb2O5 (2.5 and 5 mol%) required at least 12 h Nb-substituted glasses were found to be compatible with BMMSCs. BGSN1 significantly enhanced cell proliferation after 4 d of treatment. Concentrations of 1 and 2.5 mol% Nb2O5 stimulated osteogenic differentiation of BMMSCs after 21 d of treatment |
| Fares et al[30], 2024 | To evaluate the impact of different materials for filling bone defects following anterior cruciate ligament (ACL) reconstruction surgery with bone-patellar tendon-bone (BPTB) graft | In humans | Osteopure® allograft from resected human femoral head treated by sterilization at 25 kGy Glassbone® BG, 100% synthetic, a mixture of 45% SiO2, 24.5% CaO, 25.5% Na2O, and 6% Collapat® II, a spongy bone graft composed of a collagen structure in which hydroxyapatite granules are dispersed | A prospective, monocentric cohort study was conducted with 102 adult athletes who underwent ACL reconstruction using the same arthroscopically-assisted BPTB, with a minimum follow-up of two years. Three groups based on the type of bone substitute GB group (G1): 45S5 BG ceramic Glassbone™ (n = 36; 35.29%); CP group (G2): collagen and hydroxyapatite bone void filler in sponge-shaped Collapat® II (n = 34; 33.33%); OP group (G3) treated human bone graft Osteopure® (n = 32; 31.37%). Patients were assessed based on their ability to kneel, the presence of donor site pain, and palpation of the defect | The percentage of Glassbone™ and Collapat® patients who kneeled comfortably was significantly higher than that of Osteopure® patients (77.78% and 76.5%, vs 65.6%, respectively) |
| Lu et al[31], 2018 | To investigate the remodeling of resorbable bone cements in a stringent model of mechanically loaded tibial plateau defects in sheep | In vivo | Melt-derived 45S5 BG with fast- and slow-resorbing ceramic mini-granules (CG, 85% β-tricalcium phosphate/15% hydroxyapatite) ground to 100-300 μm diameter and biphasic PEUR composites Nanocrystalline hydroxyapatite (nHA) The resulting composite bone grafts were denoted as CG/nHA-PEUR and BGCG/nHA-PEUR CG/nHA-PEUR cement contained 55wt% CG, 24.3 wt% nHA, and 20.7 wt% PEUR, whereas BGCG/nHA-PEUR cement contained 37.5 wt% BG, 22.5 wt% CG, 21.6 wt% nHA, and 18.4 wt% PEUR | Eight sheep, with two types of bone defects in each posterior limb. The defects included a non-weight-bearing femoral plug defect on the medial and lateral distal condyles of both femurs (n = 16 per group, two defects with a 6 mm diameter and a 16 mm depth) and a weight-bearing tibial plateau slot defect (n = 8 per group) approximately 50% of the total anterior to posterior tibial depth with 6 mm height. Each sheep received both grafts (BGCG/nHA-PEUR or CG/nHA-PEUR) in separate extremities, with graft placement alternating between animals. Micro-CT analysis was conducted in the immediate postoperative period, and at 4, 8, 12, and 16 wk | CG/nHA-PEUR cements mechanically stabilized the tibial plateau defects and remodeled to form new bone at 16 wk, with early weight-bearing. Cements containing BG particles were resorbed and showed fibrous tissue filling the defect. These findings represent the first report of a settable bone cement that remodels to form new bone while providing mechanical stability in a stringent large animal model of weight-bearing bone defects near a joint |
| Diba et al[32], 2019 | To investigate the feasibility of synthesizing novel hybrid particles by exploiting the strong interactions between alendronate and 45S5 BG | In vitro and in vivo | 45S5 BG: a mean particle size of 2.0 ± 1.2 μm. Alendronic acid (4-amino-1-hydroxybutane-1,1-diphosphonic acid) powder. 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES; ≥ 99.5%), and 2-(N-morpholino)ethanesulfonic acid hydrate (MES hydrate; ≥ 99.5%). Sodium hyaluronate powder (1.01-1.8 MDa) Injectable cohesive pastes: particles mixed with an aqueous solution of sodium hyaluronate (26 mg mL−1). A particle/solution ratio (g/mL) of 0.75. Final composition (wt%): HP1-7 (ALN 62.3 ± 0.6; Ca 11.4 ± 0.0; Na 12.8 ± 0.0; Si < 2; P < 1) HP2-7 (ALN 25.5 ± 9.8; Ca 16.7±0.3; Na 34.7 ± 0.0; Si 7.3 ± 0.3; P 9.9 ± 0.2) | A cylindrical defect (2.5 mm diameter and 5 mm depth) was created in the bilateral femoral condyle of osteoporotic male rats (n = 8 per experimental group) and filled with HP1-7 and HP2-7 hybrid particle pastes. Positive control: 45S5 BG | The hybrid particles released alendronate and inorganic elements (Ca, Na, Si, and P) in a controlled manner, exhibited a strong anti-osteoclastic activity in vitro, and stimulated the regeneration of osteoporotic bone in vivo |
| Prado Ferraz et al[33], 2017 | To evaluate the in vitro osteogenic and osteoinductive potentials of BioS-2P and its ability to promote in vivo bone repair | In vitro and in vivo | Biosilicate®: 23.75 Na2O; 23.75 CaO; 48.5 SiO2; 4 Composition (mol%): BioS-2P (23.3 Na2O; 25.8 CaO; 49.2 SiO2; 1.7 45S5 BG (24.4 Na2O; 26.9 CaO; 46.1 SiO2; 2.6 | BioS-2P and 45S5 BG were cut into 3 mm thick discs and ground with silicon carbide paper to a grit of 400 (~35 μm). MSCs were obtained from the femur of two male Wistar rats and cultured on both types of discs and on polystyrene (control group). CSDs with a 5 mm diameter were created in 15 male Wistar rats and implanted with scaffolds. Evaluation occurred at 4, 8, and 12 wk (n = 5 per period). BioS-2P scaffolds seeded with unlabeled MSCs were implanted into calvarial defects and evaluated 8 wk later | Extracellular matrix mineralization increased in cells cultured on BioS-2P compared with 45S5 BG (P = 0.029) |
| Zhang et al[34], 2017 | To compare the osteogenic capacity and effects of 45S5 BG scaffolds reinforced with ZnO/B2O3 (ZB), called BG-ZB, with pure 45S5 BG. | In vivo | BG-ZB: 30 SiO2; 28 CaO; 2 45S5/ZBx powders were homogeneously mixed with paraffin microspheres (porogen) of ~350 and ~500 μm diameter. BGs scaffolds manufactured with different porogens: 45S5/ZB0-350, 45S5/ZB4-350, and 45S5/ZB4-500 | Thirty-six adult male rabbits were randomly separated into three groups according to the scaffolds (45S5/ZB0-350, 45S5/ZB4-350, and 45S5/ZB4-500). Each animal underwent surgery for a CSD (Ø 6 × 10 mm) in the bilateral distal femur, with two different implants inserted into the right and left femurs | Open porosity decreased with the addition of 4% ZB, but the percentage of interconnected pores (> 50 μm) increased with increasing porogen size from 350 to 500 μm. Stronger scaffolds containing 4% ZB and 500 μm porogen were beneficial for osteogenic capacity. In contrast, both scaffolds with smaller pore sizes exhibited a low level of new bone growth (< 32%) after 6-12 wk of implantation |
| Westhauser et al[35], 2019 | To evaluate the effects of 0106-B1-BG and 45S5 BG on osteogenic differentiation, viability, and proliferation of MSCs in vitro and in vivo in severe combined immunodeficient (SCID) mice | In vitro and in vivo | Borosilicate glass (0106-B1-BG) (wt%): 37.5% SiO2, 22.6% CaO, 5.9% Na2O, 4% 45S5 BG (wt%): 45%SiO2, 24.5% CaO, 24.5% Na2O, 6% | Ten scaffolds per BG type were seeded with MSCs. Two scaffolds per BG type were implanted without MSCs as a control (total of 24 scaffolds). Four scaffolds were implanted per animal (female SCID mice), with two subcutaneous pockets created on the forelimbs and two on the hindlimbs Evaluation occurred after 10 wk | In vitro: both 45S5 BG and 0106-B1-BG were comparable in terms of MSC proliferation, viability, and osteogenic differentiation In vivo: 0106-B1-BG scaffolds were significantly superior to 45S5 BG in terms of osteoid quantity and maturation and angiogenic gene expression patterns |
| Jing et al[36], 2018 | To investigate the relationship between icariin-doped 45S5 BG seeded with ASCs and angiogenesis of rat EPCs, in rat calvarial bone defect | In vitro and in vivo | 45S5 BG (wt%): 45% SiO2, 24.5% Na2O, 24.5% CaO, and 6% Pure 45S5 BG scaffolds were used for comparison | A 8 mm diameter calvarial defect was created in the dorsal portion of the parietal bone in twenty male Sprague-Dawley rats, which were allocated into four groups: Group A (control, no implant), Group B (45S5 BG), Group C (45S5 BG/ASCs, 45S5 BG seeded with ASCs), and Group D (icariin/45S5 BG/ASCs, icariin/45S5 BG seeded with ASCs). Evaluation after 12 wk | Treatment with icariin was optimal in promoting VEGF secretion from ASCs, and it was hypothesized to promote angiogenesis of rat EPCs. This suggests a paracrine role for VEGF in mediating the interaction between icariin-induced ASCs and EPCs |
| Westhauser et al[37], 2016 | To evaluate the bone formation potential of three different types of hBMSC-seeded polymer-coated 45S5 BG scaffolds in 3D using standardized protocols | In vitro and in vivo | Three types of 3D-polymer coated 45S5 BG scaffolds: Group A - scaffold coated in 5% w/v gelatin solution, (50 °C). Group B - scaffold coated in 5% w/v cross-linked gelatin-genipin (99:1) solution (50 ºC) Group C - scaffold coated in 5% w/v PHBV solution (room temperature) | Each group (A-C) had four identical scaffolds differing only in the type of polymer coating. Scaffolds had a nominal size of 5 × 5 × 5 mm and were implanted subcutaneously on the back above the upper and lower extremities of three female SCID mice. Evaluated 8 wk after surgery. hBMSCs from human bone marrow aspirate were seeded onto each scaffold | All groups exhibited bone formation and good infiltration of connective tissue cells, as well as a dense vascularization network. A-group showed a greater amount of bone. C-group, and especially B-group, exhibited a high dissolution. Both B- and C-groups showed more singular bone formation with no signs of interconnectivity |
| Moreira et al[38], 2018 | To evaluate the effect of low-intensity laser therapy (LLLT) on the healing of bone defects filled with autogenous bone or 45S5 BG | In vivo | 45S5 BG Biogran® Biomet 3i | A 5 mm diameter CSD was created on the calvaria of sixty adult male rats were divided into six groups (n = 10): group C (control, blood clot); group LLLT (LLLT-GaAlAs, wavelength of 780 nm, power of 100mW, energy density of 210 J/cm2 per point for 60 seconds/point, in five points, only once, after creation of the surgical defect); group AB (autogenous bone); group AB+LLLT (autogenous bone + LLLT); group BG (45S5 BG); group BG+LLLT (45S5 BG + LLLT). Evaluation after 30 d | The highest ANFB was recorded in the LLLT group (47.67% ± 8.66%), followed by the AB+LLLT (30.98% ± 16.59%) and BG+LLLT (31.13% ± 16.98%) groups. There was a statistically significant difference in ANFB values between group C and the other groups, except for the BG group (P > 0.05). There was no statistically significant difference in ANFB values between group AB and the other groups, between group AB+LLLT and groups BG and BG+LLLT, and between groups BG and BG+LLLT. The highest area of remaining particles was found in the BG group (25.15% ± 4.82%), followed by the BG+LLLT group (17.06% ± 9.01%), and there was no significant difference between the groups |
Most studies conducted in the last decade were designed to compare 45S5 BG with other cements or scaffolds, such as: (1) BGPN2.6; (2) Nb-substituted 45S5 BG; (3) Empty cavity; (4) CPC; (5) Magnesium- and strontium-doped BG; (6) BGNb; (7) A glass derived from the composition of 45S5 BG, Collapat® II, and Osteopure®; (8) Slow-resorbing ceramic granules, biphasic compounds of PEUR, and nHA; (9) Biosilicate® BioS-2P; (10) 45S5 BG scaffolds reinforced with BG-ZB; (11) Borosilicate glass 0106-B1; (12) Icariin-doped 45S5 BG seeded with ASCs; (13) 3D polymer-coated 45S5 BG scaffolds: gelatin-coated, cross-linked gelatin-coated, or PHBV-coated; and (14) LLLT in autogenous grafts.
We observed variation in the concentration of 45S5 BG in the reviewed studies. Research in regenerative medicine highlights the importance of determining and applying optimal concentrations[17]. This is essential to confirm the vascularization process in composites made of this biodegradable polymer, indicating a potential area for future research.
Only one study has assessed the interaction between alendronate and 45S5 BG[32]. As described in that study, the hybrid particles released alendronate and inorganic elements (Ca, Na, Si, and P) in a controlled manner. This controlled release exhibited a strong anti-osteoclastic effect in vitro and stimulated the regeneration of the osteoporotic femur in Wistar rats.
Only one study, Fares et al[30], investigated the outcomes in patients who underwent ACL reconstruction with patellar tendon autograft and various materials. Patients who received Glassbone® or Collapat® II grafts reported experiencing less pain and greater kneeling comfort than those who received the Osteopure® graft. No significant differences were observed among the three groups in knee function scores (International Knee Documentation Committee - IKDC and Lysholm) and anterior knee pain. The authors reported no wound healing complications. At the end of the 2-year follow-up, the type of material used had no effect on functionality.
Souza et al[26] compared the performance of BGPN2.6 with that of 45S5 BG for repairing CSD calvarial defects in rats. They comprehensively assessed biocompatibility, cell adhesion, and osteoblast cell proliferation in the presence of BGPN2.6. In animal models, micro-CT scans revealed that the application of BGPN2.6 almost completely regenerated the CSD within 8 wk, achieving over 90% coverage. In comparison, standard 45S5 BG achieved only 66% coverage. These results clearly demonstrate that Nb-containing BG is a safe and effective biomaterial for bone replacement in the treatment of CSD, with significant implications for regenerative medicine and orthopedics. This research provides encouraging evidence for the applicability of 45S5 BG in the treatment of CSD.
Continuing their research on the addition of Nb to 45S5 BG, Souza et al[3] tested rods made of different types of glass (BGPN1.3, BGPN2.6, and 45S5 BG) in rat tibiae. Their findings made important contributions, such as demonstrating the non-toxicity of Nb to hESCs and a significant increase in osteogenic capacity when adding up to 1.3 mol% of Nb2O5 to 45S5 BG. The substitution of an equivalent amount of Nb2O5 for phosphorus enhanced the osteostimulation of 45S5 BG.
The use of Nb combined with 45S5 BG has attracted the interest of researchers. Lopes et al[10] demonstrated that 45S5 BG with Nb at concentrations of 1 and 2.5 mol% stimulated osteogenic differentiation of BMMSCs after 21 d of treatment. BGNb is osteoconductive and osteostimulative. These results indicate that the bioglass (BGNb) is suitable for biomedical applications.
In another experiment investigating the effects of adding components to 45S5 BG, Esfahanizadeh et al[29] compared the elements strontium and magnesium to standard 45S5 BG. At 4 wk, the group treated with magnesium-doped 45S5 BG showed greater bone formation. At 8 wk, the group treated with strontium-doped 45S5 BG showed better results. The addition of strontium and magnesium into the composition of 45S5 BG improved bone regeneration compared to standard 45S5 BG. It should be noted that the rate of bone regeneration was higher than that of 45S5 BG, but without statistically significant differences. This finding may be attributed to the effect of magnesium and strontium ions in inhibiting osteoclastic activity, as well as the inherent ability of 45S5 BG to enhance angiogenesis and stimulate the secretion of growth and osteogenic factors.
Thomas; Anbarasu[27], who also focused on CSD in rat calvaria, demonstrate the growing research interest in this type of injury. They found that 45S5 BG achieved a cell viability rate of over 70%, confirming its cell compatibility. Furthermore, CBCT revealed a significant increase in VGi (P < 0.001) and a reduction in ROI (P < 0.001) from the fourth to the eighth week, indicating the potential of 45S5 BG for bone regeneration in CSD.
Zhang et al[34] addressed CSD in rabbit femurs and found that the strongest scaffolds, containing 4% low-melting ZB in 45S5 BG and 500 μm pores, were particularly beneficial for osteogenic capacity. This was accompanied by accelerated bone growth (6-18 wk), with the material itself showing mild resorption. In contrast, scaffolds with smaller pore sizes showed lower bone growth (< 32% after 6-12 wk). These results suggest a promising application of 45S5 BG in clinical settings, particularly in mechanically loaded bone defects.
Regenerative medicine depends on ongoing advancements to improve its principles and applications, including the development of complementary approaches to address bone defects. One example is the use of LLLT in bone lesions, which has shown promising results[39-41]. In this editorial, we highlight the study by Moreira et al[38], who used LLLT to heal CSDs filled with a blood clot, autogenous bone, or 45S5 BG. With the protocol used, LLLT did not increase ANFB when associated with autogenous bone or 45S5 BG. This underscores the need for further research and improvement of complementary methods until a consensus is reached.
The use of scaffolds, cements, and compounds (whether synthetic, natural, or in 3D formation) has been the focus of research aimed at developing artifacts to assist in surgical procedures for bone defects. Ma et al[28] evaluated the results of a CSC composed of 35% tricalcium silicate, 30% 45S5 BG (particulates with two sizes), and 35% calcium sulfate. They observed that this composite is suitable for clinical applications because it is a self-setting material. The authors observed that the addition of 45S5 BG increased mechanical strength and the ability to induce apatite formation. This outcome was expected, given the well-known properties of 45S5 BG. According to their results, there is evidence of in vivo efficacy and potential for clinical applications of silicate-based composite bone cements.
Two studies investigated the subcutaneous insertion of scaffolds. These studies caught our attention because they diverged from typical biomaterial research in bone regenerative medicine. Westhauser et al[35] implanted subcutaneous scaffolds in rats to observe the behavior of 45S5 BG (as described in Table 1). Interestingly, the authors found that both 45S5 BG and 0106-B1-BG had similar effects on the proliferation, viability, and osteogenic differentiation of MSCs. However, 0106-B1-BG outperformed 45S5 BG in terms of osteoid quantity and maturation, as well as angiogenic gene expression patterns. In another study, Westhauser et al[37] implanted subcutaneous 45S5 BG scaffolds coated with gelatin, cross-linked gelatin, and PHBV after seeding with hMSC in SCID mice. They observed bone neoformation in all groups and suggested that the gelatin coating on these implants was more stable than on group A (Table 1). This lack of stability hinders the effective interaction of the 45S5 BG surface with the surrounding tissues, thereby interfering with the formation of new tissue. Bone neoformation plays a stabilizing role for the implant. If bone neoformation is insufficient, mechanical integrity will not improve, resulting in reduced bone formation and increased mechanical destruction. Westhauser et al[37] then proposed conducting mechanical tests on the scaffolds to test their hypothesis linking structural deficit to reduced bone formation. Alternatively, it is likely that scaffolds with pores larger than 500 µm in diameter do not induce bone formation[42].
45S5 BG is a bioactive (osteoconductive) and versatile biomaterial capable of inducing bone growth in animal soft tissues. The findings of Yuan et al[43] warrant further research on the osteoinductivity of 45S5 BG, its osteoinduction mechanism, and the relationship between osteoinduction and osteoconduction.
Xynos et al[44] demonstrated the activation of genes involved in osteoblast metabolism and bone homeostasis. This was achieved through a specific transcriptional program activated in human osteoblasts after treatment with ionic products derived from the dissolution of 45S5 BG. These genes have multiple functions, including the induction of osteoblast proliferation, as exemplified by the RCL gene, which acts as a growth promoter. Moreover, these genes are involved in the remodeling of the extracellular matrix (such as metalloproteinases), play specialized functions (such as CD44), and facilitate cellular interactions, both between cells and with the extracellular matrix.
In regenerative therapy, the ability of scaffolds to be colonized by osteoblasts is extremely important, as a 45S5 BG substrate can serve as a model for previously modified tissues in bioengineering. As shown by Xynos et al[45], 45S5 BG stimulated the growth and osteogenic differentiation of primary human osteoblasts. Prado Ferraz et al[33] evaluated BioS-2P and 45S5 BG cylinders and found a similar cell growth pattern in both materials. Another interesting finding was that the BioS-2P scaffold stimulated bone formation to such an extent that its combination with MSCs could not enhance it further. In another study, rat calvarial osteoblasts cultured on BioS-1P and 45S5 BG showed identical proliferation rates[46]. These findings strongly suggest that the presence of one or two crystalline phases does not affect the ability of Biosilicate® to sustain cell adhesion and proliferation. Granito et al[47] demonstrated osteogenic activity in 45S5 BG and Biosilicate® but found no significant difference in morphometry between them, suggesting the need for further research. In both cases, growth dynamics accompanied the growth of 45S5 BG.
Icariin seeded with ASCs is another element added to 45S5 BG to treat calvarial defects. A study[36] showed that this combination significantly improved neobone formation, while also displaying excellent osteogenic and angiogenic properties. This emphasizes the potential of this combination as a viable option for regenerating large bone defects.
In orthopedic regenerative medicine, the repair of tibial plateau fractures often requires extensive mechanical fixation and protected weight-bearing for 10 wk. This is because the lack of stability of existing grafts. For bone lesions near joints, the use of a biomaterial that hardens rapidly after implantation can stabilize the fracture with minimal use of rigid implants. Moreover, this biomaterial must stimulate the neobone formation and undergo remodelling at a rate that maintains bone integrity. Developing biomaterials that provide mechanical stability for fractures while facilitating bone remodeling remains a significant challenge in bone tissue engineering.
Lu et al[31] demonstrated that CG/nHA-PEUR grafts and BGCG/nHA-PEUR grafts with ceramic granules improved handling properties by reducing polymer tackiness. Both groups hardened within 20 s, resulting in a rigid cement that could not be manually compressed. We highlight the innovations of this research: the development of the first settable bone cement that not only offers mechanical stability but also remodels to form new bone in a large, stringent animal model, particularly for bone defects near a joint. In animals that tolerated the first few weeks of early loading, the CG/nHA-PEUR cements demonstrated effective mechanical stabilization of tibial plateau defects and underwent remodeling to form new bone within 16 wk. In contrast, cements containing 45S5 BG particles were resorbed and filled the defect with fibrous tissue. Additionally, CG/nHA-PEUR cements remodeled at a significantly faster rate at the full weight-bearing tibial plateau site compared to the femoral condyle site, which was mechanically protected in the same animal. These findings, along with mechanical tests, suggest that incorporating 45S5 BG into composites renders the material more brittle.
BG materials and composites may be applicable in load-bearing orthopedic injuries. Wheeler et al[48] observed that 45S5 BG had greater shear strength, greater bone growth, no decrease in trabecular bone thickness over time, and maintenance of mechanical integrity.
This editorial aimed to provide a comprehensive and up-to-date analysis of the applications of 45S5 BG in regenerative medicine. We have reviewed a diverse range of applications in scientific research. Below, we summarize the main findings and observations from these studies.
Several studies have compared 45S5 BG with other biomaterials. The addition of niobium and other elements generally improves osteogenesis and biocompatibility of materials. These observations demonstrate the safety and efficacy of 45S5 BG as a bone substitute for the treatment of severe defects. However, results have varied over time, suggesting that the choice of these elements may depend on the specific needs of the application. Scaffolds and cements have demonstrated potential in clinical applications because of their rapid hardening ability and their ability to induce the formation of apatite deposits. The incorporation of LLLT, subcutaneous scaffold inserts, and mechanical stabilization of fractures highlights the importance of further research to improve complementary methods in bone regenerative medicine. In summary, these studies suggest that 45S5 BG and related materials have great potential in regenerative medicine and the treatment of bone defects. Modifications and combinations of these materials may optimize bone regeneration in various clinical applications.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Dentistry, oral surgery and medicine
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Watanabe T, Japan S-Editor: Gong ZM L-Editor: A P-Editor: Li X
| 1. | Shahgholi M, Oliviero S, Baino F, Vitale-Brovarone C, Gastaldi D, Vena P. Mechanical characterization of glass-ceramic scaffolds at multiple characteristic lengths through nanoindentation. J Eur Ceram Soc. 2016;36:2403-2409. [DOI] [Full Text] |
| 2. | Gao C, Peng S, Feng P, Shuai C. Bone biomaterials and interactions with stem cells. Bone Res. 2017;5:17059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 502] [Cited by in RCA: 383] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 3. | Souza L, Lopes JH, Encarnação D, Mazali IO, Martin RA, Camilli JA, Bertran CA. Comprehensive in vitro and in vivo studies of novel melt-derived Nb-substituted 45S5 bioglass reveal its enhanced bioactive properties for bone healing. Sci Rep. 2018;8:12808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Midha S, Kim TB, van den Bergh W, Lee PD, Jones JR, Mitchell CA. Preconditioned 70S30C bioactive glass foams promote osteogenesis in vivo. Acta Biomater. 2013;9:9169-9182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Wang X, Lu Z, Jia L, Chen J. Preparation of porous titanium materials by powder sintering process and use of space holder technique. J Iron Steel Res Int. 2017;24: 97-102. [DOI] [Full Text] |
| 6. | Zhang J, Tong D, Song H, Ruan R, Sun Y, Lin Y, Wang J, Hou L, Dai J, Ding J, Yang H. Osteoimmunity-Regulating Biomimetically Hierarchical Scaffold for Augmented Bone Regeneration. Adv Mater. 2022;34:e2202044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 136] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 7. | Qi J, Wang Y, Chen L, Wen F, Huang L, Rueben P, Zhang C, Li H. 3D-printed porous functional composite scaffolds with polydopamine decoration for bone regeneration. Regen Biomater. 2023;10:rbad062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 8. | Barrère F, van der Valk CM, Meijer G, Dalmeijer RA, de Groot K, Layrolle P. Osteointegration of biomimetic apatite coating applied onto dense and porous metal implants in femurs of goats. J Biomed Mater Res B Appl Biomater. 2003;67:655-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 146] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | Hench LL. Bioactive glasses: Fundamentals, technology and applications. In: Handbook of Bioceramics and Biocomposites. Springer, 2015: 41-62. [DOI] [Full Text] |
| 10. | Lopes JH, Souza LP, Domingues JA, Ferreira FV, de Alencar Hausen M, Camilli JA, Martin RA, de Rezende Duek EA, Mazali IO, Bertran CA. In vitro and in vivo osteogenic potential of niobium-doped 45S5 bioactive glass: A comparative study. J Biomed Mater Res B Appl Biomater. 2020;108:1372-1387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Hench LL. The story of Bioglass. J Mater Sci Mater Med. 2006;17:967-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1545] [Cited by in RCA: 1032] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 12. | Hench LL, Splinter RJ, Allen WC, Greenlee TK. Bonding mechanisms at the interface of ceramic prosthetic materials. J Biomed Mater Res. 1969;3: 117-141. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2160] [Cited by in RCA: 2169] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 13. | Hench LL, Wilson J. An introduction to bioceramics. In: World Scientific. 1986. [DOI] [Full Text] |
| 14. | Jones JR. Reprint of: Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2015;23 Suppl:S53-S82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 274] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 15. | Baino F, Fiorilli S, Vitale-Brovarone C, Verné E. Bioactive glasses: Special applications outside the skeletal system. J Non Cryst Solids. 2016;432: 15-30. [DOI] [Full Text] |
| 16. | Day RM, Boccaccini AR. Effect of particulate bioactive glasses on human macrophages and monocytes in vitro. J Biomed Mater Res A. 2005;73:73-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Rizwan M, Hamdi M, Basirun WJ. Bioglass® 45S5-based composites for bone tissue engineering and functional applications. J Biomed Mater Res A. 2017;105:3197-3223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 18. | Jones JR. Review of bioactive glass: from Hench to hybrids. Acta Biomater. 2013;9:4457-4486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1488] [Cited by in RCA: 1079] [Article Influence: 89.9] [Reference Citation Analysis (0)] |
| 19. | Baino F, Verné E. Glass-based coatings on biomedical implants: a state-of-the-art review. Biomed Glas. 2017;3: 1-17. [RCA] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 20. | Jones JR, Brauer DS, Hupa L, Greenspan DC. Bioglass and Bioactive Glasses and Their Impact on Healthcare. Int J Appl Glas Sci. 2016;7: 423-434. [DOI] [Full Text] |
| 21. | Hench LL. Bioactive Materials for Gene Control. In: New Materials and Technologies for Healthcare. Imperial College Press, 2011: 25-48. [DOI] [Full Text] |
| 22. | Rust KR, Singleton GT, Wilson J, Antonelli PJ. Bioglass middle ear prosthesis: long-term results. Am J Otol. 1996;17:371-374. [PubMed] |
| 23. | Ilharreborde B, Morel E, Fitoussi F, Presedo A, Souchet P, Penneçot GF, Mazda K. Bioactive glass as a bone substitute for spinal fusion in adolescent idiopathic scoliosis: a comparative study with iliac crest autograft. J Pediatr Orthop. 2008;28:347-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Lindfors NC, Koski I, Heikkilä JT, Mattila K, Aho AJ. A prospective randomized 14-year follow-up study of bioactive glass and autogenous bone as bone graft substitutes in benign bone tumors. J Biomed Mater Res B Appl Biomater. 2010;94:157-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Jung S, Day DE, Day T, Stoecker W, Taylor P. Treatment of non-healing diabetic venous stasis ulcers with bioactive glass nanofibers. Wound Repair Regen. 2011;19:A30-A30. |
| 26. | de Souza LPL, Lopes JH, Ferreira FV, Martin RA, Bertran CA, Camilli JA. Evaluation of effectiveness of 45S5 bioglass doped with niobium for repairing critical-sized bone defect in in vitro and in vivo models. J Biomed Mater Res A. 2020;108:446-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | Thomas NG, Anbarasu A. Cone-Beam Computed Tomography-Assisted Evaluation of the Bone Regenerative Potential of Modulated Sol-Gel-Synthesized 45S5 Bioglass Intended for Alveolar Bone Regeneration. J Pharm Bioallied Sci. 2022;14:S123-S126. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 28. | Ma B, Huan Z, Xu C, Ma N, Zhu H, Zhong J, Chang J. Preparation and in vivo evaluation of a silicate-based composite bone cement. J Biomater Appl. 2017;32:257-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Esfahanizadeh N, Montazeri M, Nourani MR, Harandi M. Use of bioactive glass doped with magnesium or strontium for bone regeneration: A rabbit critical-size calvarial defects study. Dent Res J (Isfahan). 2022;19:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 30. | Fares A, Hardy A, Bohu Y, Meyer A, Karam K, Lefevre N. The impact of bone graft type used to fill bone defects in patients undergoing ACL reconstruction with bone-patellar tendon-bone (BPTB) autograft on kneeling, anterior knee pain and knee functional outcomes. Eur J Orthop Surg Traumatol. 2024;34:181-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 31. | Lu S, McGough MAP, Shiels SM, Zienkiewicz KJ, Merkel AR, Vanderburgh JP, Nyman JS, Sterling JA, Tennent DJ, Wenke JC, Guelcher SA. Settable polymer/ceramic composite bone grafts stabilize weight-bearing tibial plateau slot defects and integrate with host bone in an ovine model. Biomaterials. 2018;179:29-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 32. | Diba M, Camargo WA, Zinkevich T, Grünewald A, Detsch R, Kabiri Y, Kentgens APM, Boccaccini AR, van den Beucken JJJP, Leeuwenburgh SCG. Hybrid particles derived from alendronate and bioactive glass for treatment of osteoporotic bone defects. J Mater Chem B. 2019;7:796-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Prado Ferraz E, Pereira Freitas G, Camuri Crovace M, Peitl O, Dutra Zanotto E, de Oliveira PT, Mateus Beloti M, Luiz Rosa A. Bioactive-glass ceramic with two crystalline phases (BioS-2P) for bone tissue engineering. Biomed Mater. 2017;12:045018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Zhang L, Ke X, Lin L, Xiao J, Yang X, Wang J, Yang G, Xu S, Gou Z, Shi Z. Systematic evaluation of the osteogenic capacity of low-melting bioactive glass-reinforced 45S5 Bioglass porous scaffolds in rabbit femoral defects. Biomed Mater. 2017;12:035010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Westhauser F, Widholz B, Nawaz Q, Tsitlakidis S, Hagmann S, Moghaddam A, Boccaccini AR. Favorable angiogenic properties of the borosilicate bioactive glass 0106-B1 result in enhanced in vivo osteoid formation compared to 45S5 Bioglass. Biomater Sci. 2019;7:5161-5176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 36. | Jing X, Yin W, Tian H, Chen M, Yao X, Zhu W, Guo F, Ye Y. Icariin doped bioactive glasses seeded with rat adipose-derived stem cells to promote bone repair via enhanced osteogenic and angiogenic activities. Life Sci. 2018;202:52-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 37. | Westhauser F, Weis C, Prokscha M, Bittrich LA, Li W, Xiao K, Kneser U, Kauczor HU, Schmidmaier G, Boccaccini AR, Moghaddam A. Three-dimensional polymer coated 45S5-type bioactive glass scaffolds seeded with human mesenchymal stem cells show bone formation in vivo. J Mater Sci Mater Med. 2016;27:119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 38. | Moreira GS, Machado Alves PH, Esper LA, Sbrana MC, da Silva Dalben G, Neppelenbroek KH, Fraga de Almeida ALP. Effect of Low-Level Laser on the Healing of Bone Defects Filled with Autogenous Bone or Bioactive Glass: In Vivo Study. Int J Oral Maxillofac Implants. 2018;33:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 39. | Rosso MPO, Oyadomari AT, Pomini KT, Della Coletta BB, Shindo JVTC, Ferreira Júnior RS, Barraviera B, Cassaro CV, Buchaim DV, Teixeira DB, Barbalho SM, Alcalde MP, Duarte MAH, Andreo JC, Buchaim RL. Photobiomodulation Therapy Associated with Heterologous Fibrin Biopolymer and Bovine Bone Matrix Helps to Reconstruct Long Bones. Biomolecules. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 40. | Rosso MPO, Buchaim DV, Pomini KT, Coletta BBD, Reis CHB, Pilon JPG, Duarte Júnior G, Buchaim RL. Photobiomodulation Therapy (PBMT) Applied in Bone Reconstructive Surgery Using Bovine Bone Grafts: A Systematic Review. Materials (Basel). 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 41. | Della Coletta BB, Jacob TB, Moreira LAC, Pomini KT, Buchaim DV, Eleutério RG, Pereira ESBM, Roque DD, Rosso MPO, Shindo JVTC, Duarte MAH, Alcalde MP, Júnior RSF, Barraviera B, Dias JA, Andreo JC, Buchaim RL. Photobiomodulation Therapy on the Guided Bone Regeneration Process in Defects Filled by Biphasic Calcium Phosphate Associated with Fibrin Biopolymer. Molecules. 2021;26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 42. | Chang B, Song W, Han T, Yan J, Li F, Zhao L, Kou H, Zhang Y. Influence of pore size of porous titanium fabricated by vacuum diffusion bonding of titanium meshes on cell penetration and bone ingrowth. Acta Biomater. 2016;33:311-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 43. | Yuan H, de Bruijn JD, Zhang X, van Blitterswijk CA, de Groot K. Bone induction by porous glass ceramic made from Bioglass (45S5). J Biomed Mater Res. 2001;58:270-276. [PubMed] [DOI] [Full Text] |
| 44. | Xynos ID, Edgar AJ, Buttery LD, Hench LL, Polak JM. Gene-expression profiling of human osteoblasts following treatment with the ionic products of Bioglass 45S5 dissolution. J Biomed Mater Res. 2001;55:151-157. [PubMed] [DOI] [Full Text] |
| 45. | Xynos ID, Hukkanen MV, Batten JJ, Buttery LD, Hench LL, Polak JM. Bioglass 45S5 stimulates osteoblast turnover and enhances bone formation In vitro: implications and applications for bone tissue engineering. Calcif Tissue Int. 2000;67:321-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 560] [Cited by in RCA: 413] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 46. | Moura J, Teixeira LN, Ravagnani C, Peitl O, Zanotto ED, Beloti MM, Panzeri H, Rosa AL, de Oliveira PT. In vitro osteogenesis on a highly bioactive glass-ceramic (Biosilicate). J Biomed Mater Res A. 2007;82:545-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 47. | Granito RN, Ribeiro DA, Rennó AC, Ravagnani C, Bossini PS, Peitl-Filho O, Zanotto ED, Parizotto NA, Oishi J. Effects of biosilicate and bioglass 45S5 on tibial bone consolidation on rats: a biomechanical and a histological study. J Mater Sci Mater Med. 2009;20:2521-2526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 48. | Wheeler DL, Montfort MJ, McLoughlin SW. Differential healing response of bone adjacent to porous implants coated with hydroxyapatite and 45S5 bioactive glass. J Biomed Mater Res. 2001;55:603-612. [PubMed] [DOI] [Full Text] |