INTRODUCTION
Mycobacterium houstonense (M. houstonense) belongs to the nontuberculous mycobacterium group. Infection caused by M. houstonense is prone to recurrence. In clinical practice, the pathogen of infectious osteomyelitis is mainly Staphylococcus aureus, and osteomyelitis caused by M. houstonense is extremely rare and easily overlooked by clinical physicians. There are few reports on cases of osteomyelitis caused by M. houstonense. Once it enters bone tissue, M. houstonense will multiply in it, causing inflammation and bone destruction, ultimately leading to bone infection. In addition, some other factors may also increase the risk of infection, such as weakened immune system, long-term use of antibiotics, diabetes, rheumatoid arthritis, and other chronic diseases. The transmission routes of M. houstonense bone infection mainly include contact transmission and blood transmission. Contact transmission refers to the direct or indirect contact with infected parts or pollutants of M. houstonense, such as contaminated instruments or hands during surgery, injection, and trauma. Blood transmission refers to the infection of bone tissue by M. houstonense through blood, such as bacteria reaching and colonizing bone tissue through blood flow, causing infection. In addition, M. houstonense can also spread through natural environments such as water sources and soil; however, this pathway is relatively rare. M. houstonense bone infection is a serious disease that requires timely diagnosis and treatment. M. houstonense is a relatively rare pathogen. Osteomyelitis induced by M. houstonense usually has an insidious onset. In the early stage, patients may only show mild pain, swelling, or local discomfort. These symptoms are often easily overlooked or misdiagnosed as other common bone problems. As the disease progresses, the pain gradually intensifies and may affect the patient's activity ability and even lead to limb dysfunction. The diagnosis of this type of osteomyelitis is relatively difficult. Traditional diagnostic methods such as blood tests and X-rays may not be able to detect the presence of M. houstonense in a timely and accurate manner. At this time, advanced molecular biology detection technologies, such as next-generation sequencing (NGS), may help improve the accuracy of diagnosis. Here, we report a case of osteomyelitis diagnosed by metagenomic NGS (mNGS).
CASE PRESENTATION
Chief complaints
Six months of fluid oozing from a surgical wound on the left lower limb with no obvious cause.
History of present illness
She denied a history of other diseases such as hepatitis B, tuberculosis, or tumors. At the same time, she also denied a history of blood transfusion and allergy.
History of past illness
She denied a travel history to places affected by an epidemic or areas with a high incidence of infectious diseases.
Personal and family history
All members of her family are in good health and have no history of tuberculosis or other related diseases.
Physical examination
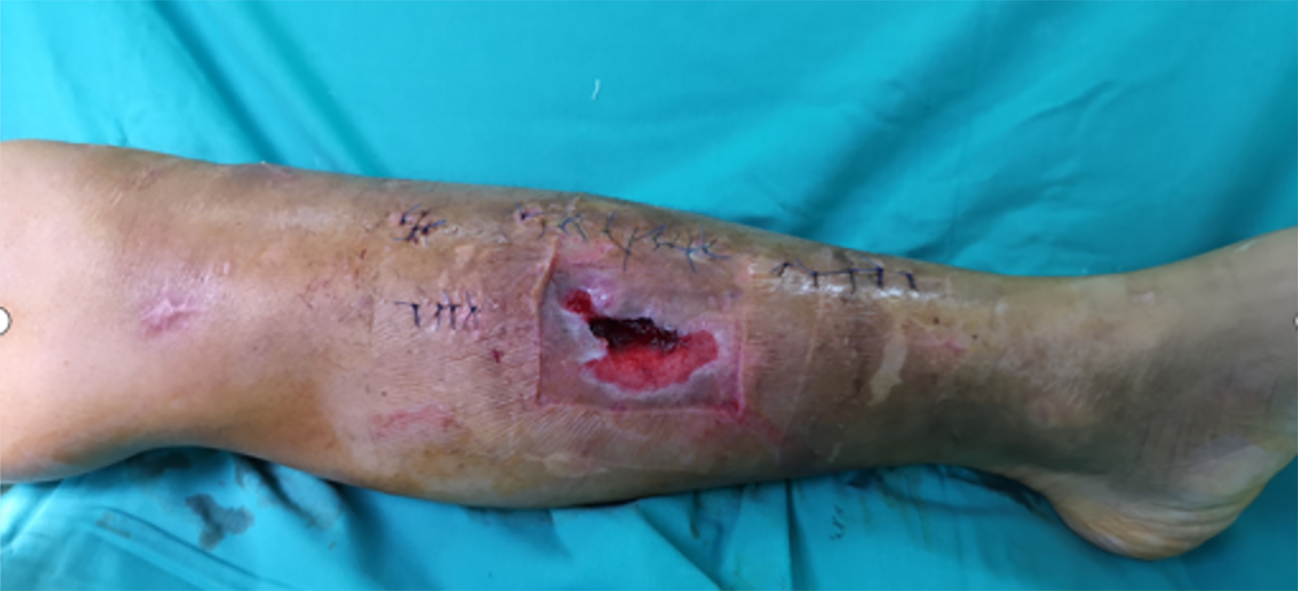
Admission examination revealed skin lesions on the left lower limb near the knee end (Figure 1).
Figure 1 Soft tissue lesions.
The wound defect was dark red, with mild redness and swelling of the surrounding skin. Local skin defects have irregular shapes with uneven edges. The wound defect was about 3 centimeters long, 2 centimeters wide, and up to 0.5 centimeters deep. Moderate swelling was observed around the wound, with disappearance of local skin lines. The skin around the wound felt numb and the pain was dull.
Laboratory examinations
The patient underwent routine blood, interleukin 6, blood biochemistry, coagulation, thyroid function, autoantibody, and tumor markers tests, all of which were negative.
Imaging examinations
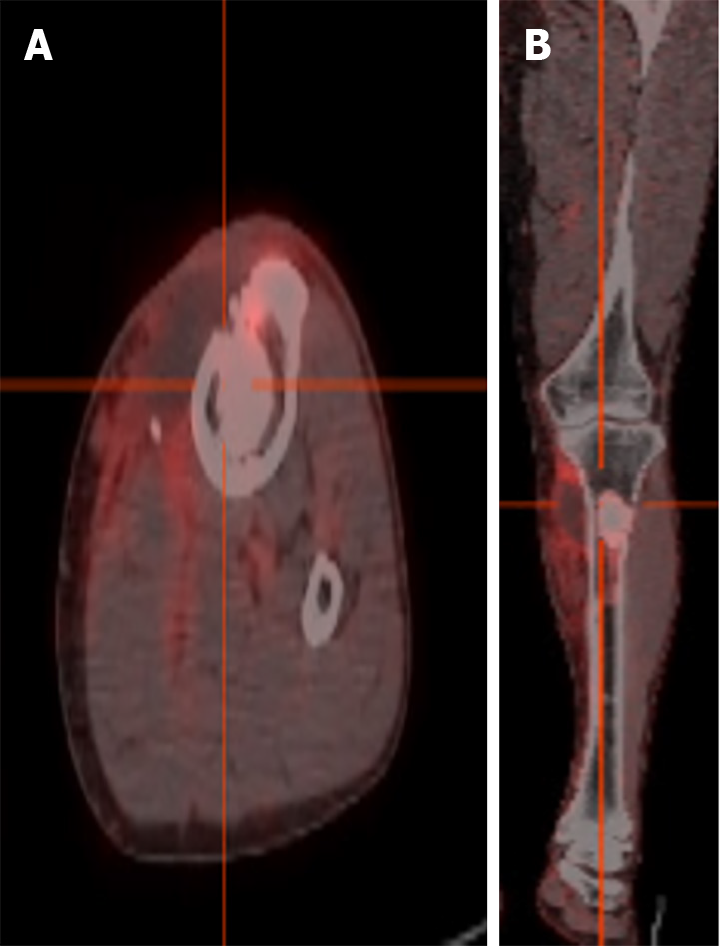
Positron emission tomography-computed tomography showed left tibial osteomyelitis, local soft tissue infection, and local sinus formation (Figure 2A and B).
Figure 2 Positron emission tomography/computed tomography: Osteomyelitis of the upper tibia, local soft tissue infection.
A and B: A circular high-density shadow could be seen in the proximal tibia, with clear boundaries and locally increased fluorodeoxyglucose metabolism, shown in transverse (A) and coronal (B) views.
MULTIDISCIPLINARY EXPERT CONSULTATION
Based on the results, we recommended orthopedic debridement surgery on the 4th day of hospitalization. At the same time, the internal medicine department provided anti-infective treatment and actively clarified the etiology.
FINAL DIAGNOSIS
Osteomyelitis caused by M. houstonense.
TREATMENT
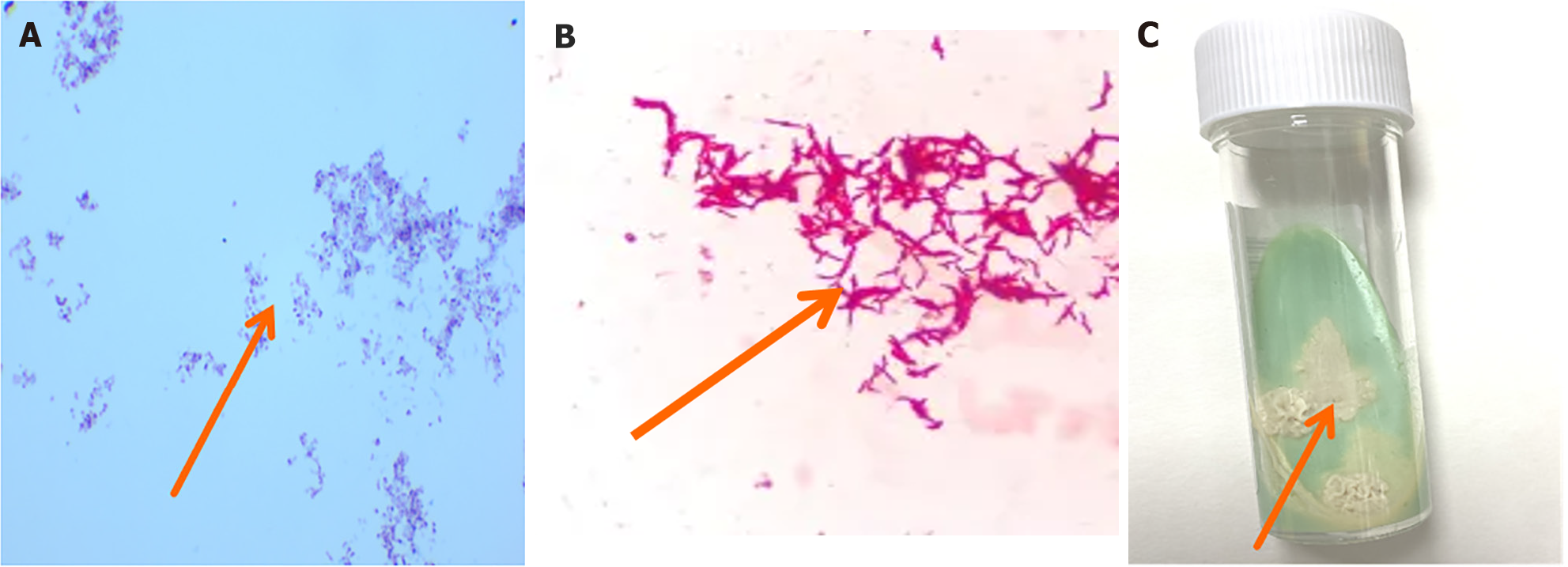
Based on the patient’s condition and relevant examination results, we have consulted an orthopedic physician and recommended that the patient undergo orthopedic debridement while receiving anti-infective treatment. Positive acid fast staining of bacterial colonies in pus culture suggested the possibility of infection with Nocardia or nontuberculous mycobacteria (Figure 3A and B). As tuberculosis infection could not be excluded, the patient received a dose of 300 mg isoniazid administered once daily, a dose of 450 mg rifampicin once daily, a dose of 750 mg ethambutol once daily, and a dose of 500 mg pyrazinamide three times a day.
Figure 3 Cultivation of nontuberculous mycobacteria and microscopic images.
A: Positive acid fast staining of pus, with a large number of red elongated hyphae (100 ×); B: Under the microscope, slender, red, rod-shaped nontuberculous mycobacteria could be seen, with relatively scattered distribution and some bacterial cells slightly curved (400 ×); C: On the 12th day of observation, a unique scene appeared on Roche medium. Rice yellowish dry bacterial colonies appeared on top. This rice yellow color was very eye-catching and stood out, particularly against the background of the culture medium. The colony appeared dry and lacked a moist luster.
OUTCOME AND FOLLOW-UP
To further clarify the pathogenic bacteria, mNGS was performed. There are six genes of M. houstonense, with the following gene sequences: GGGTGAGCGTGCCCATGAGCTCGAAGAGCTTCTTGCGTTG, GCTCAAGCCGTCGATCAGGTCCGGGCTCGCATCCGCGTAC, CTCAACCAGCGCGGGCTGGCCGGCCCGAACACCGGCATCT, CATCGGTGCGGAACTCGACGGCCCCGGTGATCCCGGCCGG, GCTTCAGCTTCTCAGGCGAGAGCATTCCGACCGCAGAAGG, GATCTGCCCGTTGGGCACCGTGTACATCTCACCCTCGGAT.
As shown in Figure 3C, we conducted further cultivation. This is consistent with the previous mNGS results. Therefore, according to relevant diagnosis and treatment guidelines. The anti-infection regimen was adjusted to amikacin combined with levofloxacin and cefoxitin combined with lenazolam intravenous drip for 6 weeks. After discharge, the patient continued to receive sequential oral SMZ combined with levofloxacin treatment for 3 months. Rice yellow dry colonies appeared on Roche medium on the 12th day (Figure 3C). This result was consistent with the results of acid fast staining and mNGS in pus.
DISCUSSION
The patient in this case has a history of surgical trauma, repeated debridement surgeries, a long course of disease, and multiple episodes of pus like exudation from the wound after surgery. The use of multiple antibiotics has shown poor therapeutic effects, and it is necessary to consider the possibility of low virulence pathogen infections, such as mycobacterium, Nocardia, or fungi, which are difficult to distinguish in clinical practice. The pathogen was ultimately confirmed to be M. houstonense through pus culture and mNGS results. The second-generation gene sequencing is a fast and reliable identification method for fast-growing mycobacterium[1,2]. Some common symptoms of nontuberculous mycobacterium bone infection caused by M. houstonense are as follows: (1) Pain: The common symptom of bone infection is pain, usually manifested as persistent or dull pain[3,4]. The severity and location of pain vary among individuals, but usually worsen as the condition progresses[5,6]; (2) Redness and swelling: The infected area may experience redness and swelling, as well as inflammatory reactions caused by bacterial proliferation[7,8]. The severity and extent of redness and swelling may also vary among individuals[9,10]; (3) Fever: The infected area may experience fever, which is caused by inflammation caused by bacterial proliferation[11,12]. The degree and duration of fever may also vary among individuals[13,14]; (4) Sinus formation: This is caused by inflammation and tissue damage caused by infection[15,16], which may affect the patient's mobility and quality of life; and (5) Muscle atrophy: Long-term inactivity or bed rest may lead to muscle atrophy, which is caused by muscle atrophy due to lack of exercise[17,18]. Muscle atrophy may affect the patient's recovery and quality of life[19,20]. It should be noted that not all patients will experience these symptoms, and the frequency and severity of their occurrence may vary from person to person[21-23]. Doctors should make preliminary judgments based on the patient's medical history, symptoms, and signs[24,25]. For example, whether the patient has symptoms such as pain, redness, and fever, as well as signs of pain and swelling in the infected area. Some relevant laboratory tests are necessary[26,27]. These indicators can indicate the presence and severity of infection. Further imaging examinations such as X-rays, computed tomography scans, and magnetic resonance imaging are needed to understand the location and extent of infection, as well as the degree of bone damage and soft tissue infection. These examination results help determine the diagnosis and treatment plan for infection. The pathological tissue of the infected site should be obtained through tissue biopsy and pathological examination should be performed. Pathological examination can determine the cause and type of infection, as well as the inflammatory response and tissue damage of infected tissues. Bacterial culture and drug sensitivity testing are needed to determine the type of infected bacteria and their antibiotic sensitivity and resistance[28]. These examination results will help guide doctors in selecting appropriate antibiotics for treatment.
CONCLUSION
Osteomyelitis is a serious infectious disease of the bones. When M. houstonense becomes the pathogenic factor, the challenges it brings are even more severe. In terms of treatment, osteomyelitis induced by M. houstonense is also full of challenges. Due to the particularity of this pathogen, conventional antibiotic treatment may be ineffective. Doctors need to select sensitive antibiotics for individualized treatment according to the results of the pathogen's drug sensitivity test. However, the treatment process is often long and complex, and patients need to have sufficient patience and compliance. In some cases, surgical treatment such as debridement and bone transplantation may be necessary to remove infected tissues and promote bone repair. In conclusion, osteomyelitis induced by M. houstonense is a complex and challenging disease. Early diagnosis, individualized treatment, and active rehabilitation measures are the keys to dealing with this disease. At the same time, medical researchers also need to make continuous efforts to explore more effective diagnostic and treatment methods and bring more hope to patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Infectious diseases
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade B
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Azmi AS S-Editor: Liu JH L-Editor: Filipodia P-Editor: Yu HG