Published online Nov 18, 2024. doi: 10.5312/wjo.v15.i11.1001
Revised: October 16, 2024
Accepted: November 5, 2024
Published online: November 18, 2024
Processing time: 170 Days and 7.1 Hours
In this editorial, we comment on the paper by Muthu et al published in the recent issue of the journal. This editorial review focusses on the use of adipose-derived stem cells (ADSCs) in knee osteoarthritis treatment. We discuss the differences between the stromal vascular fraction and microfragmented adipose tissue and highlight the results of clinical studies comparing both treatments and the use of hyaluronic acid, platelet-rich plasma, and bone marrow aspirate concentrate. The use of expanded ADSCs is also discussed; moreover, concerns regarding treatment with ADSCs, particularly the heterogeneity of published studies and the need to standardize protocols to explore clinical potential is explored.
Core Tip: Adipose tissue has been described being superior to bone marrow as a source mesenchymal stem cell due to its lower invasiveness and higher cell content. Hence, products derived from the adipose tissue for the treatment of knee osteoarthritis re
- Citation: de Sousa EB, Gabbi Filho JPA, Gameiro VS, Baptista LS. Adipose-derived stem cells and knee osteoarthritis: New perspectives, old concerns. World J Orthop 2024; 15(11): 1001-1006
- URL: https://www.wjgnet.com/2218-5836/full/v15/i11/1001.htm
- DOI: https://dx.doi.org/10.5312/wjo.v15.i11.1001
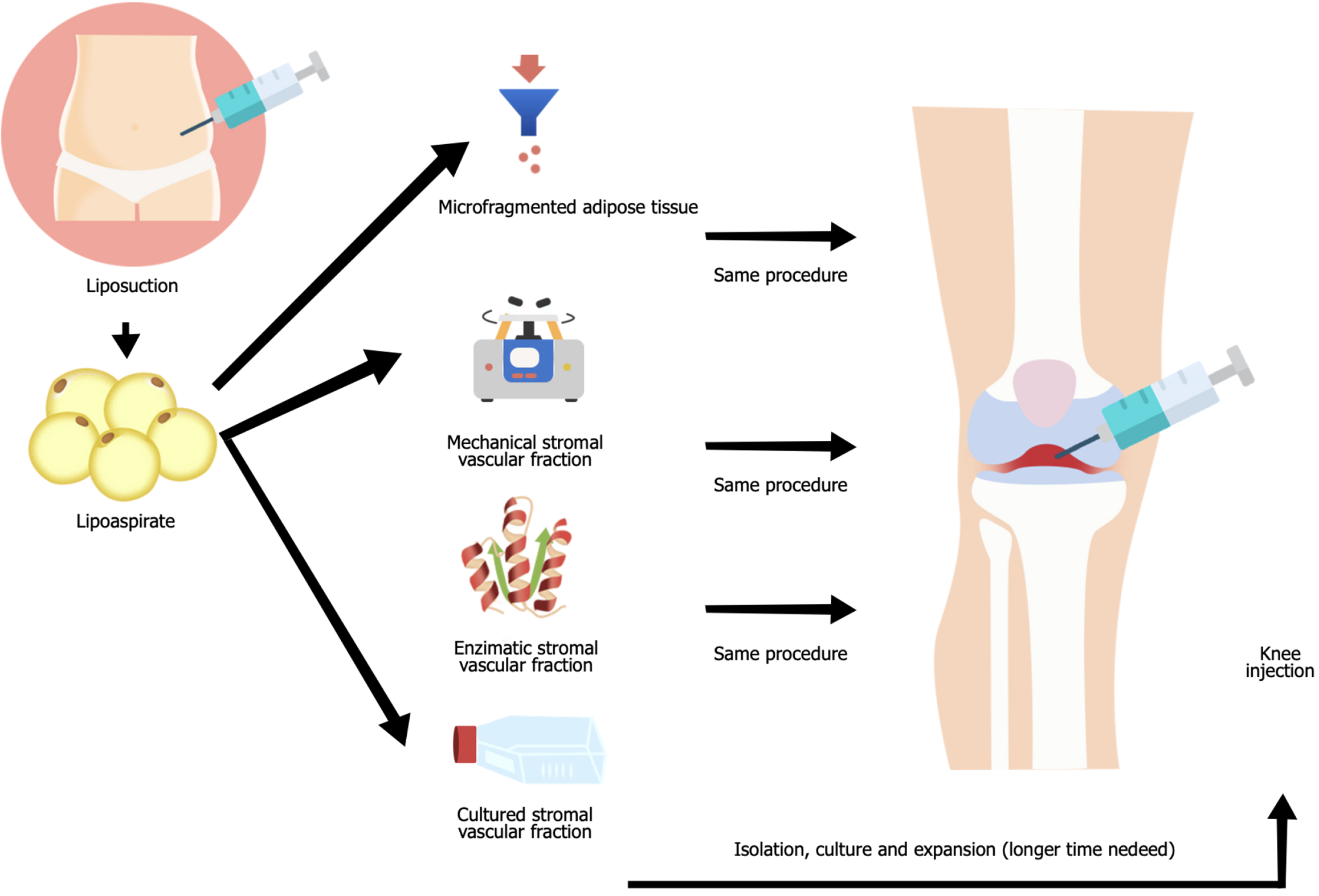
Knee osteoarthritis (KOA) is a chronic disease that affects the whole synovial joint, leading to cartilage degeneration, synovial inflammation, and subchondral bone thickening[1,2]. KOA treatment involves conservative (non-operative) measures, including education, analgesics and anti-inflammatory drugs, corticosteroids, and physical therapy and exercise. Surgical measures include arthroscopy, osteotomy, and total joint replacement[3,4]. In the last decades, interest in the use of orthobiologics, biologic materials for orthopedic disease treatment, has increased. These include platelet-rich plasma (PRP), bone marrow concentrate, and mesenchymal stem cells owing to their potential role in relieving KOA symptoms, slowing disease progression, and regenerating articular cartilage[5-7]. In the systematic review by Muthu et al[8] in 2023, adipose tissue was described as superior to bone marrow as a mesenchymal stem cell source in terms of safety and KOA improvement. However, the authors recommended future trials to confirm their findings. A thorough literature analysis regarding the use of products derived from adipose tissue for KOA treatment leads to the insight that they have treatment potential; however, the heterogeneity of protocols and published studies reveals concerns similar to those reported with other orthobiologic therapies. Beginning with their nomenclature, papers describing various treatments as adipose-derived stem cells (ADSCs) are common. However, ADSC can be observed in distinct proportions in microfragmented adipose tissue (MFAT) and stromal vascular fraction (SVF). For their suitable isolation and expansion in culture, ADSC must be isolated from SVF[9]. Moreover, these sources of ADSC (Figure 1) may differ in their clinical effect[10,11]. Another concern involves patient stratification aiming to predict outcomes[12], especially in KOA as the disease presents different phenotypes[13]. This review aimed to highlight the differences between various ADSC treatments and to present the most recent studies involving their use in clinical practice.
The SVF, a cell fraction of adipose tissue without adipocytes, consists of heterogeneous nonadipocyte cell types such as fibroblasts, vascular cells, macrophages, and plastic adherent cells amenable to culture, called ADSC[14-16]. The SVF contains factors that enhance and stimulate regenerative pathways including angiogenesis, cell proliferation, and differentiation[17] and is predominantly obtained through collagenase digestion, centrifugation, washing, and filtration[15,16]. MFAT is a type of adipose tissue processing wherein the lipoaspirate is fragmented into 0.2-0.8 clusters, maintaining stromal cells intertwined in the extracellular matrix content, including ADSCs and pericytes[18]. MFAT produced by different systems may lead to distinct cellular content, consequently affecting the cytokine profile[16,19]. Besides, MFAT extracted from patients with obesity exhibits greater pro-inflammatory cytokine patterns and effects[20]. ADSC are fibroblastic-like cells isolated from the SVF using enzymatic or mechanical processing of adipose tissue[21]. ADSC popularity rose given their ease of harvest, yielding up to 10% of nucleated cells compared to 0.001%-0.01% found in bone marrow-derived stem cells; high proliferative potential and expansion; and low rate of complications[22]. ADSC can be differentiated in vitro into adipocytes, osteoblasts, chondroblasts, and myocytes[23]. Moreover, ADSCs do not lose their chondrogenic potential and expansion properties with age, and have greater anti-inflammatory properties[24].
Jeyaraman et al[25] affirmed that level I evidence studies involving the use of SVF for KOA treatment were available and suggested establishing standardized protocols following regulatory requirements. SVF isolation can be alternatively performed using mechanical or physical forces to modify adipose tissue structural integrity and to avoid the potential side effects of enzymatic digestion, preserving cells in their native environment[17,26]. Boada-Pladellorens et al[27] suggested that SVF was a safe and promising treatment for KOA, indicating that the products need standardization and cell number homogenization. This finding was confirmed by Goncharov et al[28], noting the safety and efficacy of SVF in improving pain, symptoms, and mobility in patients with KOA and presenting with few or no adverse effects. Goncharov et al[28] also indicated the need to evaluate the study design, sample size, and method variability, prioritizing patient safety. Aletto et al[29] reported excellent clinical and radiographical results using intra-articular SVF for KOA treatment.
Kim et al[30] reported that arthroscopically implanted SVF used in the treatment of Outerbridge grade 3-4 cartilage lesions in KOA can result in pain relief and cartilage regeneration, which correlated to magnetic resonance imaging outcomes at 12 months. Santoprete et al[31] concluded that SVF is a safe and effective procedure with low morbidity for patients with KOA [Kellgren-Lawrence grade (K-L) ≥ 2]. Hong et al[32] indicated that “superior to hyaluronic acid (HA)” SVF treatment was safe, relieved pain, improved function, and repaired cartilage in patients with K-L grades 3-4 KOA. Bolia et al[33] suggested that both bone marrow aspirate concentrate (BMAC) and SVF presented short-term symptomatic relief in patients with KOA; however, SVF resulted in better pain reduction. Gobbi et al[34] concluded that one MFAT injection improved clinical, functional, and quality of life outcomes in K-L grade 2-4 KOA patients at two years. Similarly, Yu et al[35] noted that autologous MFAT improved knee pain and function 9-12 months after injections; no adverse effects were observed after 18 months compared to baseline. Wu et al[36] observed that compared to HA, treatment with MFAT following arthroscopic surgery was safe and effective given better improvement in pain and function between 12 and 24 months in patients with KOA. Ulivi et al[37] concluded that MFAT and arthroscopic debridement improved functional outcomes and magnetic resonance imaging appearance compared to isolated arthroscopy in patients with KOA.
Comparing KOA treatment at six months using PRP and MFAT revealed clinical improvements without differences in outcomes[38,39]. However, a prospective comparative trial concluded that intra-articular injection of BMAC and ADSCs improved pain and function in patients with KOA at six months, without notable differences between them[40]. Oeding et al[41] noted that PRP was superior to HA for KOA treatment, confirming the results of Belk et al[42], who also su
Kim et al[46] affirmed that expanded/cultured ADSC led to significant pain relief and functional improvement in patients with K-L grade 3 KOA, but suggested that long-term follow-up was required to explore the disease-modifying effects and their duration. Comparing ADSC and leukocyte-poor PRP for KOA treatment, both resulted in good clinical outcomes at six months, but ADSC was superior at twelve and twenty-four months[47]. Huang et al[48] concluded that the potential risks and side effects of ADSC must be explored, although it presents promising results in KOA treatment. Furthermore, Issa et al[49] observed that ADSC was a safe and effective treatment, presenting short and possibly long-term results on pain and functional outcomes for patients with KOA. Their results corroborate those of Yang et al[50] which indicated that single or multiple injections of both ADSC or SVF were safe and improved pain in patients with KOA. Schweich-Adami et al[51] reported the treatment of one patient with KOA with expanded ADSC, resulting in an improvement in pain and quality of life. Finally, Yokota et al[52] observed that both the intra-articular injection of SVF and ADSC in patients with KOA led to clinical improvement; however, ADSC revealed superior results in terms of improvement of pain and symptoms, suggesting that a clinical trial should be conducted for further validation.
KOA treatment using adipose tissue and associated cells has been increasing currently owing to the ease of harvesting and low morbidity. Moreover, adipose tissue presents higher mesenchymal stem cell content than bone marrow. Based on published studies, ADSC presents better clinical outcomes than HA, PRP, and BMAC. However, most studies describing the potential use of ADSC present heterogeneous protocols for harvesting and delivery. Hence, concerns similar to other orthobiological treatments persist, and more studies are required to establish adequate protocols for their clinical use.
| 1. | Giorgino R, Albano D, Fusco S, Peretti GM, Mangiavini L, Messina C. Knee Osteoarthritis: Epidemiology, Pathogenesis, and Mesenchymal Stem Cells: What Else Is New? An Update. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 157] [Reference Citation Analysis (0)] |
| 2. | Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393:1745-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1458] [Cited by in RCA: 2658] [Article Influence: 443.0] [Reference Citation Analysis (0)] |
| 3. | Lim WB, Al-Dadah O. Conservative treatment of knee osteoarthritis: A review of the literature. World J Orthop. 2022;13:212-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 52] [Article Influence: 17.3] [Reference Citation Analysis (7)] |
| 4. | Brumat P, Kunšič O, Novak S, Slokar U, Pšenica J, Topolovec M, Mihalič R, Trebše R. The Surgical Treatment of Osteoarthritis. Life (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 5. | Crane DM, Oliver KS, Bayes MC. Orthobiologics and Knee Osteoarthritis: A Recent Literature Review, Treatment Algorithm, and Pathophysiology Discussion. Phys Med Rehabil Clin N Am. 2016;27:985-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Mavrogenis AF, Karampikas V, Zikopoulos A, Sioutis S, Mastrokalos D, Koulalis D, Scarlat MM, Hernigou P. Orthobiologics: a review. Int Orthop. 2023;47:1645-1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 7. | Weber AE, Bolia IK, Trasolini NA. Biological strategies for osteoarthritis: from early diagnosis to treatment. Int Orthop. 2021;45:335-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Muthu S, Patil SC, Jeyaraman N, Jeyaraman M, Gangadaran P, Rajendran RL, Oh EJ, Khanna M, Chung HY, Ahn BC. Comparative effectiveness of adipose-derived mesenchymal stromal cells in the management of knee osteoarthritis: A meta-analysis. World J Orthop. 2023;14:23-41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 34] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 9. | Ranmuthu CDS, Ranmuthu CKI, Khan WS. Evaluating the Current Literature on Treatments Containing Adipose-Derived Stem Cells for Osteoarthritis: a Progress Update. Curr Rheumatol Rep. 2018;20:67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Ossendorff R, Menon A, Schildberg FA, Randelli PS, Scheidt S, Burger C, Wirtz DC, Cucchi D. A Worldwide Analysis of Adipose-Derived Stem Cells and Stromal Vascular Fraction in Orthopedics: Current Evidence and Applications. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 11. | Perucca Orfei C, Boffa A, Sourugeon Y, Laver L, Magalon J, Sánchez M, Tischer T, Filardo G, de Girolamo L. Cell-based therapies have disease-modifying effects on osteoarthritis in animal models. A systematic review by the ESSKA Orthobiologic Initiative. Part 1: adipose tissue-derived cell-based injectable therapies. Knee Surg Sports Traumatol Arthrosc. 2023;31:641-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 27] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 12. | Epanomeritakis IE, Khan WS. Adipose-derived regenerative therapies for the treatment of knee osteoarthritis. World J Stem Cells. 2024;16:324-333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 13. | Ma J, Zhang K, Ma X, Wang H, Ma C, Zhang Y, Liu R. Clinical phenotypes of comorbidities in end-stage knee osteoarthritis: a cluster analysis. BMC Musculoskelet Disord. 2024;25:299. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Baptista LS. Adipose stromal/stem cells in regenerative medicine: Potentials and limitations. World J Stem Cells. 2020;12:1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Schipper JAM, van Laarhoven CJHCM, Schepers RH, Tuin AJ, Harmsen MC, Spijkervet FKL, Jansma J, van Dongen JA. Mechanical Fractionation of Adipose Tissue-A Scoping Review of Procedures to Obtain Stromal Vascular Fraction. Bioengineering (Basel). 2023;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 16. | Tang Q, Zhao XS, Guo A, Cui RT, Song HL, Qi ZY, Pan Y, Yang Y, Zhang FF, Jin L. Therapeutic applications of adipose-derived stromal vascular fractions in osteoarthritis. World J Stem Cells. 2022;14:744-755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Vargel İ, Tuncel A, Baysal N, Hartuç-Çevik İ, Korkusuz F. Autologous Adipose-Derived Tissue Stromal Vascular Fraction (AD-tSVF) for Knee Osteoarthritis. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 18. | Van Genechten W, Vuylsteke K, Martinez PR, Swinnen L, Sas K, Verdonk P. Autologous Micro-Fragmented Adipose Tissue (MFAT) to Treat Symptomatic Knee Osteoarthritis: Early Outcomes of a Consecutive Case Series. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 19. | Greenwood V, Clausen P, Matuska AM. Micro-fragmented adipose tissue cellular composition varies by processing device and analytical method. Sci Rep. 2022;12:16107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 20. | Cavallo C, Boffa A, de Girolamo L, Merli G, Kon E, Cattini L, Santo E, Grigolo B, Filardo G. Bone marrow aspirate concentrate quality is affected by age and harvest site. Knee Surg Sports Traumatol Arthrosc. 2023;31:2140-2151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 21. | Al-Ghadban S, Artiles M, Bunnell BA. Adipose Stem Cells in Regenerative Medicine: Looking Forward. Front Bioeng Biotechnol. 2021;9:837464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 22. | Kunze KN, Burnett RA, Wright-Chisem J, Frank RM, Chahla J. Adipose-Derived Mesenchymal Stem Cell Treatments and Available Formulations. Curr Rev Musculoskelet Med. 2020;13:264-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 23. | Locke M, Windsor J, Dunbar PR. Human adipose-derived stem cells: isolation, characterization and applications in surgery. ANZ J Surg. 2009;79:235-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 184] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 24. | Agarwal N, Mak C, Bojanic C, To K, Khan W. Meta-Analysis of Adipose Tissue Derived Cell-Based Therapy for the Treatment of Knee Osteoarthritis. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 25. | Jeyaraman M, Maffulli N, Gupta A. Stromal Vascular Fraction in Osteoarthritis of the Knee. Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 26. | De Francesco F, Gravina P, Busato A, Farinelli L, Soranzo C, Vidal L, Zingaretti N, Zavan B, Sbarbati A, Riccio M, Gigante A. Stem Cells in Autologous Microfragmented Adipose Tissue: Current Perspectives in Osteoarthritis Disease. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 27. | Boada-Pladellorens A, Avellanet M, Pages-Bolibar E, Veiga A. Stromal vascular fraction therapy for knee osteoarthritis: a systematic review. Ther Adv Musculoskelet Dis. 2022;14:1759720X221117879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 28. | Goncharov EN, Koval OA, Nikolaevich Bezuglov E, Encarnacion Ramirez MJ, Engelgard M, Igorevich EI, Saporiti A, Valentinovich Kotenko K, Montemurro N. Stromal Vascular Fraction Therapy for Knee Osteoarthritis: A Systematic Review. Medicina (Kaunas). 2023;59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Aletto C, Oliva F, Maffulli N. Knee intra-articular administration of stromal vascular fraction obtained from adipose tissue: A systematic review. J Clin Orthop Trauma. 2022;25:101773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Kim YS, Oh SM, Suh DS, Tak DH, Kwon YB, Koh YG. Arthroscopic Implantation of Adipose-Derived Stromal Vascular Fraction Improves Cartilage Regeneration and Pain Relief in Patients With Knee Osteoarthritis. Arthrosc Sports Med Rehabil. 2023;5:e707-e716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 31. | Santoprete S, Marchetti F, Rubino C, Bedini MG, Nasto LA, Cipolloni V, Pola E. Fresh autologous stromal tissue fraction for the treatment of knee osteoarthritis related pain and disability. Orthop Rev (Pavia). 2021;13:9161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Hong Z, Chen J, Zhang S, Zhao C, Bi M, Chen X, Bi Q. Intra-articular injection of autologous adipose-derived stromal vascular fractions for knee osteoarthritis: a double-blind randomized self-controlled trial. Int Orthop. 2019;43:1123-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 127] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 33. | Bolia IK, Bougioukli S, Hill WJ, Trasolini NA, Petrigliano FA, Lieberman JR, Weber AE. Clinical Efficacy of Bone Marrow Aspirate Concentrate Versus Stromal Vascular Fraction Injection in Patients With Knee Osteoarthritis: A Systematic Review and Meta-analysis. Am J Sports Med. 2022;50:1451-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 34. | Gobbi A, Dallo I, Rogers C, Striano RD, Mautner K, Bowers R, Rozak M, Bilbool N, Murrell WD. Two-year clinical outcomes of autologous microfragmented adipose tissue in elderly patients with knee osteoarthritis: a multi-centric, international study. Int Orthop. 2021;45:1179-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 35. | Yu GR, Zhang MZ, Aiyer A, Tang X, Xie M, Zeng LR, Zhao YG, Li B, Yang YF. Repair of the acute deltoid ligament complex rupture associated with ankle fractures: a multicenter clinical study. J Foot Ankle Surg. 2015;54:198-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 36. | Wu CZ, Shi ZY, Wu Z, Lin WJ, Chen WB, Jia XW, Xiang SC, Xu HH, Ge QW, Zou KA, Wang X, Chen JL, Wang PE, Yuan WH, Jin HT, Tong PJ. Mid-term outcomes of microfragmented adipose tissue plus arthroscopic surgery for knee osteoarthritis: A randomized, active-control, multicenter clinical trial. World J Stem Cells. 2023;15:1063-1076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 37. | Ulivi M, Meroni V, Viganò M, Colombini A, Lombardo MDM, Rossi N, Orlandini L, Messina C, Sconfienza LM, Peretti GM, Mangiavini L, de Girolamo L. Micro-fragmented adipose tissue (mFAT) associated with arthroscopic debridement provides functional improvement in knee osteoarthritis: a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2023;31:3079-3090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 38. | Baria M, Pedroza A, Kaeding C, Durgam S, Duerr R, Flanigan D, Borchers J, Magnussen R. Platelet-Rich Plasma Versus Microfragmented Adipose Tissue for Knee Osteoarthritis: A Randomized Controlled Trial. Orthop J Sports Med. 2022;10:23259671221120678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 39. | Baria M, Barker T, Durgam S, Pedroza A, Flanigan D, Jia L, Kaeding C, Magnussen R. Microfragmented Adipose Tissue Is Equivalent to Platelet-Rich Plasma for Knee Osteoarthritis at 12 Months Posttreatment: A Randomized Controlled Trial. Orthop J Sports Med. 2024;12:23259671241233916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 40. | Pintore A, Notarfrancesco D, Zara A, Oliviero A, Migliorini F, Oliva F, Maffulli N. Intra-articular injection of bone marrow aspirate concentrate (BMAC) or adipose-derived stem cells (ADSCs) for knee osteoarthritis: a prospective comparative clinical trial. J Orthop Surg Res. 2023;18:350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 41. | Oeding JF, Varady NH, Fearington FW, Pareek A, Strickland SM, Nwachukwu BU, Camp CL, Krych AJ. Platelet-Rich Plasma Versus Alternative Injections for Osteoarthritis of the Knee: A Systematic Review and Statistical Fragility Index-Based Meta-analysis of Randomized Controlled Trials. Am J Sports Med. 2024;52:3147-3160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 42. | Belk JW, Kraeutler MJ, Houck DA, Goodrich JA, Dragoo JL, McCarty EC. Platelet-Rich Plasma Versus Hyaluronic Acid for Knee Osteoarthritis: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Am J Sports Med. 2021;49:249-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 299] [Article Influence: 74.8] [Reference Citation Analysis (0)] |
| 43. | Belk JW, Lim JJ, Keeter C, McCulloch PC, Houck DA, McCarty EC, Frank RM, Kraeutler MJ. Patients With Knee Osteoarthritis Who Receive Platelet-Rich Plasma or Bone Marrow Aspirate Concentrate Injections Have Better Outcomes Than Patients Who Receive Hyaluronic Acid: Systematic Review and Meta-analysis. Arthroscopy. 2023;39:1714-1734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 46] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 44. | Russo A, Cortina G, Condello V, Collarile M, Orlandi R, Gianoli R, Giuliani E, Madonna V. Autologous micro-fragmented adipose tissue injection provides significant and prolonged clinical improvement in patients with knee osteoarthritis: a case-series study. J Exp Orthop. 2023;10:116. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 45. | Screpis D, Natali S, Farinelli L, Piovan G, Iacono V, de Girolamo L, Viganò M, Zorzi C. Autologous Microfragmented Adipose Tissue for the Treatment of Knee Osteoarthritis: Real-World Data at Two Years Follow-Up. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 46. | Kim KI, Lee MC, Lee JH, Moon YW, Lee WS, Lee HJ, Hwang SC, In Y, Shon OJ, Bae KC, Song SJ, Park KK, Kim JH. Clinical Efficacy and Safety of the Intra-articular Injection of Autologous Adipose-Derived Mesenchymal Stem Cells for Knee Osteoarthritis: A Phase III, Randomized, Double-Blind, Placebo-Controlled Trial. Am J Sports Med. 2023;51:2243-2253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 48] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 47. | Khoury MA, Chamari K, Tabben M, Alkhelaifi K, Papacostas E, Marín Fermín T, Laupheimer M, D Hooghe P. Knee Osteoarthritis: Clinical and MRI Outcomes After Multiple Intra-Articular Injections With Expanded Autologous Adipose-Derived Stromal Cells or Platelet-Rich Plasma. Cartilage. 2023;14:433-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 48. | Huang Z, Zhang S, Cao M, Lin Z, Kong L, Wu X, Guo Q, Ouyang Y, Song Y. What is the optimal dose of adipose-derived mesenchymal stem cells treatment for knee osteoarthritis? A conventional and network meta-analysis of randomized controlled trials. Stem Cell Res Ther. 2023;14:245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 49. | Issa MR, Naja AS, Bouji NZ, Sagherian BH. The role of adipose-derived mesenchymal stem cells in knee osteoarthritis: a meta-analysis of randomized controlled trials. Ther Adv Musculoskelet Dis. 2022;14:1759720X221146005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 50. | Yang Y, Lan Z, Yan J, Tang Z, Zhou L, Jin D, Jin Q. Effect of intra-knee injection of autologous adipose stem cells or mesenchymal vascular components on short-term outcomes in patients with knee osteoarthritis: an updated meta-analysis of randomized controlled trials. Arthritis Res Ther. 2023;25:147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 51. | Schweich-Adami LC, Silva RAD, Baranoski A, Kassuya CAL, Antoniolli-Silva ACMB, Oliveira RJ. Effects of Adipose-derived Stem Cells in the Treatment of Knee Osteoarthritis: A Case Report in Brazil's Unified Health System. Rev Bras Ortop (Sao Paulo). 2024;59:e471-e474. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 52. | Yokota N, Hattori M, Ohtsuru T, Otsuji M, Lyman S, Shimomura K, Nakamura N. Comparative Clinical Outcomes After Intra-articular Injection With Adipose-Derived Cultured Stem Cells or Noncultured Stromal Vascular Fraction for the Treatment of Knee Osteoarthritis. Am J Sports Med. 2019;47:2577-2583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |