Published online Jun 18, 2023. doi: 10.5312/wjo.v14.i6.458
Peer-review started: December 21, 2022
First decision: April 13, 2023
Revised: April 26, 2023
Accepted: May 15, 2023
Article in press: May 15, 2023
Published online: June 18, 2023
Processing time: 179 Days and 11.2 Hours
Over the past years, patient specific instrumentation (PSI) for total knee arthroplasty (TKA) has been implemented and routinely used. No clear answer has been given on its associated cost and cost-effectiveness when compared to conventional instrumentation (CI) for TKA.
To compare the cost and cost-effectiveness of PSI TKA compared to CI TKA.
A literature search was performed in healthcare, economical healthcare, and medical databases (MEDLINE, EMBASE, CINAHL, Web of Science, Cochrane Li
Thirty-two studies were included into the systematic review. Two were included in the meta-analysis. 3994 PSI TKAs and 13267 CI TKAs were included in the sample size. The methodological quality of the included studies, based on Consensus on Health Economic Criteria-scores and risk of bias, ranged from average to good. PSI TKA costs less than CI TKA when considering mean operating room time and its associated costs and tray sterilization per patient case. PSI TKA costs more compared to CI TKA when considering imaging and production costs. Considering total costs per patient case, PSI TKA is more expensive in comparison to CI TKA. Meta-analysis comparing total costs for PSI TKA, and CI TKA showed a significant higher cost for PSI TKA.
Cost for PSI and CI TKA can differ when considering distinct aspects of their implementation. Total costs per patient case are increased for PSI TKA when compared to CI TKA.
Core Tip: Patient specific instrumentation (PSI) for total knee arthroplasty (TKA) has become a frequently used technique for performing TKA. In the past decade the use of PSI TKA has not proven superior nor inferior when compared to conventional instrumentation (CI) for TKA in terms of prosthetic alignment, prosthetic survival, and patient satisfaction. However, PSI TKA has been associated with a higher healthcare cost. In this review, we critically analysed the cost of PSI TKA compared to CI TKA, focusing on all facets of their cost.
- Citation: Dorling IM, Geenen L, Heymans MJLF, Most J, Boonen B, Schotanus MGM. Cost-effectiveness of patient specific vs conventional instrumentation for total knee arthroplasty: A systematic review and meta-analysis. World J Orthop 2023; 14(6): 458-470
- URL: https://www.wjgnet.com/2218-5836/full/v14/i6/458.htm
- DOI: https://dx.doi.org/10.5312/wjo.v14.i6.458
Total knee arthroplasty (TKA) has become a commonplace surgery worldwide. Indication for TKA is disabling osteoarthritis which is often accompanied by pain, reduced range of motion and intra-articular wear and tear[1]. New technology has emerged during the past decades to improve surgical techniques and equipment. Among these developments, patient specific instrumentation (PSI) for TKA was brought to the market, which could possibly prove superior to conventional instrumentation (CI) for TKA in terms of outliers in alignment. PSI may be used as a drill guide for pin placement and/or as cutting blocks to perform the femoral and tibial bony resections.
Numerous studies have been conducted on this subject with differing results[2-10]. For example, femoral and tibial implant alignment precision has shown improved[6], declined[7] or not differing when compared to CI TKA[2-5]. Decreased blood loss[4,8], shortened hospital stay[6], and reduced operating time[3,6,8] have been reported more consistently when using PSI TKA compared to CI TKA. These factors could be associated with decreased healthcare costs and an improved efficiency of patient care. Additionally, no difference in patient reported outcome measures between PSI TKA and CI TKA have been found in a 1-, 2-, 3- and 5-year follow-up study done by Schoenmakers et al[10]. Clinically, PSI TKA has thus far not proved to be superior to CI TKA. However, some logistic improvements have been suggested, which may lead to a decreased cost of surgery.
Studies on cost-effectiveness of PSI show mixed results[11,12]. The included outcomes of these studies differ and thus do not allow for a concluding statement on the cost-effectiveness of PSI TKA.
The aim of this study was to systematically review literature about the cost-effectiveness of PSI for TKA and perform a meta-analysis on the available data. Thereby, the aim was to provide an evidence-based answer on the cost-effectiveness of PSI TKA compared to CI TKA.
This systematic review was performed in accordance with PRISMA statement and in line with the five-step approach for constructing a review on cost-effectiveness by PeerJ[13], Moher et al[14], Shamseer et al[15], Van Mastrigt et al[16], Thielen et al[17], and Wijnen et al[18]. The following research question was postulated:
Is PSI TKA cost-effective when compared to CI TKA when used for adults with disabling osteoart
The following databases were systematically searched: OVID MEDLINE, EMBASE.com, CINAHL (via EBSCO), The Cochrane Library and The Cochrane Central of register for Controlled Trials (CENTRAL)/Wiley, Web of Science/Clarivate Analytics and Econlit. Additionally, ongoing unpublished trials were identified on clinical trials.gov, the World Health Organization trial registry portal and PROSPERO. Reference lists of all papers about cost effectiveness of PSI TKA and/or CI TKA have been searched manually to ensure no papers were missed in the search. No search limitations were applied. The search strategy was in accordance with the Cochrane Highly Sensitive Search Strategy[20].
Studies meeting the following criteria were included into the review: (1) TKA for disabling osteoarthritis in adults; (2) PSI TKA and/or CI TKA; and (3) Cost-analysis and/or cost-effectiveness for TKA. Articles that included patients undergoing TKA for any other reason than osteoarthritis were excluded.
All searched and eligible articles were independently reviewed by two reviewers (ID, LG) using RefWorks[21]. The studies searched in the databases were de-duplicated and selected titles were examined on titles and abstracts based on the eligibility criteria named above. Thereafter the reviewers examined the full text independently and decided whether articles should be included or excluded. Disagreement regarding the inclusion or exclusion was resolved by discussion between the reviewers and if required a third reviewer (MS) was consulted. Once articles were included the two reviewers (ID, LG) objectively and independently extracted data from the included articles. The reviewers were blinded to each other’s extractions. The following data items were obtained during the extraction process, if available: Study ID (author, year), number of patients (n), age of patients in years (mean), number of female and male patients, country of study conduction, study design, length of hospital stay, operation time, tray sterilization cost, PSI production cost, imaging cost, staff cost, hospital stay cost, data on Quality-Adjusted Life Years (QALYs) and Incremental Cost-Effectiveness Ratio (ICER). Other study results include length of hospital stay, operation duration, readmission rates and patient characteristics and the costs tied to these.
All costs were converted to 2018 United States Dollars using a web-based tool. This tool was developed by the Campbell and Cochrane Economics Methods Group and the Evidence for Policy and Practice Information and Coordinating Centre (v.1.6, updated last April 2019)[22]. If studies reported an index year, this year was used for conversion. This tool was able to adjust costs up to 2018. Studies performed after 2018 were therefor indexed to 2018 despite them reporting later index years. If studies did not specify an index year, it was set using the year in which the last patients were included. If this was not applicable due to study design, year of study receival (in revised form) was used.
Included studies were assessed by the two reviewers separately (ID, LG) for quality and risk of bias (RoB). The assessment for RoB was performed using the Cochrane Risk of Bias Tool. For randomised studies the RoB Tool 2.0 was used, for non-randomised studies the RoB-I Tool was used. Both tools were retrieved from the Cochrane Handbook for Systematic Reviews of Interventions[23]. Evidence level of the included articles has been determined using the level of Evidence Guidelines from the Oxford centre for Evidence-Based Medicine[24].
To evaluate the methodological quality of economic evaluations the Consensus on Health Economic Criteria (CHEC) list was used[25]. The CHEC-list scores range from 0 to 19 (0 being the lowest score and 19 being the highest).
Agreement on RoB, methodological quality and evidence level was reached through discussion between the two reviewers (ID, LG) after separate and individual assessment was performed.
Studies have been stratified by geographical location, year of conduction, study design, level of evidence, RoB and CHEC scores. All costs, including QALYs and ICER values, have been adjusted for inflation using a web-based tool by Campbell and Cochrane Economics Methods Group and the Evidence for Policy and Practice Information and Coordinating Centre (v.1.6, updated last April 2019)[22]. Due to differing sourcing of costs and a variety of countries of study conduction, results were expected to show heterogeneity. Results are therefore not presented as means. The reported costs are presented in ranges.
Review Manager version 5.3 (2014) was used to perform a meta-analysis of the study data. For the meta-analysis, a fixed effects model was used and relative risk was reported with a 95% confidence interval (CI), which was used when there was similarity in included study execution. Mean difference was selected as the effect measure. Results were statistically significant if P ≤ 0.05. To quantify the statistical heterogeneity in the studies, the I2 value was used. I2 values of > 75% were interpreted as high heterogeneity[26]. Only if studies were sufficiently clinically, methodologically, and statistically homogenous, the data was pooled in a meta-analysis.
Data which could be included into the meta-analysis but was presented as ranges with a 95%CI were converted to standard deviations.
An extensive overview of the search can be found in Supplementary Table 1. The first search was conducted on April 26, 2021. The second and last search was conducted on January 24, 2022. The systematic search of the databases resulted in 16454 studies. The manual search of the relevant reference lists resulted in 6 additional examined studies. De-duplication was performed which resulted in a remainder of 10366 studies. These were screened on titles and abstracts by the two independent reviewers. Of these studies, 15 possible relevant titles had unavailable abstracts. They were sought for retrieval by a clinical epidemiologist (MH). Six of these titles were not retrieved. Out of all screened studies, 81 articles were eligible for full text analysis. After full-text analysis, 28 articles were included into the study. Three more studies were included after reference-list analysis. A second search was conducted with the exact search terms on January 24, 2022, to include any new literature. One additional study was eligible for inclusion. This resulted in the inclusion of a total of 32 studies. A full overview of the study selection procedure is shown in the PRISMA-flowchart in Figure 1. An overview of the included studies is presented in Table 1.
| Ref. | Country of conduction | Study design | Studied TKA types | Type of economic analysis |
| Attard et al[44], 2019 | United Kingdom | RCT | PSI TKA vs CI TKA vs Single use inserts for TKA | Financial study |
| Barrack et al[31], 2012 | United States | Retrospective | PSI TKA vs CI TKA | Financial study |
| Cotter et al[42], 2022 | United States | Retrospective | CI TKA vs other1 TKA | Financial study |
| Dakin et al[43], 2012 | United Kingdom | Retrospective RCT-analysis | CI TKA | Cost-effectiveness analysis |
| Dakin et al[33], 2020 | United Kingdom | Retrospective | CI TKA vs Total hip arthroplasty | Cost-effectiveness analysis |
| DeHaan et al[49], 2014 | United States | Retrospective | PSI TKA vs CI | Financial study |
| Elmallah et al[29], 2017 | United States | Retrospective comparative cohort | CI TKA vs Total hip arthroplasty | Cost-effectiveness analysis |
| Goldberg et al[30], 2019 | United States | Retrospective | CI TKA vs other TKA | Financial decision model |
| Jenkins et al[32], 2013 | United Kingdom | Prospective | CI TKA vs Total hip arthroplasty | Cost-effectiveness analysis |
| Konopka et al[37], 2018 | United States | Retrospective | CI TKA vs Total hip arthroplasty | Cost-effectiveness analysis |
| Krummenauer et al[35], 2009 | Germany | Prospective | CI TKA | Cost-effectiveness analysis |
| Lionberger et al[27], 2014 | United States | RCT | PSI TKA vs CI TKA | Financial study |
| Losina et al[46], 2009 | United States | Retrospective population analysis | CI TKA | Cost-effectiveness analysis |
| Moerenhout et al[36], 2021 | Switzerland | Case control, retrospective chart | PSI TKA vs CI TKA | Financial study |
| Mont et al[48], 2012 | United States | Prospective controlled trial | CI TKA vs other TKA | Financial study |
| Navarro Espigares and Hernández Torres[47], 2008 | Spain | Prospective cohort | CI TKA vs Total hip arthroplasty | Cost-effectiveness analysis |
| Nunley et al[11], 2012 | United States | Retrospective | PSI TKA vs CI TKA | Financial study |
| Peersman et al[53], 2014 | Belgium | Retrospective | CI TKA vs other TKA | Cost-effectiveness analysis |
| Räsänen et al[45], 2007 | Finland | Prospective | CI TKA vs Total hip arthroplasty | Cost-effectiveness analysis |
| Rorabeck and Murray[50], 1996 | Canada | Retrospective | CI TKA | Financial study |
| Schilling et al[28], 2017 | Australia | Retrospective | CI TKA | Cost-effectiveness analysis |
| Siegel et al[40], 2015 | United States | Retrospective | CI TKA vs other TKA | Financial study |
| Slover et al[54], 2006 | United States | Theoretical cohort | CI TKA vs other TKA | Cost-effectiveness analysis |
| Slover et al[12], 2012 | United States | Retrospective | PSI TKA vs CI TKA | Financial decision model |
| Stan et al[34], 2015 | Romania | Retrospective | CI TKA vs other TKA | Cost-effectiveness analysis |
| Teeter et al[38], 2019 | Canada | RCT | PSI TKA vs CI TKA | Financial study |
| Thienpont et al[41], 2015 | Belgium | Retrospective | PSI TKA vs CI TKA | Financial study |
| Thomas et al[56], 2022 | United States | Retrospective | PSI TKA vs CI TKA | Financial study |
| Tibesku et al[51], 2013 | Germany | Retrospective | PSI TKA vs CI TKA | Financial study |
| Waimann et al[39], 2014 | United States | Prospective | CI TKA | Cost-effectiveness analysis |
| Watters et al[52], 2011 | United States | Retrospective | PSI TKA vs CI TKA | Financial study |
| Xie et al[55], 2010 | Singapore | Non-randomized prospective observational cohort | CI TKA vs other TKA | Cost-effectiveness analysis |
The characteristics and patient demographic of the 32 included studies are summarized in Supplementary Table 2[11,12,27-56].
Of the included studies three were randomised controlled trials[27,38,44], twenty-one were retrospective studies[11,12,28-31,33,34,36,37,40-43,46,49-52,56], seven were prospective studies[32,35,39,45,47,48,55] and one was a theoretical cohort study[54]. Publication years ranged from 1998 to 2020. Of the included studies sixteen were cost-effect analyses, fifteen were financial studies, and two studies used financial decision models.
Eleven studies directly compared PSI TKA to CI TKA. Eight papers compared CI TKA to another type of TKA (robot-assisted, unicondylar, single-use). Six papers only analysed CI TKA. Six papers compared CI TKA to total hip arthroplasty. One single paper compared PSI TKA to CI TKA and single-use instruments for TKA.
Twelve studies assessed costs from a societal perspective[12,32-35,37,39,43,46,53-55], all other studies assessed costs from a hospital perspective. Studies obtained cost estimates by either using their own hospital data set, diagnosis-related group codes, Medicare data (for studies conducted in the United States) or other national cost databases.
The main outcome measure was QALYs for sixteen studies, TKA procedure related costs for eleven studies, and surgical instrumentation and sterilization costs for five studies.
In all studies determining cost-effectiveness based on QALYs, quality of life before and after TKA was determined using health-related quality of life (HRQoL) scores. Thirteen studies used the EQ-5D, SF-6D, SF-12, SF-36, 15D HRQoL or WOMAC to determine QALY gain for TKA. Three other studies determining QALY gain for TKA used a Markov model design.
The total sample size consisted of 19331 patients and 19360 performed TKAs. Of these, 3994 PSI TKAs and 13267 were CI TKAs. All other 2099 TKAs were either unicondylar, computer-assisted, single-use or robot-assisted TKAs.
All three randomised studies had some concerns for RoB[27,38,44]. This was mainly caused by the presence of randomization bias. Thirteen non-randomised studies were assessed, of which eleven had a moderate risk of bias[11,31,36,41,42,48,49,51,52,55,56]. One single study showed a low risk of bias[40] and one study showed serious risk of bias[34]. The main domains in which studies showed moderate risk of bias were the possible presence of bias in measurement of outcome and bias in the selection of participants. The 17 remaining studies could not be assessed for risk of bias as they did not compare two or more interventions or used an economical model without patient population (e.g. Markov-model) to calculate cost-effectiveness[12,25,26,28-30,32,33,35,37,39,43,45-47,50,53,54].
The three included randomised studies have an evidence level of II[27,38,44]. One single study, a case control study, has a level of evidence of IV[36]. All other included studies have an evidence level of III[11,25,26-30,31,32,34,37,39-43,45-55]. The level of evidence and RoB of the included studies have been summarized in Supplementary Table 3.
Economic quality of the included studies according to the CHEC-score were moderate. CHEC-scores ranged from 11 to 17, with an average of 14. CHEC-scores are not available for two of the included studies (Rorabeck and Murray[50], Nunley et al[11])[11,50]. The scoring-system was not applicable for studies in which no costing-models or mean costs were used and/or presented. Basis for lower CHEC-scores was due to inappropriate study design for an economic analysis, a lack of incremental analysis of costs and outcomes, a lack of sensitivity analysis of costs and outcomes, a lack of discounting of costs for appropriate index years and a lack of analysis on ethical aspects of intervention costs. A summary of CHEC scores per study can be found in Supplementary Table 4.
Reported costs and inflation-adjusted costs, are summarized in Supplementary Table 5.
Imaging costs were defined as all imaging needed for patients preoperatively. For CI TKA, this included standard X-ray imaging of the affected knee. Imaging costs for CI TKA ranged from $52.51 to $901.48[39,42,50,54]. Imaging for PSI TKA was defined as MRI-imaging needed to model the PSI. The reported costs ranged from $226.79 to $13942.40[12,27,31,41,44,49,51]. A single study directly compared imaging costs for PSI TKA and CI TKA[51]. The study reported an increased imaging cost of $12091 for PSI TKA.
PSI production costs were defined as the cost of producing the PSI model. Costs were reported to range from $377.98 to $119.35 per model[12,31,44,49,51,52]. The types of PSI models used in the included studies were the patient-specific cutting blocks from MyKnee (Medacta), KneePlan (Symbios), Visionaire (Smith & Nephew’s) and Zimmer-Biomet’s Signature knee system. For CI TKA, no additional production costs were reported.
Sterilization costs were defined as the cost of sterilizing surgical instrument trays per surgical case. Studies have reported a decrease in tray usage when TKA is performed using PSI. The amount of trays needed for CI TKA in all studies ranged from 6 to 34 trays per patient case[41,48,51,52]. For PSI TKA the amount of trays needed per patient case ranged from 1 to 5 trays[41,49,51,52].
A decrease of 4 trays per case have been reported in two studies when using PSI TKA[49,51]. A decrease of 5 trays per case have been reported in two other studies when using PSI TKA[41,52]. Tray sterilization costs ranged from $353.89 to $1533.32 per case for CI TKA[30,41,42,44,51,52]. For PSI TKA, tray sterilization costs ranged from $67.60 to $495.50 per case[36,44,49,52]. In three studies costs of tray sterilization between PSI TKA and CI TKA were directly compared. All three studies reported cost decrease when using PSI TKA[44,51,52]. However, they do not report the amount of decrease in a monetary value.
Operating room (OR) time was defined as time from patients entering the OR to leaving the OR, patient preparation time combined with surgery time, and time from the start of the patient case to the start of the next patient case.
When using CI TKA, studies reported mean OR times that ranged from 63.10 ± 38.02 min to 141.3 ± 22.1 min[11,41,42,44,49,51,52]. When using PSI TKA, studies reported mean OR times that ranged from 58.10 ± 23.04 min to 148.2 ± 16.2 min.
Six studies directly compared mean OR time differences between CI and PSI TKA. One study reported a prolonged mean OR time of 2 min, resulting in associated $1985 in costs when using PSI TKA[41]. The remaining five studies reported a reduced mean OR time when using PSI TKA[40,44,49,51,52]. Time savings reported in these studies ranged from 5 min to 30 min when using PSI TKA.
Additionally, five studies reported the comparison of OR time and their associated costs between CI TKA and PSI TKA. One study does not give exact OR times for CI or PSI TKA. They reported that PSI TKA usage is associated with a mean OR time decrease of 11 min which translated to saving $226.54 per patient case[31]. The three remaining studies observe a decrease in mean OR time when using PSI TKA[49,51,52]. The decrease in mean OR time per patient case for PSI TKA was reported to be between 13 and 30 min[49,51,52]. The associated decrease in cost is respectively between $117.36 and $1416.38[49,51,52].
One study reports lower readmission rates when using PSI TKA compared to CI TKA[56]. Patients who received a TKA using PSI had statistically significant (P ≤ 0.05) lower readmission rates at 30 d (2.03% vs 2.92%), 60 d (2.65% vs 4.02%), 90 d (3.19% vs 4.62%), and at one year (6.46% vs 8.76%).
Total costs for TKA were defined as the sum of costs per patient case for OR time, inpatient stay, sterile processing, surgeon cost, imaging costs, PSI production, TKA implant, and/or postoperative care.
Nine studies solely reported total costs for CI TKA[28-30,32,39,42,43,47,55]. The reported mean cost for CI TKA ranged from $1391 to $29163.16 per patient case.
One study solely reported total cost for PSI TKA[27]. The reported mean cost for PSI TKA was $12642.27 per patient case.
Four studies compared mean PSI TKA costs directly to mean CI TKA costs[38,51,52,56]. In three of the comparing studies, PSI TKA was more costly than CI TKA[38,51,52]. In one study PSI TKA was less costly than CI TKA[56].
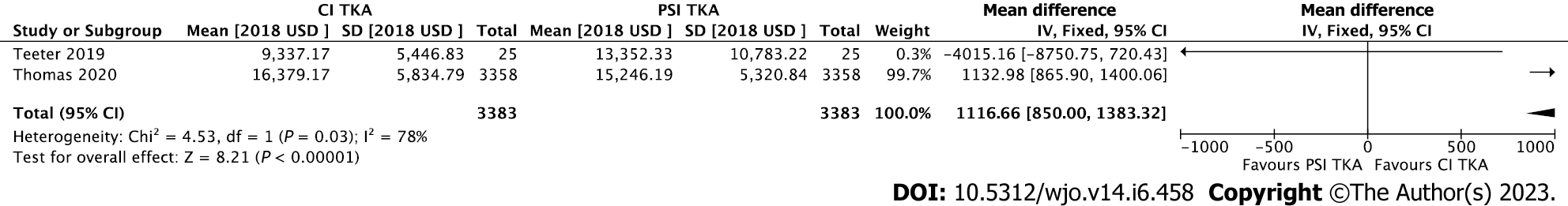
The first study found a mean costs per patient case of $9337.17 ± 5446.83 for CI TKA compared to $13352.33 ± 10783.22 for PSI TKA[38]. The following studies found these mean costs per patient case to be respectively; $8177.77 compared to $8264.76[51], $2989.46 compared to $3608.84[52], and $16379.17 (16182.84-16577.55) compared to $15246.19 (15067.24-15427.18)[56]. An overview of total TKA costs for PSI and CI is presented in Figure 2.
QALY gain was determined as the amount of QALYs gained in a certain number of years after TKA. Seven studies reported data on this for CI TKA[28,29,34,35,37,45,46], no studies reported data on QALY gain for PSI TKA. QALY gain was determined using the EQ-5D in three studies and the SF-12, SF-6D, 15D HRQol and SF-36 were used in the remaining four studies. QALY gain per year for CI TKA was reported as; 0.77/1 year[28], 0.768/1 year[29], 2.6/1 year[34], 0.17/1 year[37], 0.359/1 year[45], 2.93/0.25 years[35] and 1/7.957 years[46].
Costs per QALY were determined in eight of the included studies. QALY outcomes were based on EQ-5D in four studies[34,35,43,47], EQ-5D-3L in two studies[32,33], 15D HRQoL in one study[45] and a Markov model in one other study[12].
One study compared PSI and CI TKA directly[12]. PSI TKA costs $4700/QALY compared to $2900/QALY for CI TKA. Costs per QALY gained determined for CI TKA ranged from $1275.84 per QALY to > $20000 per QALY[32-35,43,45,47].
ICER per QALY was defined as the price per QALY gained for TKA. Four studies presented data on this for CI TKA[29,46,53,55]. No studies presented this data for PSI TKA. ICER per QALY ranged from $20090.25/QALY to $76384.09/QALY.
A meta-analysis was performed using the data on total cost for PSI TKA and CI TKA. Two studies were included into the meta-analysis[38,56]. Two other studies (Tibesku et al[51], Watters et al[52]) comparing total cost for both techniques could not be included into the analysis due to lack of ranges or standard deviation[51,52].
The forest plot is presented in Figure 3. Study heterogeneity was high, with an I2 of 78% (P = 0.03). The overall effect was in favour of CI TKA, with a significant mean difference of $1132.98 [850.00-1383.32] 95%CI (P > 0.00001) less when compared to PSI TKA.
The goal of this systematic review was to assess the cost-effectiveness of PSI TKA compared to CI TKA. Additionally, results were pooled into a meta-analysis. The study aimed to combine the most accurate results available on this topic to produce a clear answer as to whether PSI TKA is a cost-effective alternative to CI TKA.
The main conclusion is that there is a lack of literature on PSI TKA when it comes to data on QALYs and ICER. Furthermore, data on costs of PSI TKA and CI TKA show heterogeneity. Due to this, no definite conclusion can be drawn on the cost-effectiveness of PSI TKA when directly compared to CI TKA. This systematic review has shown that PSI TKA costs less when considering OR time and tray sterilization. PSI TKA costs are increased when considering imaging, production, and costs per total patient case.
There was a large heterogeneity in costs due to differences in calculation methods of costs and patient charges per study. Additionally, country of study conduction has an impact on prices. Furthermore, differences in patient population (such as age, comorbidities, anatomic variance of knee joints) per study could influence the cost outcome. In this systematic review costs were directly compared, but the abovementioned factors should be considered when interpreting the outcome of these comparisons. For example, studies performed in the United States often reported much higher costs than studies performed in Europe.
A meta-analysis was performed, the results were heterogeneous and therefore did not provide conclusive evidence. Furthermore, no meta-analysis could be performed on the QALY or ICER data as no studies were found that directly compared these for PSI TKA and CI TKA. In the future, studies should directly assess and directly compare cost-effectiveness using QALYs and ICER.
Multiple studies have investigated whether PSI TKA is superior to CI TKA when it comes to prosthetic placement, peri- and post-operative outcomes. Schotanus et al[57] described that PSI TKA was ready for primetime after performing a comparative study with CI TKA. The study compared four different PSI TKA systems to CI TKA in a total of 117 knees. PSI TKA was showed to have a lower number of significant outliers[57]. Predescu et al[58] performed a comparative study, where PSI and CI TKA were compared in a population of 80 patients. They found that PSI TKA did not prove superior however posed as an alternative for CI TKA or computer assisted TKA[58]. Furthermore, Sassoon et al[59] described in their systematic review that PSI TKA has not reliably demonstrated improvement of postoperative limb or component alignment[59]. Despite this, PSI TKA has remained popular due to its relatively easy production and especially its usefulness as an additional planning tool in anatomically challenging cases. This systematic review shows that in most cases, more costs are associated with the use of PSI TKA. The results of this review could be taken into consideration when making decisions on whether and when to use PSI TKA.
One single study included into this systematic review determined additional costs for readmission rates related to either PSI or CI use for TKA[55]. The results from this study showed that CI TKA was associated with higher readmission rates, which is associated with increased costs of $6753 ± 175[60]. Thus, more investigation into cost-effectiveness related to readmission rates could be useful for future decision-making regarding instrumentation choices in general. This should also be reconsidered now that robotics are being introduced for TKA at a rapid pace, which entails the additional necessary costs[61].
Results from this systematic review were extracted from a variety of countries. As a result of this, pricing may vary for developing countries or countries with different types of health care pricing systems.
In regards to study quality, multiple studies received sponsoring or funding through prosthetic and/or orthopaedic manufacturers[28,30,33,41,42,46,50]. Minimal influence on the results is expected due to these sponsorships since not all authors were associated with the sponsorships, nor did they receive any payment for the conducted study. Additionally, variety in definition of utilities per study should be considered as pricing may not be based on the exact same parameters per case. Therefore, not all costs in this systematic review should be considered as directly comparable to any or every country. However, these costs are an indication of true cost of PSI and CI TKA worldwide.
This systematic review provided a detailed overview of relevant literature on costs and cost-effectiveness of PSI compared to CI TKA. Systematic reviews on PSI TKA which investigate facets of its costs, such as tray sterilization, are available[59]. However, this systematic review aimed to present relevant literature on all facets of costs associated with PSI TKA and CI TKA.
The strength of this systematic review is its methodological quality. It was executed in accordance with the five-step approach for constructing a review on cost-effect by Van Mastrigt et al[16] and the PRISMA statement[13-18]. Furthermore, a large patient population and all the attributed costs for CI and PSI TKA were analysed.
Its limitation is, however, the possibility of biased results due to exclusion of non-full texts.
This study showed that costs for PSI TKA and CI TKA can differ when considering different aspects of their implementation in a hospital setting. A comprehensive overview of the contributing components to the pricing of CI and PSI TKA is provided. When considering total costs, PSI TKA is more costly when directly compared to CI TKA.
Over the years, extensive research into the clinical outcomes of patient specific instrumentation (PSI) for total knee arthroplasty (TKA) compared to conventional instrumentation (CI) for TKA have been performed. Clinically, the instrumentation techniques are considered equal. However, decreased operating time and sterilization tray usage have been reported when using PSI TKA. These factors could influence the healthcare cost.
Multiple studies into the cost and cost-effectiveness of PSI and CI TKA have been performed since its introduction. Most studies consider specific aspects of their costs, such as: Additional imaging costs, PSI production costs, operating time costs, and tray sterilization costs. Furthermore, studies on Quality Adjusted Life Years (QALY) and Incremental Cost Effectiveness Ratio (ICER) for PSI and CI TKA have been performed. Despite the abundance of research, no clear overview or comparison has been presented. The motivation for this systematic review was to give a clear overview of the cost and cost-effectiveness of PSI TKA compared to CI TKA.
The objective of this research was to present the different aspects of cost of PSI TKA and CI TKA. Furthermore, cost-effectiveness was investigated. By doing this, the secondary objective was to advise orthopaedic surgeons in their decision making when choosing either PSI TKA or CI TKA.
A systematic literature search was performed in healthcare, economical healthcare, and medical databases (MEDLINE, EMBASE, CINAHL, Web of Science, Cochrane Library, EconLit). Relevant literature included randomised controlled trials, retrospective studies, prospective studies, observational studies, and case control studies. Data extraction was performed to obtain the following results: ICER, QALYs, total costs, imaging costs, production costs, sterilization associated costs, surgery duration associated costs and readmission rates and associated costs. Meta-analysis was performed for outcomes with sufficient data.
Thirty-two studies were included into the systematic review. Two were included in the meta-analysis. 3994 PSI TKAs and 13267 CI TKAs were included in the sample size. We found that when considering mean OR time and its associated costs and tray sterilization per patient case, PSI TKA costs less than CI TKA. PSI TKA is more costly compared to CI TKA when considering imaging and production costs. Considering total costs per patient case, PSI TKA is more expensive in comparison to CI TKA. Meta-analysis comparing total costs for PSI TKA, and CI TKA showed a significant higher cost for PSI TKA.
This study showed that costs for PSI TKA and CI TKA can differ when considering different aspects of their implementation. When directly comparing PSI and CI TKA, results showed that total costs per patient case are more for PSI TKA.
Based on the results presented, we recommend orthopaedic surgeons worldwide make careful decisions when deciding on which instrumentation technique to use for TKA. In anatomically challenging cases PSI is a helpful planning modality for TKA. However, this systematic review showed that the total cost of its implementation is higher per patient case. Surgeons are advised to take the cost-effectiveness and total cost into consideration.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: Netherlands
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Oommen AT, India S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Gademan MG, Hofstede SN, Vliet Vlieland TP, Nelissen RG, Marang-van de Mheen PJ. Indication criteria for total hip or knee arthroplasty in osteoarthritis: a state-of-the-science overview. BMC Musculoskelet Disord. 2016;17:463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 183] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 2. | Roh YW, Kim TW, Lee S, Seong SC, Lee MC. Is TKA using patient-specific instruments comparable to conventional TKA? A randomized controlled study of one system. Clin Orthop Relat Res. 2013;471:3988-3995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 3. | Chareancholvanich K, Narkbunnam R, Pornrattanamaneewong C. A prospective randomised controlled study of patient-specific cutting guides compared with conventional instrumentation in total knee replacement. Bone Joint J. 2013;95-B:354-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 4. | Boonen B, Schotanus MG, Kerens B, van der Weegen W, van Drumpt RA, Kort NP. Intra-operative results and radiological outcome of conventional and patient-specific surgery in total knee arthroplasty: a multicentre, randomised controlled trial. Knee Surg Sports Traumatol Arthrosc. 2013;21:2206-2212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 5. | Abane L, Anract P, Boisgard S, Descamps S, Courpied JP, Hamadouche M. A comparison of patient-specific and conventional instrumentation for total knee arthroplasty: a multicentre randomised controlled trial. Bone Joint J. 2015;97-B:56-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 6. | Noble JW Jr, Moore CA, Liu N. The value of patient-matched instrumentation in total knee arthroplasty. J Arthroplasty. 2012;27:153-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 164] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 7. | Chen JY, Yeo SJ, Yew AK, Tay DK, Chia SL, Lo NN, Chin PL. The radiological outcomes of patient-specific instrumentation vs conventional total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2014;22:630-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Bali K, Walker P, Bruce W. Custom-fit total knee arthroplasty: our initial experience in 32 knees. J Arthroplasty. 2012;27:1149-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Schotanus MGM, Boonen B, van der Weegen W, Hoekstra H, van Drumpt R, Borghans R, Vos R, van Rhijn L, Kort NP. No difference in mid-term survival and clinical outcome between patient-specific and conventional instrumented total knee arthroplasty: a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2019;27:1463-1468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Schoenmakers DAL, Schotanus MGM, Boonen B, Kort NP. Consistency in patient-reported outcome measures after total knee arthroplasty using patient-specific instrumentation: a 5-year follow-up of 200 consecutive cases. Knee Surg Sports Traumatol Arthrosc. 2018;26:1800-1804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Nunley RM, Ellison BS, Ruh EL, Williams BM, Foreman K, Ford AD, Barrack RL. Are patient-specific cutting blocks cost-effective for total knee arthroplasty? Clin Orthop Relat Res. 2012;470:889-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 164] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 12. | Slover JD, Rubash HE, Malchau H, Bosco JA. Cost-effectiveness analysis of custom total knee cutting blocks. J Arthroplasty. 2012;27:180-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | PeerJ. PRISMA-P (Preferred Reporting Items for Systematic review and Meta-analysis Protocol) 2015 checklist: recommended items to address in a systematic review protocol. Available from: https://peerj.com/articles/4598/#supp-5. |
| 14. | Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15040] [Cited by in RCA: 15826] [Article Influence: 1582.6] [Reference Citation Analysis (1)] |
| 15. | Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7463] [Cited by in RCA: 8226] [Article Influence: 822.6] [Reference Citation Analysis (0)] |
| 16. | van Mastrigt GA, Hiligsmann M, Arts JJ, Broos PH, Kleijnen J, Evers SM, Majoie MH. How to prepare a systematic review of economic evaluations for informing evidence-based healthcare decisions: a five-step approach (part 1/3). Expert Rev Pharmacoecon Outcomes Res. 2016;16:689-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 144] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 17. | Thielen FW, Van Mastrigt G, Burgers LT, Bramer WM, Majoie H, Evers S, Kleijnen J. How to prepare a systematic review of economic evaluations for clinical practice guidelines: database selection and search strategy development (part 2/3). Expert Rev Pharmacoecon Outcomes Res. 2016;16:705-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 18. | Wijnen B, Van Mastrigt G, Redekop WK, Majoie H, De Kinderen R, Evers S. How to prepare a systematic review of economic evaluations for informing evidence-based healthcare decisions: data extraction, risk of bias, and transferability (part 3/3). Expert Rev Pharmacoecon Outcomes Res. 2016;16:723-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 133] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 19. | Booth A, Clarke M, Dooley G, Ghersi D, Moher D, Petticrew M, Stewart L. The nuts and bolts of PROSPERO: an international prospective register of systematic reviews. Syst Rev. 2012;1:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 638] [Cited by in RCA: 895] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 20. | Lefebvre C, Manheimer E, Glanville J. Searching for studies. In: Higgins JPT, Thomas J, Chandler J, cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane Handbook of Systematic Reviews of Interventions. John Wiley and Sons; 2008: 95-150. Available from: https://handbook-5-1.cochrane.org/chapter_6/6_searching_for_studies.htm. |
| 21. | Refworks-Copyright 2022. ProQuest LLC. Available from: Available from: https://www.refworks.com/refworks2/?. |
| 22. | CCEMG. CCEMG-EPPI-Centre Cost Converter’ (v.1.6 Last update: 29 April 2019). Available from: https://eppi.ioe.ac.uk/costconversion/. |
| 23. | Higgins J, Altman D. Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series. Edited by: Higgins JP, Green S. 2008. John Wiley & Sons. 2008;. [DOI] [Full Text] |
| 24. | Jeremy Howick, Iain Chalmers, Paul Glasziou, Trish Greenhalgh, Carl Heneghan, Alessandro Liberati, Ivan Moschetti, Bob Phillips, and Hazel Thornton. “The 2011 Oxford CEBM Levels of Evidence (Introductory Document)” Oxford Centre for Evidence-Based Medicine. Available from: https://www.cebm.ox.ac.uk/resources/Levels-of-evidence/ocebm-levels-of-evidence. |
| 25. | Evers S, Goossens M, de Vet H, van Tulder M, Ament A. Criteria list for assessment of methodological quality of economic evaluations: Consensus on Health Economic Criteria. Int J Technol Assess Health Care. 2005;21:240-245. [PubMed] |
| 26. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46387] [Article Influence: 2108.5] [Reference Citation Analysis (3)] |
| 27. | Lionberger DR, Crocker CL, Chen V. Patient specific instrumentation. J Arthroplasty. 2014;29:1699-1704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Schilling CG, Dowsey MM, Petrie DJ, Clarke PM, Choong PF. Predicting the Long-Term Gains in Health-Related Quality of Life After Total Knee Arthroplasty. J Arthroplasty. 2017;32:395-401.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Elmallah RK, Chughtai M, Khlopas A, Bhowmik-Stoker M, Bozic KJ, Kurtz SM, Mont MA. Determining Cost-Effectiveness of Total Hip and Knee Arthroplasty Using the Short Form-6D Utility Measure. J Arthroplasty. 2017;32:351-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 30. | Goldberg TD, Maltry JA, Ahuja M, Inzana JA. Logistical and Economic Advantages of Sterile-Packed, Single-Use Instruments for Total Knee Arthroplasty. J Arthroplasty. 2019;34:1876-1883.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Barrack RL, Ruh EL, Williams BM, Ford AD, Foreman K, Nunley RM. Patient specific cutting blocks are currently of no proven value. J Bone Joint Surg Br. 2012;94:95-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 32. | Jenkins PJ, Clement ND, Hamilton DF, Gaston P, Patton JT, Howie CR. Predicting the cost-effectiveness of total hip and knee replacement: a health economic analysis. Bone Joint J. 2013;95-B:115-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 253] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 33. | Dakin H, Eibich P, Beard D, Gray A, Price A, ACHE Study team. The use of patient-reported outcome measures to guide referral for hip and knee replacement: Part 2–a cost-effectiveness analysis. Bone Joint J. 2020;102. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 34. | Stan G, Orban H, Orban C. Cost Effectiveness Analysis of Knee Osteoarthritis Treatment. Chirurgia (Bucur). 2015;110:368-374. [PubMed] |
| 35. | Krummenauer F, Wolf C, Günther KP, Kirschner S. Clinical benefit and cost effectiveness of total knee arthroplasty in the older patient. Eur J Med Res. 2009;14:76-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 36. | Moerenhout K, Allami B, Gkagkalis G, Guyen O, Jolles BM. Advantages of patient-specific cutting guides with disposable instrumentation in total knee arthroplasty: a case control study. J Orthop Surg Res. 2021;16:188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Konopka JF, Lee YY, Su EP, McLawhorn AS. Quality-Adjusted Life Years After Hip and Knee Arthroplasty: Health-Related Quality of Life After 12,782 Joint Replacements. JB JS Open Access. 2018;3:e0007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 38. | Teeter MG, Marsh JD, Howard JL, Yuan X, Vasarhelyi EM, McCalden RW, Naudie DDR. A randomized controlled trial investigating the value of patient-specific instrumentation for total knee arthroplasty in the Canadian healthcare system. Bone Joint J. 2019;101-B:565-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 39. | Waimann CA, Fernandez-Mazarambroz RJ, Cantor SB, Lopez-Olivo MA, Zhang H, Landon GC, Siff SJ, Suarez-Almazor ME. Cost-effectiveness of total knee replacement: a prospective cohort study. Arthritis Care Res (Hoboken). 2014;66:592-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 40. | Siegel GW, Patel NN, Milshteyn MA, Buzas D, Lombardo DJ, Morawa LG. Cost Analysis and Surgical Site Infection Rates in Total Knee Arthroplasty Comparing Traditional vs. Single-Use Instrumentation. J Arthroplasty. 2015;30:2271-2274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 41. | Thienpont E, Paternostre F, Van Wymeersch C. The indirect cost of Patient-Specific Instruments. Acta Orthop Belg. 2015;81:462-470. [PubMed] |
| 42. | Cotter EJ, Wang J, Illgen RL. Comparative Cost Analysis of Robotic-Assisted and Jig-Based Manual Primary Total Knee Arthroplasty. J Knee Surg. 2022;35:176-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 43. | Dakin H, Gray A, Fitzpatrick R, Maclennan G, Murray D; KAT Trial Group. Rationing of total knee replacement: a cost-effectiveness analysis on a large trial data set. BMJ Open. 2012;2:e000332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 140] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 44. | Attard A, Tawy GF, Simons M, Riches P, Rowe P, Biant LC. Health costs and efficiencies of patient-specific and single-use instrumentation in total knee arthroplasty: a randomised controlled trial. BMJ Open Qual. 2019;8:e000493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 45. | Räsänen P, Paavolainen P, Sintonen H, Koivisto AM, Blom M, Ryynänen OP, Roine RP. Effectiveness of hip or knee replacement surgery in terms of quality-adjusted life years and costs. Acta Orthop. 2007;78:108-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 285] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 46. | Losina E, Walensky RP, Kessler CL, Emrani PS, Reichmann WM, Wright EA, Holt HL, Solomon DH, Yelin E, Paltiel AD, Katz JN. Cost-effectiveness of total knee arthroplasty in the United States: patient risk and hospital volume. Arch Intern Med. 2009;169:1113-21; discussion 1121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 438] [Cited by in RCA: 441] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 47. | Navarro Espigares JL, Hernández Torres E. Cost-outcome analysis of joint replacement: evidence from a Spanish public hospital. Gac Sanit. 2008;22:337-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 48. | Mont MA, Pivec R, Johnson AJ, Issa K. Single-use cutting blocks and trials lower costs in primary total knee arthroplasty. Surg Technol Int. 2012;22:331-335. [PubMed] |
| 49. | DeHaan AM, Adams JR, DeHart ML, Huff TW. Patient-specific vs conventional instrumentation for total knee arthroplasty: peri-operative and cost differences. J Arthroplasty. 2014;29:2065-2069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 50. | Rorabeck CH, Murray P. The cost benefit of total knee arthroplasty. Orthopedics. 1996;19:777-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 51. | Tibesku CO, Hofer P, Portegies W, Ruys CJ, Fennema P. Benefits of using customized instrumentation in total knee arthroplasty: results from an activity-based costing model. Arch Orthop Trauma Surg. 2013;133:405-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 52. | Watters TS, Mather RC 3rd, Browne JA, Berend KR, Lombardi AV Jr, Bolognesi MP. Analysis of procedure-related costs and proposed benefits of using patient-specific approach in total knee arthroplasty. J Surg Orthop Adv. 2011;20:112-116. [PubMed] |
| 53. | Peersman G, Jak W, Vandenlangenbergh T, Jans C, Cartier P, Fennema P. Cost-effectiveness of unicondylar vs total knee arthroplasty: a Markov model analysis. Knee. 2014;21 Suppl 1:S37-S42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 54. | Slover J, Espehaug B, Havelin LI, Engesaeter LB, Furnes O, Tomek I, Tosteson A. Cost-effectiveness of unicompartmental and total knee arthroplasty in elderly low-demand patients. A Markov decision analysis. J Bone Joint Surg Am. 2006;88:2348-2355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 55. | Xie F, Lo NN, Tarride JE, O'Reilly D, Goeree R, Lee HP. Total or partial knee replacement? Cost-utility analysis in patients with knee osteoarthritis based on a 2-year observational study. Eur J Health Econ. 2010;11:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 56. | Thomas S, Patel A, Patrick C, Delhougne G. Total Hospital Costs and Readmission Rate of Patient-Specific Instrument in Total Knee Arthroplasty Patients. J Knee Surg. 2022;35:113-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 57. | Schotanus MG, Boonen B, Kort NP. Patient specific guides for total knee arthroplasty are ready for primetime. World J Orthop. 2016;7:61-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 58. | Predescu V, Prescura C, Olaru R, Savin L, Botez P, Deleanu B. Patient specific instrumentation vs conventional knee arthroplasty: comparative study. Int Orthop. 2017;41:1361-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 59. | Sassoon A, Nam D, Nunley R, Barrack R. Systematic review of patient-specific instrumentation in total knee arthroplasty: new but not improved. Clin Orthop Relat Res. 2015;473:151-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 131] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 60. | Urish KL, Qin Y, Li BY, Borza T, Sessine M, Kirk P, Hollenbeck BK, Helm JE, Lavieri MS, Skolarus TA, Jacobs BL. Predictors and Cost of Readmission in Total Knee Arthroplasty. J Arthroplasty. 2018;33:2759-2763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 61. | Rajan PV, Khlopas A, Klika A, Molloy R, Krebs V, Piuzzi NS. The Cost-Effectiveness of Robotic-Assisted Versus Manual Total Knee Arthroplasty: A Markov Model-Based Evaluation. J Am Acad Orthop Surg. 2022;30:168-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |