Published online Apr 18, 2023. doi: 10.5312/wjo.v14.i4.218
Peer-review started: January 13, 2023
First decision: February 2, 2023
Revised: February 10, 2023
Accepted: April 4, 2023
Article in press: April 4, 2023
Published online: April 18, 2023
Processing time: 95 Days and 8.1 Hours
Endoprosthetic distal femoral replacement (DFR) is a well-established salvage procedure following resection of malignant tumors within the distal femur. Use of an all-polyethylene tibial (APT) component is cost-effective and avoids failure due to locking-mechanism issues and backside wear, but limits modularity and the option for late liner exchange. Due to a paucity of literature we sought to answer three questions: (1) What are the most common modes of implant failure for patients undergoing cemented DFR with APT for oncologic indications? (2) What is the survivorship, rate of all-cause reoperation, and rate of revision for aseptic loosening of these implants? And (3) Is there a difference in implant survivorship or patient demographics between cemented DFRs with APT performed as a primary reconstruction vs those performed as a revision procedure?
To assess outcomes of cemented DFRs with APT components used for oncologic indications.
After Institutional Review Board approval, a retrospective review of consecutive patients who underwent DFR between December 2000 to September 2020 was performed using a single-institutional database. Inclusion criteria consisted of all patients who underwent DFR with a GMRS® (Global Modular Replacement System, Stryker, Kalamazoo, MI, United States) cemented distal femoral endoprosthesis and APT component for an oncologic indication. Patients undergoing DFR for non-oncologic indications and patients with metal-backed tibial components were excluded. Implant failure was recorded using Henderson's classification and survivorship was reported using a competing risks analysis.
55 DFRs (55 patients) with an average age of 50.9 ± 20.7 years and average body mass index of 29.7 ± 8.3 kg/m2 were followed for 38.8 ± 54.9 mo (range 0.2-208.4). Of these, 60.0% were female and 52.7% were white. The majority of DFRs with APT in this cohort were indicated for oncologic diagnoses of osteogenic sarcoma (n = 22, 40.0%), giant cell tumor (n = 9, 16.4%), and metastatic carcinoma (n = 8, 14.6%). DFR with APT implantation was performed as a primary procedure in 29 patients (52.7%) and a revision procedure in 26 patients (47.3%). Overall, twenty patients (36.4%) experienced a postoperative complication requiring reoperation. The primary modes of implant failure included Henderson Type 1 (soft tissue failure, n = 6, 10.9%), Type 2 (aseptic loosening, n = 5, 9.1%), and Type 4 (infection, n = 6, 10.9%). There were no significant differences in patient demographics or rates of postoperative complications between the primary procedure and revision procedure subgroups. In total, 12 patients (21.8%) required a revision while 20 patients (36.4%) required a reoperation, resulting in three-year cumulative incidences of 24.0% (95%CI 9.9%-41.4%) and 47.2% (95%CI 27.5%-64.5%), respectively.
This study demonstrates modest short-term survivorship following cemented DFR with APT components for oncologic indications. Soft tissue failure and endoprosthetic infection were the most common postoperative complications in our cohort.
Core Tip: The current study demonstrates modest short-term survivorship following cemented distal femoral replacement with all-polyethylene tibial components for oncologic indications. Approximately one third of patients experienced a postoperative complication. The most common modes of implant failure were soft tissue failure and endoprosthetic infection.
- Citation: Christ AB, Chung BC, Urness M, Mayer LW, Gettleman BS, Heckmann ND, Menendez LR. Clinical outcomes of cemented distal femur replacements with all-polyethylene tibial components for oncologic indications. World J Orthop 2023; 14(4): 218-230
- URL: https://www.wjgnet.com/2218-5836/full/v14/i4/218.htm
- DOI: https://dx.doi.org/10.5312/wjo.v14.i4.218
Endoprosthetic reconstruction of the distal femur has been used as a limb-salvage procedure to treat oncologic processes of the distal femur for nearly five decades[1], and is currently considered standard of practice for this indication. Improvements in design, such as a rotating hinge mechanism and ongrowth collars adjacent to the femoral cut surface, have improved survivorship with regards to aseptic loosening, and are now included in most modern systems[2-4]. However, implant design and research regarding fixation has focused primarily on the femoral side[5-7].
Metal-backed and all-polyethylene tibial components are available, both of which can be fixed to the bone with fully cemented, hybrid, or in some cases cementless fashion. However, there is a paucity of literature examining the survivorship of distal femoral replacements (DFRs) with respect to the type of tibial component or fixation used[8,9]. Furthermore, the majority of available studies fail to describe the type of tibial component or fixation used[10,11]. Therefore, the purpose of this study is to assess outcomes of cemented DFRs with all-polyethylene tibia (APT) components used for oncologic indications.
Specifically, we sought to answer the following research questions: (1) What are the most common modes of implant failure for patients undergoing cemented DFR with APT for oncologic indications? (2) What is the survivorship, rate of all-cause reoperation, and rate of revision for aseptic loosening of these implants? and (3) Is there a difference in implant survivorship or patient demographics between cemented DFRs with APT performed as a primary reconstruction vs those performed as a revision procedure?
After Institutional Review Board approval (IRB HS-20-00396), a retrospective review of consecutive patients who underwent DFR between December 2000 to September 2020 was performed using a single-institutional database. The DFR was performed either as the primary treatment for the disease in question, or as a revision of a previous failed surgery (indications included recurrence, fracture, etc.). We then defined reoperation as any subsequent procedure, including manipulation under anesthesia, that was performed after placement of the DFR. Revision of the DFR was defined as a subsequent procedure which specifically required exchange or removal of femoral or tibial components. Inclusion criteria consisted of all patients who underwent DFR with a GMRS® (Global Modular Replacement System, Stryker, Kalamazoo, MI, United States) cemented distal femoral endoprosthesis and APT component for an oncologic indication. Patients were excluded if undergoing DFR for non-oncologic indications or if a different implant design was used. Patients were then stratified into two groups based on whether the index procedure was a primary reconstruction or a revision of a previous DFR. Patients who had undergone previous incisional biopsies or arthroscopic procedures without reconstruction on the operative limb prior to DFR implantation were classified in the primary reconstruction cohort.
Thorough review of patient medical records and operative reports was performed to obtain patient demographic information including comorbidities, age at the time of surgery, sex, race/ethnicity, body mass index (BMI), and American Society of Anesthesiologists score. Operative reports were reviewed to obtain surgical variables including the indication for surgery, a comprehensive surgical history of the operative limb, surgical approach, implants utilized, and operative time. The primary outcome was implant survivorship, with all-cause reoperation and revision total knee arthroplasty as endpoints. Given the primary purpose of the present study was to characterize early complications and implant longevity in the setting of limb-salvage, functional and patient-reported outcome measures were not collected.
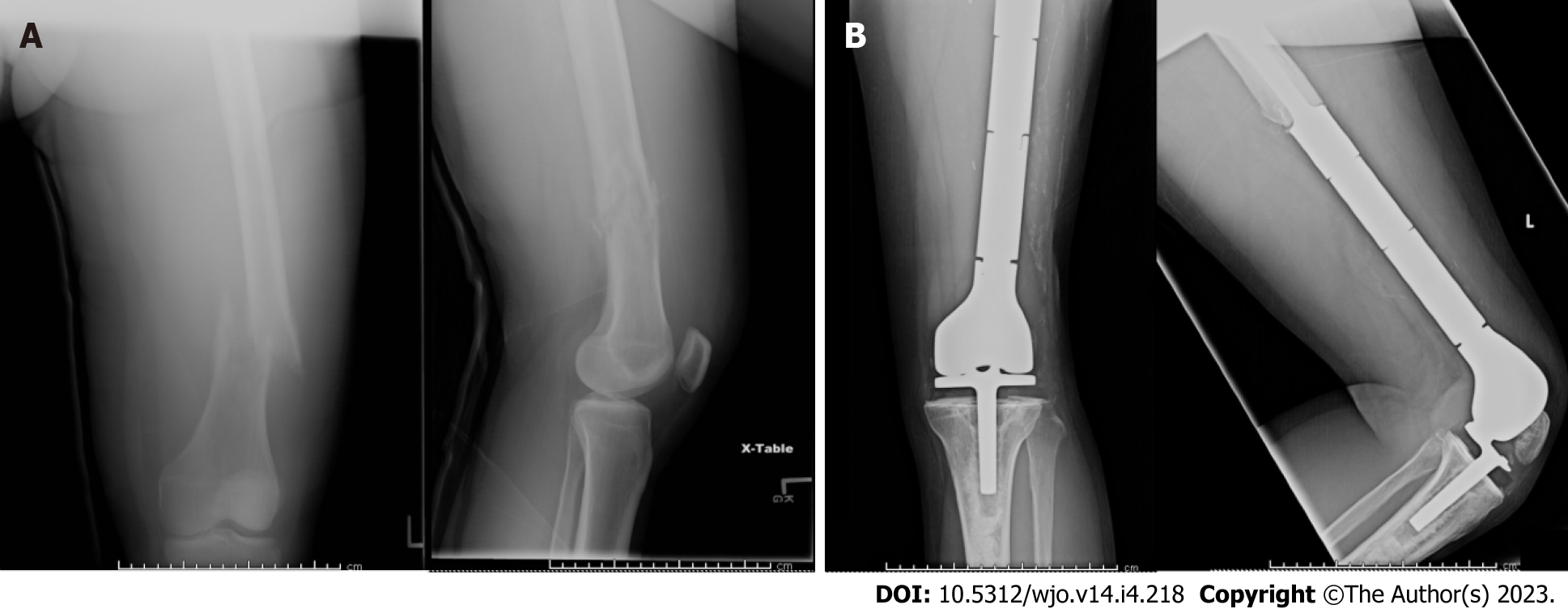
The Stryker GMRS® (Global Modular Replacement System, Stryker, Kalamazoo, MI, United States) was utilized for all cases in this series. This system is designed to assist in the reconstruction of large segmental and osteoarticular defects about the knee joint, particularly in the setting of tumors, previously failed arthroplasty, and traumatic injury. In this system, the standard distal femoral components can be paired with either a modular rotating-hinge tibial baseplate or APT component, the latter of which was used selectively in the present cohort (Figure 1). Multiple cemented stem options are available, including straight, curved, and long curved geometries, both with and without extra-cortical porous-coated intercalated body sections. This construct can be further customized with the use of extension pieces, available in over a dozen sizes, for the optimization of leg length.
Medial and lateral parapatellar approaches were utilized based upon previous biopsy incisions and location of the neoplasm. Following oncologic resection, the femur and tibia were prepared using conventional jigs and reamers, and the femur was reamed in a sequential manner to accommodate the appropriate stem diameter and length. Trial implants were inserted to assess appropriate range of motion, limb length, and patellar tracking prior to insertion of the final implant. Polymethylmethacrylate cement was used for fixation in all cases.
Each patient’s clinical course was followed in detail to characterize postoperative complications and the need for reoperations or revision surgery. Given the complex patient demographics, we decided to not have a minimum follow-up in order to capture all patients who underwent this reconstruction. No follow-up was chosen over the conventional two-year minimum to capture early postoperative complications in this high-risk cohort of patients undergoing limb-salvage procedures, especially those with metastatic disease or prior failed reconstruction. Following the index procedure, any procedure on the affected limb that did not involve removal or alteration of the endoprosthesis was classified as a reoperation. Revision procedures were defined as any surgery involving removal or replacement of any prosthetic component. Implant failure was categorized based on the Henderson classification of failure of limb salvage after reconstructive surgery for bone tumors[12].
In total, 92 patients were identified during the study period and screened for inclusion. Eleven patients (12.0%) with a metal-backed tibial baseplate and 26 patients (28.3%) who underwent DFR for a non-oncologic indication were excluded, leaving 55 patients (59.8%) who were included in the final analysis. The mean age of the cohort was 50.9 ± 20.7 years (range, 16-88 years) and mean BMI was 29.7 ± 8.3 kg/m2 (range, 16.4-52.9). The average follow-up of the study cohort was 38.8 ± 54.9 mo (range 0.2-208.4 mo), with a total of 21 patients (38.2%) possessing a minimum follow-up of 2 years (Table 1).
| Demographic variable | Value |
| Age (mean ± SD) | 50.9 ± 20.7 yr |
| Gender | |
| Male | 22 (40.0) |
| Female | 33 (60.0) |
| Race/Ethnicity | |
| White | 29 (52.7) |
| Hispanic or Latino | 9 (16.4) |
| Black | 4 (7.3) |
| Asian | 5 (9.1) |
| Other | 8 (14.5) |
| ASA score | |
| 1 | 10 (18.2) |
| 2 | 21 (38.2) |
| 3 | 21 (38.2) |
| 4 | 3 (5.5) |
| Body mass index (mean ± SD) | 29.7 ± 8.3 kg/m2 |
| Follow-up (mean ± SD) | 38.8 ± 54.9 mo |
DFR with APT implantation was performed as a primary procedure in 29 patients (52.7%) and a revision procedure in 26 patients (47.3%), with a median operative time of 178 minutes across the entire cohort. For the 26 patients who underwent DFR with APT implantation as a revision procedure, the average number of previous surgeries on the affected limb was 2.0 ± 1.3 (range 1-5) (Table 2). The primary procedures for the revision were as follows: distal femoral replacement[12], open reduction internal fixation for pathologic fracture[6], Cryoablation +/- curettage[6], and soft tissue resection[2] (Table 3). The majority of DFRs with APT in this cohort were indicated for oncologic diagnoses of osteogenic sarcoma (n = 22, 40.0%), giant cell tumor (n = 9, 16.4%), and metastatic carcinoma (n = 8, 14.6%) (Table 2).
| Operative variable | Value |
| Procedure type | |
| Primary | 29 (52.7) |
| Revision | 26 (47.3) |
| Surgical indications | |
| Osteogenic sarcoma | 22 (40.0) |
| Giant cell tumor | 9 (16.4) |
| Metastatic carcinoma | 8 (14.6) |
| Soft-tissue sarcomaa | 4 (7.3) |
| Chondrosarcoma | |
| High-grade | 3 (5.5) |
| Low-grade | 2 (3.6) |
| Synovial chondromatosis | 2 (3.6) |
| Multiple myeloma | 2 (3.6) |
| Non-Hodgkin's lymphoma | 1 (1.8) |
| Pigmented villonodular synovitis | 1 (1.8) |
| Neoplasm of unspecified behavior | 1 (1.8) |
| Primary mode of failure, henderson classification | |
| Type 1 (soft-tissue failure) | 6 (10.9) |
| Type 2 (aseptic loosening) | 5 (9.1) |
| Type 3 (structural failure)b | 2 (3.6) |
| Type 4 (periprosthetic infection) | 6 (10.9) |
| Type 5 (tumor progression) | 1 (1.8) |
| Number of previous surgeries (mean ± SD) | 1.1 ± 1.3 surgeries |
| Primary procedure in the revision cohort (N = 26) | |
| Category | Number of patients |
| Distal femoral replacement | 12 |
| Open reduction internal fixation for pathologic fracture | 6 |
| Curettage +/- cryoablation | 6 |
| Soft tissue resection | 2 |
Statistical analyses were performed using SPSS version 26 (IBM, Armonk, New York, United States). Patient demographics, operative variables, and postoperative complications are presented as means or percentages with standard deviations or ranges where appropriate. Univariate analyses were performed to compare differences between groups using the Mann-Whitney-U test for continuous variables and Chi-square test for categorical variables or Fisher’s exact test where appropriate. Competing risk analyses were performed to evaluate the cumulative incidence of all-cause reoperation, need for revision surgery, and patient death. Competing risk analysis was conducted using the survival[13,14] and cmprsk[15] function within R (R Core Team, 2021 packages)[16]. Figure 2 and Figure 3 were produced using the package ggplot2[17,18].
In total, 20 patients (36.4%) experienced a postoperative complication requiring reoperation (Figure 4). The indications for the 26 reoperations were the following: Mechanical failure[11], non-union of prior pathological fracture[7], tumor progression[3], definitive management of a prior open reduction internal fixation for a pathologic fracture[2], local recurrence[1], infection[1], and soft tissue failure[1] (Table 4). Of these 20 cases requiring reoperation, 7 patients (12.7%) required only one reoperation, 2 patients (3.6%) required 2 reoperations, 4 patients (7.3%) required 3 reoperations, and 7 patients (12.7%) required 4+ reoperations. The primary modes of implant failure in this cohort according to Henderson’s classification included Type 1 (soft tissue failure, n = 6, 10.9%), Type 2 (aseptic loosening, n = 5, 9.1%), and Type 4 (infection, n = 6, 10.9%) (Table 2). Of the 6 patients with a soft tissue failure, 3 were due to arthrofibrosis, 2 due to extensor mechanism failures, and one was due to wound dehiscence. Regarding the patients who failed due to infection, none of the individuals were on chemotherapy when infection was identified. Finally, local recurrence of the primary bone tumor occurred in one patient who was diagnosed with a “neoplasm of unspecified behavior” and was managed with radical resection at 13.2 mo (Henderson Type 5).
| Reason for revision | |
| Category | Number of patients |
| Mechanical failure | 11 |
| Nonunion of prior pathological fracture | 7 |
| Tumor Progression | 3 |
| Definitive management of a prior open reduction internal fixation for a pathological fracture | 2 |
| Local recurrence | 1 |
| Infection | 1 |
| Soft tissue failure | 1 |
Of the five patients who required reoperation for soft tissue failure (Henderson Type 1), two patients experienced arthrofibrosis requiring manipulation under anesthesia with lysis of adhesions at 3.3 and 4.0 mo postoperatively, two patients required extensor mechanism repair for postoperative falls at 14.5 and 19.7 mo postoperatively, and one patient required multiple flaps for soft tissue reconstruction at 6.8 mo postoperatively. All six patients who underwent reoperation for infection (Henderson Type 4) were managed with serial irrigation and debridement procedures (mean 2.2 procedures, range 1-5), with two patients requiring antibiotic spacer placement and three patients undergoing soft tissue reconstruction at the time of reoperation. None of these patients required amputation.
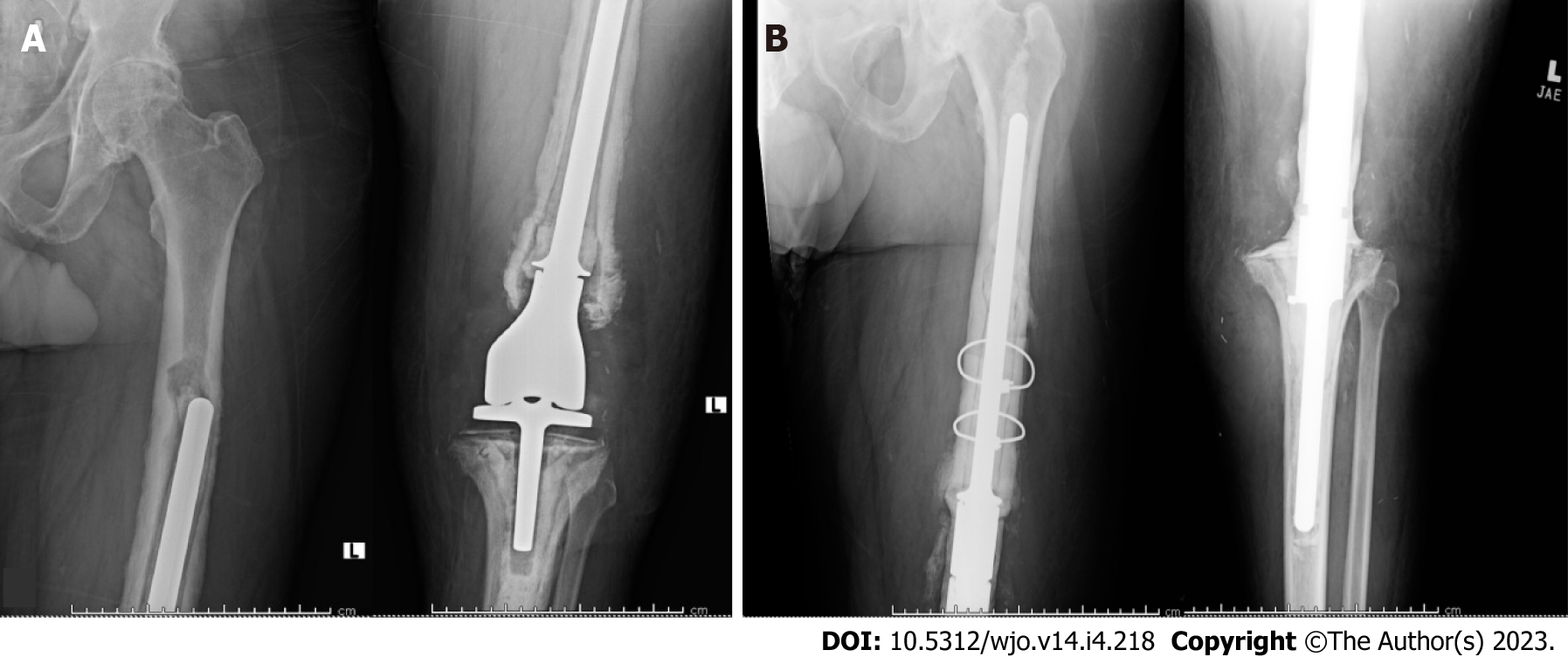
Additionally, two patients required revision surgery for corrosion and metal wear debris at 32.5 mo and 99.5 mo postoperatively (Type 3). Two cases were complicated by deep peroneal nerve palsy, which were managed nonoperatively with ankle-foot orthoses. There were no identified cases for which periprosthetic fracture was the primary indication for revision surgery with the use of these constructs.
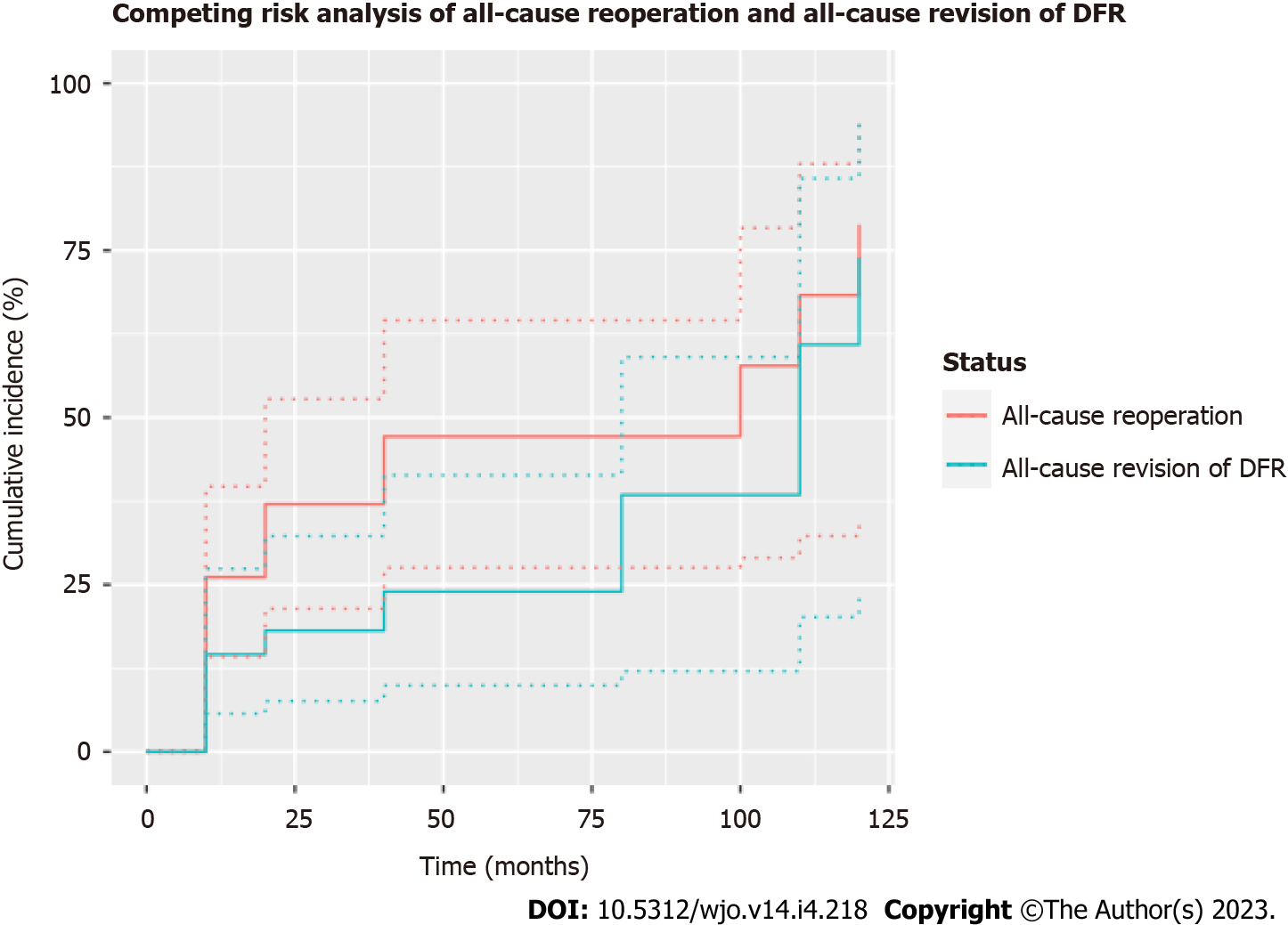
Competing risks analysis depicting the need for any revision operation (requiring exchange of either the femoral or tibial component), any reoperation, and patient death were plotted (Figure 3). In total, 12 patients (21.8%) required a revision, resulting in one- and three-year cumulative incidence of 14.6% (95%CI 5.7%-27.4%) and 24.0% (95%CI 9.9%-41.4%), respectively, with all-cause revision as the endpoint. Additionally, 20 patients (36.4%) required reoperation, resulting in one- and three-year cumulative incidences of 26.1% (95%CI 14.2%-39.7%) and 47.2% (95%CI 27.5%-64.5%), respectively, with all-cause reoperation as the endpoint. At final follow-up, one patient (1.8%) had died, with cause of death unrelated to the DFR procedure. No information regarding the date of death was available for this patient.
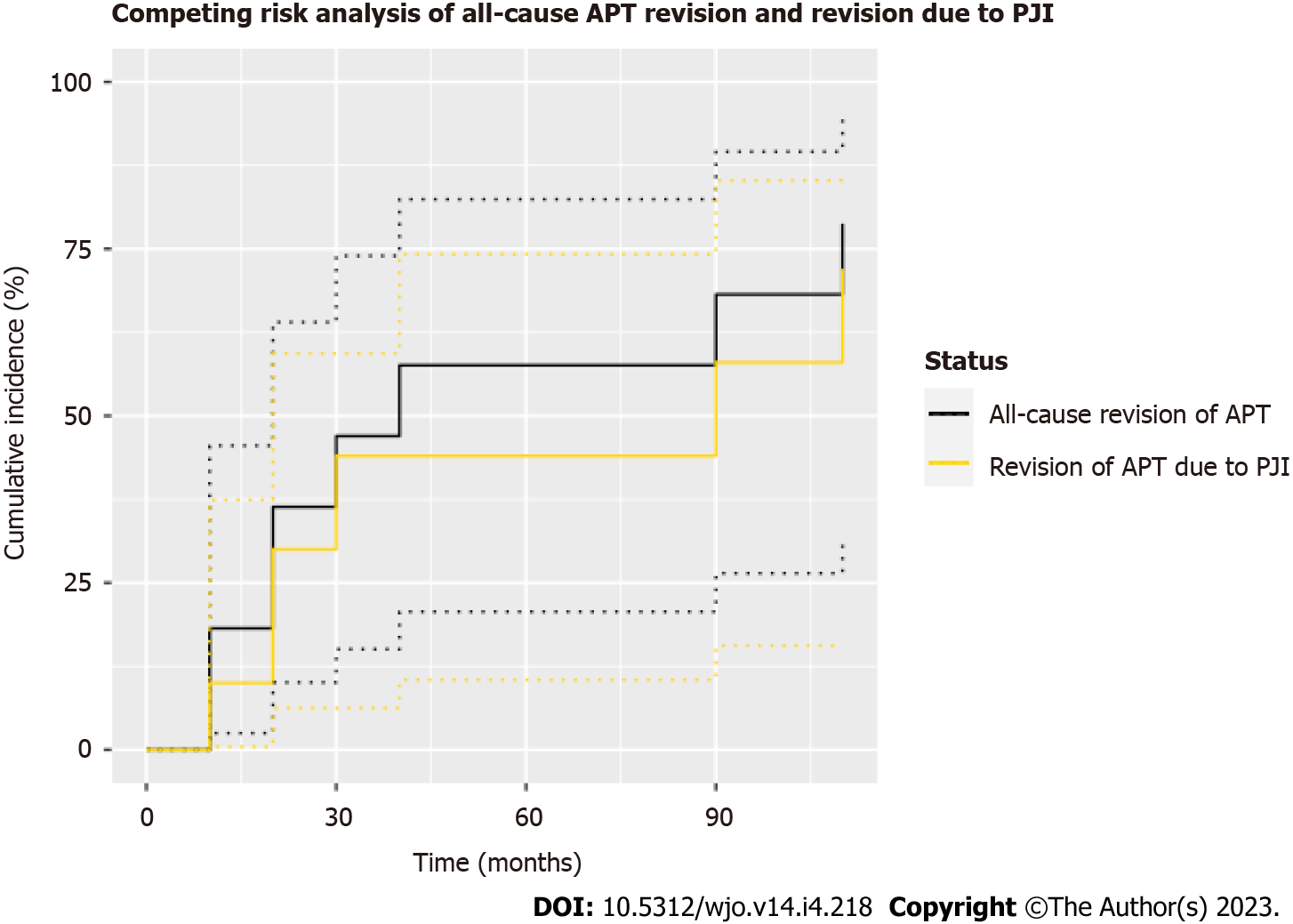
In total, 10 of the 12 patients (83.3%) who underwent revision surgery required revision of the APT component. Of these 10 patients, three (30.0%) were revised due to aseptic loosening at an average of 80.4 mo postoperatively, six (60.0%) were revised due to periprosthetic joint infection at an average of 44.8 mo, and one (10.0%) was revised due to periprosthetic fracture requiring placement of medial and lateral titanium plates at 9.6 mo. A second competing risks analysis depicting the incidence of all-cause revision of the APT component and APT component failure secondary to periprosthetic joint infection (PJI) was plotted (Figure 3). This analysis demonstrated one- and three-year cumulative incidences of 18.2% (95%CI 2.5%-45.5%) and 47.0% (95%CI 15.1%-74.0%), respectively, with all-cause revision of the APT component as the endpoint. When failure of the APT secondary to PJI was used as the endpoint, the one- and three-year cumulative incidences were 10.0% (95%CI 0.5%-37.4%) and 44.0% (95%CI 6.3%-59.3%), respectively.
No significant differences in patient demographics or reoperation rates were identified between patients for whom the index procedure was a primary reconstruction (“primary DFR”) and patients for whom DFR was performed as a revision procedure (“revision DFR”). Both cohorts had similar lengths of clinical follow-up (42.7 ± 61.4 vs 34.5 ± 47.5 mo, P = 0.946) (Table 5).
| Primary DFR (n = 29) | Revision DFR (n = 26) | P value | |
| Age (mean ± SD) | 49.7 ± 20.6 yr | 52.2 ± 21.1 yr | 0.649 |
| Gender | 0.44 | ||
| Male | 13 (44.8) | 9 (34.6) | |
| Female | 16 (55.2) | 17 (65.4) | |
| Body mass index (mean ± SD) | 29.5 ± 8.5 kg/m2 | 30.0 ± 8.4 kg/m2 | 0.567 |
| Follow-up (mean ± SD) | 42.7 ± 61.4 mo | 34.5 ± 47.5 mo | 0.946 |
| Complication requiring reoperation? | 11 (37.9) | 9 (34.6) | 0.799 |
| Total reoperations required (mean ± SD) | 1.4 ± 2.6 | 1.0 ± 1.8 | 0.624 |
Endoprosthetic reconstruction is standard-of-care for oncologic processes of the distal femur, as greater than 90% of patients can be treated with limb salvage. While this procedure is effective in restoring mobility and salvaging the limb, it has a well-known complication and survival profile[19]. Aside from septic failure, aseptic loosening has been the leading cause of failure historically, and improvements have been focused on fixation of the femoral component[5,10]. However, relatively little attention has been paid to the tibial component. Unlike the femoral component, tibial components are available in both metal and all-polyethylene, and fixation of the tibial component can be achieved in a variety of ways, including cemented, cementless, and hybrid fixation. Despite the increased utilization of DFR with APTs over time, previous studies have paid little attention to the outcomes of tibial components until recently[6,9,20]. Bukowski et al[20] showed that DFRs with APT have a significantly lower incidence of tibial revision at 10 years (1.1% vs 12.5%, HR = 0.18, P = 0.03) and no difference in infection-free survival (P = 0.72) when compared to the traditional DFR with a metal backed tibia.
APTs are monoblock cemented components that offer cost-effectiveness and surgical efficiency when compared to metal-backed tibial components[21,22]. They avoid failure due to locking-mechanism issues and backside wear, but limit modularity and the option for late liner exchange. While we theorized that APTs would suffer from some of the same failure mechanisms as metal backed tibial components, such as periprosthetic joint infection and late polyethylene wear, it is unclear whether they exhibit novel modes of failure, or whether they are more resistant to certain types of failure, such as aseptic loosening, than metal-backed components. The purpose of this study was to examine a large cohort of DFR with APT performed for oncologic indications, with specific focus on failure rate and mechanisms of the APT.
The most common modes of implant failure in this cohort were soft tissue (Type I) (10.9%) and deep infection (Type IV) (10.9%), followed by aseptic loosening (Type II) (9.1%). Aseptic loosening was evenly split between the femoral and tibial components. There were two revisions for corrosion and metal debris (Type III), and one case of tumor recurrence (Type V). The rate of infection is comparable to previous large series. Sharma demonstrated a 7.8% infection rate (Type IV), 6.5% local recurrence rate (Type V), and no aseptic loosening with line-to-line cemented femoral stems using the same implant system[23]. Henderson demonstrated 1.3% soft tissue failure (Type I), 6.4% aseptic loosening (Type II), 6.3% structural failure (Type III), and 8.3% infection (Type IV) in their cohort’s subset of distal femur replacements[12]. Our soft tissue failure rate was significantly higher, for unclear reasons. Given the referral nature of our practice, the present cohort may be inherently at greater risk for soft tissue failure due to a higher proportion of ethnic minorities from underserved areas with greater comorbid burden, many of whom require prior insurance authorization resulting in delayed time to definitive treatment. However, rates of infection, aseptic loosening, and structural failure were similar. Pala demonstrated a 26.6% overall failure rate for DFRs in their study, including 6% soft tissue failure (Type I), 5% aseptic loosening (Type II), and 9% infection (Type IV)[24]. Our series of DFR with APT for oncologic indications appears to have similar modes and rates of failure as previously published studies. We demonstrated a higher rate of soft tissue failure, the reason for which is unclear. However, it is unlikely to be due to the APT, as the rotating tibial component and axel for the APT is approximately 2.5 cm longer for the APT than the metal-backed tibia, conferring a much larger jump distance prior to dislocation, i.e. soft tissue failure leading to instability[25].
Our study demonstrated a 24% revision rate and 47% all-cause reoperation rate at 3 years. This is consistent with large reports of modern distal femoral replacements[9]. The rate of aseptic loosening was 9.1%, which was seen on both the femoral and tibial side. This appears to be consistent with previous reports for femoral-sided aseptic loosening[12,24]. However, few reports have specifically examined the tibial component, so it is unclear how this rate of aseptic loosening of the tibial component (3/55) compares with other historical groups. One recent study suggests that these components achieve durable fixation, with no cases of aseptic loosening and a small number (6) sustaining mechanical failure of the tibial component out of 125 patients[8]. This speaks to the advantage of line-to-line cement technique on the femur, and durable fixation of the APT, with predictable long-term failure like our study. They also observed one patient with polyethylene granuloma over the APT. Finally, they noted an infection rate (Type IV failure) of 10%, nearly identical to our study, and reported a 15% reoperation rate at 1 year and 30% reoperation rate at 5 years.
Finally, we found no significant differences in terms of preoperative demographics or post-operative complications in patients who received DFR with APT as a primary or revision procedure for their oncologic process. The revision cohort had 2.0 ± 1.3 (range 1-5) previous operations prior to DFR. It is surprising that the group performed as a revision procedure did not have a higher complication or reoperation rate, despite having been operated on previously. However, this finding is supported by several previous investigations. The reoperation rate of 38% in the revision DFR cohort is similar to published reports of DFR used for non-oncologic revision total knee arthroplasty, as the Mayo clinic series demonstrated a 46.3% percent all-cause reoperation rate at 10 years for non-oncologic DFR[26]. A similar reoperation rate was found by Staats and colleagues in a cohort of both oncologic and non-oncologic DFRs (36.4% at 2 years), and they were unable to detect a difference in the cumulative incidence of revision surgery in patients with oncologic vs non-oncologic disease[27]. Other studies have found no difference between primary and revision DFRs as well[28,29], indicating that previous oncologic procedures in the same field do not significantly affect outcomes after DFR.
This study has several limitations. It is a single institution, retrospective study in which statistical power is limited due to sample size. Also, given the heterogeneity of oncologic disease, specific indications, treatments, and surgical techniques could not be standardized. However, the risk of unintentional selection bias in the present study is mitigated by the composition of the current cohort, which represents a consecutive series of DFR with APT performed for oncologic indications by a single surgeon at our institution. The APT was used consistently as the primary construct of the treating surgeon in this consecutive series of patients - therefore, we can only make historical comparisons to other studies, and cannot directly compare these patients with a cohort of metal-backed tibial components performed in the same patient population. These types of studies are difficult to accomplish in orthopaedic oncology due to the heterogeneous patient population and rare diseases treated. Nevertheless, the present study provides valuable insight into the survivorship and common modes of implant failure for the DFR with APT construct utilized this high-risk patient population, and there is value in reporting these case series so that they may be analyzed in aggregate with other published reports.
Despite the inherent risk of complications and reoperations associated with oncologic surgery, DFR with APT is a reliable reconstructive option for oncologic defects of the distal femur. APTs are efficient, cost-effective, and more likely to avoid failure mechanisms related to modularity. Failures of DFR with APT, like other DFRs, are mostly related to infection, soft tissue failures, and late aseptic loosening. While we observed several cases of aseptic loosening of the tibial component, we did not observe fractures of the APT, which has been reported previously. In concordance with previous studies, we did not observe a difference in complication rates or failures between DFR with APT performed for primary and revision indications. Further studies, including cohort or randomized trials, are needed to determine the optimal tibial component for oncologic DFR.
Future prospective studies with larger sample sizes and longer term followup are necessary to determine the optimal construct for oncologic distal femoral replacement (DFR). Comparative studies investigating the differences in clinical, functional, and patient-reported outcomes between the use of metal-backed vs all-polyethylene tibial components and cemented vs cementless fixation will provide further insight into the specific failure mechanisms associated with each construct.
This study proposes that DFR with all-polyethylene tibial (APT) is a reliable reconstruction option for oncologic defects of the distal femur.
DFR with APT implantation was performed as a primary procedure in 29 patients (52.7%) and a revision procedure in 26 patients (47.3%). Overall, twenty patients (36.4%) experienced a postoperative complication requiring reoperation. In total, 12 patients (21.8%) required a revision while 20 patients (36.4%) required a reoperation, resulting in three-year cumulative incidences of 24.0% (95%CI 9.9%-41.4%) and 47.2% (95%CI 27.5%-64.5%), respectively.
A retrospective review of consecutive patients who underwent DFR with a GMRS® (Global Modular Replacement System, Stryker, Kalamazoo, MI, United States) cemented distal femoral endoprosthesis and APT component for an oncologic indication was performed using a single-institutional database. Univariate analyses were performed to compare differences between those who had a DFR performed either as the primary treatment for the disease in question vs those who had a DFR as a revision of a previous failed surgery (indications included recurrence, fracture, etc.). Competing risk analyses were performed to evaluate the cumulative incidence of all-cause reoperation, need for revision surgery, and patient death.
This study was designed to answer the following research questions: (1) What are the most common modes of implant failure for patients undergoing cemented DFR with APT for oncologic indications? (2) What is the survivorship, rate of all-cause reoperation, and rate of revision for aseptic loosening of these implants? and (3) Is there a difference in implant survivorship or patient demographics between cemented DFRs with APT performed as a primary reconstruction vs those performed as a revision procedure?
Prior studies investigating the outcomes of endoprosthetic distal femoral replacement have largely failed to describe the type of tibial component or fixation used. Unlike the femoral component, tibial components are available in both metal-backed and all-polyethylene designs, and fixation may be achieved via cemented, cementless, or hybrid fixation. Future research investigating the effect of tibial component design and fixation on clinical outcomes is critical to determining the optimal construct for oncologic DFR.
Endoprosthetic reconstruction of the distal femur has been used as a limb-salvage procedure to treat oncologic processes of the distal femur for nearly five decades, and is currently considered standard of practice for this indication. However, there is a paucity of literature examining the survivorship of DFRs with respect to the type of tibial component utilized. The purpose of this study was to report on the clinical outcomes of patients undergoing cemented DFR with all-polyethylene tibial components for oncologic indications.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gutowski CJ, United States; Mohammadpour M, Iran S-Editor: Liu JH L-Editor: A P-Editor: Yuan YY
| 1. | Simon MA, Aschliman MA, Thomas N, Mankin HJ. Limb-salvage treatment vs amputation for osteosarcoma of the distal end of the femur. J Bone Joint Surg Am. 1986;68:1331-1337. [RCA] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 329] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 2. | Unwin PS, Cannon SR, Grimer RJ, Kemp HB, Sneath RS, Walker PS. Aseptic loosening in cemented custom-made prosthetic replacements for bone tumours of the lower limb. J Bone Joint Surg Br. 1996;78:5-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 275] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 3. | Schwartz AJ, Kabo JM, Eilber FC, Eilber FR, Eckardt JJ. Cemented distal femoral endoprostheses for musculoskeletal tumor: improved survival of modular versus custom implants. Clin Orthop Relat Res. 2010;468:2198-2210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 4. | Myers GJ, Abudu AT, Carter SR, Tillman RM, Grimer RJ. Endoprosthetic replacement of the distal femur for bone tumours: long-term results. J Bone Joint Surg Br. 2007;89:521-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 206] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 5. | Christ AB, Hornicek FJ, Fabbri N. Distal femoral replacement - Cemented or cementless? J Clin Orthop Trauma. 2021;19:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Pala E, Mavrogenis AF, Angelini A, Henderson ER, Douglas Letson G, Ruggieri P. Cemented versus cementless endoprostheses for lower limb salvage surgery. J BUON. 2013;18:496-503. [PubMed] |
| 7. | Farfalli GL, Boland PJ, Morris CD, Athanasian EA, Healey JH. Early equivalence of uncemented press-fit and Compress femoral fixation. Clin Orthop Relat Res. 2009;467:2792-2799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Tayara B, Nooh A, Chalopin A, Goulding K, Turcotte RE. Outcomes of Cemented Distal Femoral Replacement Using "Line to Line" Technique With All-Polyethylene Tibial Implant for Tumors. J Arthroplasty. 2021;36:2913-2920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Ogura K, Fujiwara T, Morris CD, Boland PJ, Healey JH. Long-term competing risks for overall and cause-specific failure of rotating-hinge distal femoral arthroplasty for tumour reconstruction. Bone Joint J. 2021;103-B:1405-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Haijie L, Dasen L, Tao J, Yi Y, Xiaodong T, Wei G. Implant Survival and Complication Profiles of Endoprostheses for Treating Tumor Around the Knee in Adults: A Systematic Review of the Literature Over the Past 30 Years. J Arthroplasty. 2018;33:1275-1287.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 11. | Graulich T, Kranz C, Korallus C, Oergel M, Pacha OT, Omar M, Liodakis E, Krettek C, Panzica M. Clinical Outcome After Replacement of Distal Femur/Proximal Tibia in a Heterogeneous Patient Cohort: Function Following Tumour, Trauma, and Loosening. In Vivo. 2021;35:2275-2281. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Henderson ER, Groundland JS, Pala E, Dennis JA, Wooten R, Cheong D, Windhager R, Kotz RI, Mercuri M, Funovics PT, Hornicek FJ, Temple HT, Ruggieri P, Letson GD. Failure mode classification for tumor endoprostheses: retrospective review of five institutions and a literature review. J Bone Joint Surg Am. 2011;93:418-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 488] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 13. | Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. SBH. 2000;. [DOI] [Full Text] |
| 14. | Therneau T. A package for survival analysis in R: R package version 3.2-10. 2021. 2021. [DOI] [Full Text] |
| 15. | Gray B. cmprsk: subdistribution analysis of competing risks. 2014. R package version 2020; 2–1. |
| 16. | Ripley BD. The R project in statistical computing. MSOR Connect. 2001;1:23-25. [RCA] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 126] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Wickham H. ggplot2: Elegant Graphics for Data Analysis. Springer International Publishing. [DOI] [Full Text] |
| 18. | Team RC. R: A language and environment for statistical computing (R Version 4.0. 3, R Foundation for Statistical Computing, Vienna, Austria, 2020). 2021. [DOI] [Full Text] |
| 19. | Houdek MT, Wagner ER, Wilke BK, Wyles CC, Taunton MJ, Sim FH. Long term outcomes of cemented endoprosthetic reconstruction for periarticular tumors of the distal femur. Knee. 2016;23:167-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Bukowski BR, Tagliero AJ, Heidenreich MJ, Johnson JD, Rose PS, Houdek MT. Comparison of all-polyethylene and metal-backed modular tibial components in endoprosthetic reconstruction of the distal femur. J Surg Oncol. 2021;123:1126-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Ryan SP, Steele JR, Plate JF, Attarian DE, Seyler TM, Bolognesi MP, Wellman SS. All-Polyethylene Tibia: An Opportunity for Value-Based Care in Bundled Reimbursement Initiatives. Orthopedics. 2021;44:e114-e118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Kumar V, Hasan O, Umer M, Baloch N. Cemented all-poly tibia in resource constrained country, affordable and cost-effective care. Is it applicable at this era? Ann Med Surg (Lond). 2019;47:36-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Sharma S, Turcotte RE, Isler MH, Wong C. Cemented rotating hinge endoprosthesis for limb salvage of distal femur tumors. Clin Orthop Relat Res. 2006;450:28-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Pala E, Henderson ER, Calabrò T, Angelini A, Abati CN, Trovarelli G, Ruggieri P. Survival of current production tumor endoprostheses: complications, functional results, and a comparative statistical analysis. J Surg Oncol. 2013;108:403-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 25. | Ward WG, Haight D, Ritchie P, Gordon S, Eckardt JJ. Dislocation of rotating hinge total knee prostheses. A biomechanical analysis. J Bone Joint Surg Am. 2003;85:448-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Wyles CC, Tibbo ME, Yuan BJ, Trousdale RT, Berry DJ, Abdel MP. Long-Term Results of Total Knee Arthroplasty with Contemporary Distal Femoral Replacement. J Bone Joint Surg Am. 2020;102:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 27. | Staats K, Vertesich K, Sigmund IK, Sosa B, Kaider A, Funovics PT, Windhager R. Does a Competing Risk Analysis Show Differences in the Cumulative Incidence of Revision Surgery Between Patients with Oncologic and Non-oncologic Conditions After Distal Femur Replacement? Clin Orthop Relat Res. 2020;478:1062-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Heyberger C, Auberger G, Babinet A, Anract P, Biau DJ. Patients with Revision Modern Megaprostheses of the Distal Femur Have Improved Disease-Specific and Health-Related Outcomes Compared to Those with Primary Replacements. J Knee Surg. 2018;31:822-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Zimel MN, Farfalli GL, Zindman AM, Riedel ER, Morris CD, Boland PJ, Healey JH. Revision Distal Femoral Arthroplasty With the Compress(®) Prosthesis Has a Low Rate of Mechanical Failure at 10 Years. Clin Orthop Relat Res. 2016;474:528-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |