Published online Jan 18, 2023. doi: 10.5312/wjo.v14.i1.13
Peer-review started: September 26, 2022
First decision: October 21, 2022
Revised: November 4, 2022
Accepted: December 7, 2022
Article in press: December 7, 2022
Published online: January 18, 2023
Processing time: 108 Days and 11.2 Hours
Polydactyly is a malformation during the development of the human limb, which is characterized by the presence of more than the normal number of fingers or toes. It is considered to be one of the most common inherited hand disorders. It can be divided into two major groups: Non-syndromic polydactyly or syndromic polydactyly. According to the anatomical location of the duplicated digits, polydactyly can be generally subdivided into pre-, post-axial, and mesoaxial forms. Non-syndromic polydactyly is often inherited with an autosomal dominant trait and defects during the procedure of anterior-posterior patterning of limb development are incriminated for the final phenotype of the malforma
Core Tip: The molecular basis of hand and foot polydactyly, syndromic or non- syndromic, is diverse. There are several phenotypes of the disorder which are correlated to a specific molecular profile and other whose molecular basis is still unclear. We summarize and provide an overview of gene mutations that cause hand and foot polydactyly as an isolated disorder or as part of a syndrome and present the clinical manifestations that they cause.
- Citation: Kyriazis Z, Kollia P, Grivea I, Stefanou N, Sotiriou S, Dailiana ZH. Polydactyly: Clinical and molecular manifestations. World J Orthop 2023; 14(1): 13-22
- URL: https://www.wjgnet.com/2218-5836/full/v14/i1/13.htm
- DOI: https://dx.doi.org/10.5312/wjo.v14.i1.13
Non-syndromic (Table 1) or syndromic polydactyly (Table 2) is often inherited with an autosomal dominant trait with variable penetrance[1]. It is related with a disturbance of the anterior–posterior axial development procedure of the limb[2] and is classified into preaxial, axial (central), and postaxial polydactyly[3]. Preaxial polydactyly is defined as an extra digit affecting the radial/tibial digits while postaxial involves the ulnar/peroneal digits. The rare type of axial (central) polydactyly refers to the duplication of three central hand or foot digits. Mirror-image polydactyly and Haas-type polysyndactyly are rare and distinct types, not fitting to the three categories[4].
| Preaxial | Central | Postaxial | Complex |
| CEP290 | CPLANE1 | GLI3 | MIPOL1 |
| RPGRIP1 | ZNF141 | PITX1 | |
| TMEM216 | DACH1 | LMBR1 | |
| FBN1 | GLI1 | ||
| CEP164 | |||
| MEGF8 | |||
| LMBR1 | |||
| ZRS | |||
| GLI3 | |||
| ZNF141 | |||
| STKLD1 | |||
| GLI1 | |||
| KIAA0586 | |||
| EVC | |||
| HES1 |
| Syndrome | Mutated gene(s) |
| Bardet-Biedl | CCDC28B, ARL6, MKS1, BBS8, SDCCAG8, LZTFL1, WDPCP, BBS4, BBS12, TMEM67, BBS1, BBS2, BBS6, BBS10, BBS9, BBS7, BBS5, CEP290, TRIM32, BBIP1, ALMS1, MKKS |
| McKusick-Kaufman | MKKS |
| Carpenter | P4HB, RAB23 |
| Saethre-Chotzen | TWIST1, FGFR2 |
| Poland syndrome | - |
| Greig cephalopolysyndactyly | GLI3 |
| Short-rib polydactyly | ATD1, LBN, DYNC2H1, IFT81 |
| Pallister-Hall | GLI3 |
| Triphalangeal thumb-polydactyly | LMBR1 |
| Smith-Lemli-Opitz | DHCR7 |
Many specific phenotypes, including all types of hand and foot polydactyly, have been identified and correlated to gene mutations[5].
Since polydactyly is often a part of a syndrome, the ability to identify the potential syndromes associated with this anomaly is very important for the clinician. Additionally, it is important to distinguish between syndromic and non-syndromic cases for reasons of genetic counselling. In this paper, we review the recent progress in the molecular genetics, including clinical and molecular manifestations of disorders, and present some representative syndromes including polydactyly as a phenotype.
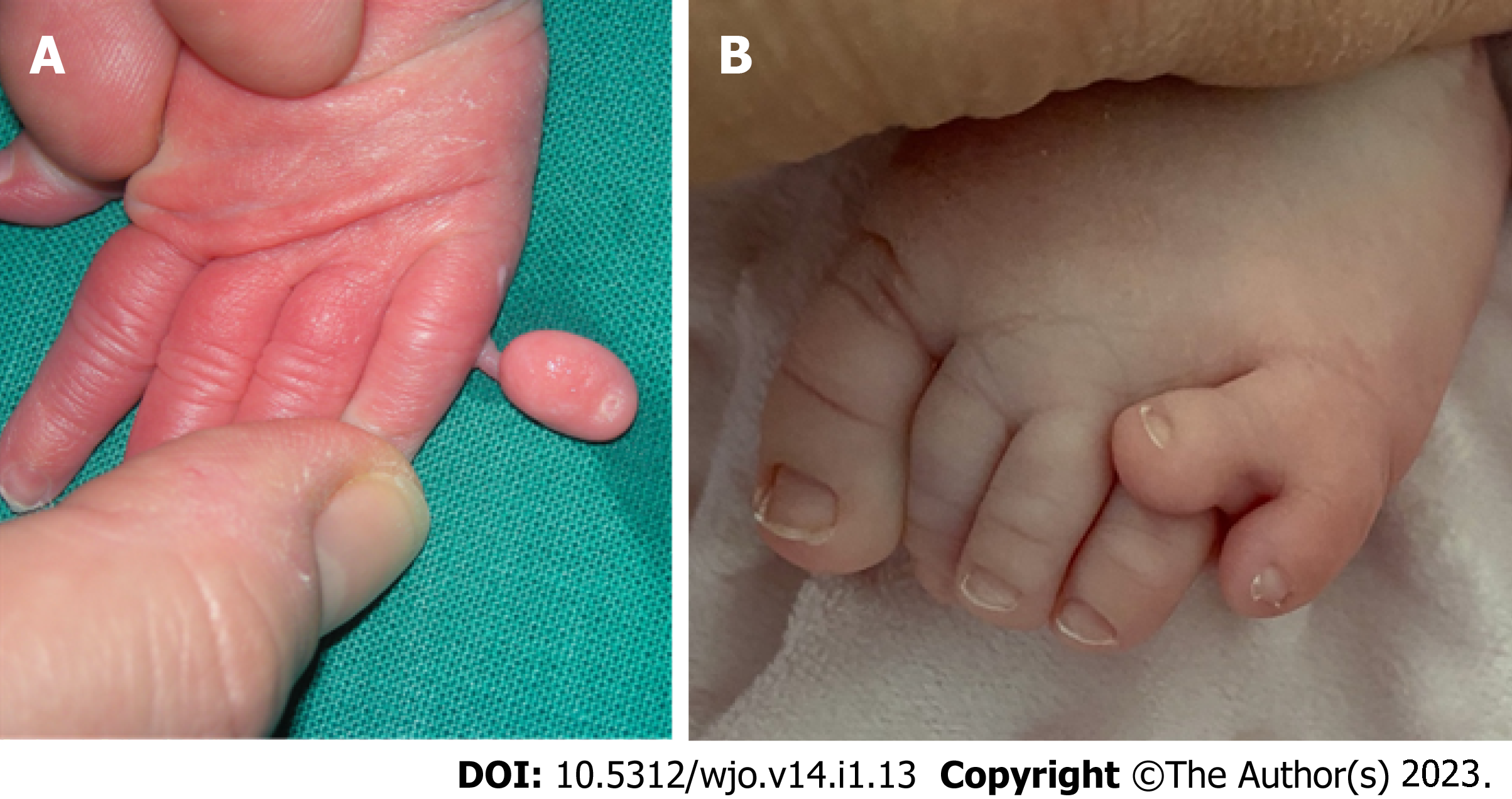
The preaxial form of polydactyly is the second most common phenotype behind the postaxial with a reported prevalence of approximately 0.8 to 2.3 in 10000 live births. It is characterized by an extra digit on the tibial/radial side of limb (Figure 1). The following classification has been suggested:
Preaxial polydactyly type I, which is thumb polydactyly (OMIM 174400)[6]—characterized by duplication of one or more skeletal elements of a biphalangeal thumb.
Preaxial polydactyly type II, which is polydactyly of a triphalangeal thumb (OMIM 174500).
Preaxial polydactyly type III, which is polydactyly of the index finger, characterized by the presence of one or two triphalangeal digits (OMIM 174600).
Preaxial polydactyly type IV and syndactyly of various degrees involving the middle and ring finger/second and third toe (OMIM 174700) or hallux polydactyly (OMIM 601759)[7].
Preaxial polydactyly type I: Thumb polydactyly is usually observed in unilateral form. In bilateral cases, hands are more often affected and the left hand is also more often affected than the right. It follows an autosomal dominant inheritance model[7]. However, a recent study in a Pakistani family has revealed a rare autosomal recessive form of preaxial polydactyly, linked to a novel variant (c.1517T>A; p. Leu506Gln) in the GLI1 gene on chromosome 12q13.3[8].
The most commonly used classification is Wassel classification which divides thumb duplication into six subtypes according to the level and the extent of duplication (partial or complete)[9]. Hallux polydactyly is known to exist as a predominant presentation or an isolated disorder. The incidence of hallux duplication is 2.4/100000 as compared to thumb polydactyly incidence in South America, which is 1.65/10000.
Preaxial type I polydactyly is caused by sequence variants in the sonic hedgehog (SHH) enhancer, called zone of polarizing activity (ZPA) regulatory sequence (ZRS), which is regulated by LMBR1 gene. Mutations in CEP290, RPGRIP1, TMEM216, FBN1, CEP1, and MEGF8 genes have been isolated and suspected to play a role in Wassel III and Wassel IV manifestations[10]. Recently, a mutation in STKLD1 gene, located on chromosome 9q34.2, was found and correlated with the disease phenotype in all members of the studied family[11]. Another molecular study of the SHH/GLI signaling axis, identified HES1 gene as a downstream modifier which can cause preaxial polydactyly[12].
Next generation sequence analysis in a large four-generation family with isolated preaxial polydactyly revealed a new ZRS mutation (g.101779T>A) which can cause the disease phenotype[13]. Another recent genetic analysis of 20 Chinese patients with preaxial polydactyly identified two novel mutations in GLI3 gene (c.G2844A) and in EVC gene (c.1409_1410del). Mutations in KIAA0586 gene, which are related with ciliopathies (OMIM 610178), were also detected[14].
Preaxial polydactyly type II: Preaxial polydactyly type II is characterized by the presence of a usually opposable triphalangeal thumb with or without additional duplication of one or more skeletal components of the thumb. The thumb appearance can differ widely in shape or it can be deviated in the radio-ulnar plane. It can also be associated with Holt-Oram syndrome and Fanconi anemia. LMBR1 and its related pathways Wnt/Notch and Hedgehog play a significant role in the development of the disorder. The disease gene locus was mapped to chromosome 7q36[15]. Mutations in the SHH regulatory factor were also reported[16]. Two mutations, a 739A>G transition near the 5- end of the ZRS and a 621C>G mutation in the ZRS of the LMBR1 gene, were identified[17]. Triphalangeal thumb-polysyndactyly can manifest as a syndrome. It is an isolated limb deformity characterized by pre- and postaxial polysyndactyly of hands and feet. Mutations in ZRS have been identified[18,19].
Preaxial polydactyly type III: Preaxial polydactyly type III is an autosomal dominant disorder which is characterized by a malformation of fingers, where the thumb is replaced by one or two triphalangeal digits with dermatoglyphic pattern specific for the index finger. It can occur unilaterally and bilaterally. No responsible gene has been identified[20].
Preaxial polydactyly type IV: Preaxial polydactyly type IV is an autosomal dominant disorder which can be described as mild duplication of the thumb, syndactyly that affects the third and fourth hand/foot fingers/toes, duplication of the first or second toes, and toes syndactyly. There are patients who have only foot malformations. GLI3 gene mutations are associated with the disorder. Genetic analysis in two families with the phenotype were found heterozygous for p.L1216PfsX31 and p.R290X mutations in the GLI3 gene[21].
Postaxial polydactyly is a frequent congenital hand malformation characterized by fifth digit duplications in hands and/or feet (Figure 2). Its prevalence is estimated between 1/630 and 1/3300 in Caucasian race and between 1/100 and 1/300 in Black race. Two phenotypic categories have been described: Type A, the extra digit is well formed and articulates with the fifth or an extra metacarpal; Type B, there is a rudimentary extra fifth digit which is usually represented by an extra skin tag. Both types can be inherited by autosomal dominant or recessive trait[22]. There are six subcategories of type A postaxial polydactyly.
Postaxial polydactyly type A1: In postaxial polydactyly type A1, the extra digit is well-formed and articulates with the fifth or a sixth metacarpal/metatarsal. Genetic analysis in an Indian family resulted in the identification of association of GLI3 gene mutations with the phenotype[23]. It was mapped to 7pl5-q11.23. Mutation in the C- and the N-terminal or the zinc finger domain of the GLI3 gene causes isolated postaxial polydactyly type A1 and is also linked to Greig cephalopolysyndactyly syndrome, while a mutation in the post-zinc finger region is incriminated for Pallister–Hall syndrome[24]. A recent genetic study in a Chinese family with isolated postaxial polydactyly revealed a new mutation of GLI3 (c.1180C>TT, p.P394fs18x)[25]. A DACH1 gene mutation was identified in a patient with bilateral postaxial polydactyly who was subjected to whole exome sequencing[26]. New mutations of the GLI1 gene have been incriminated for postaxial polydactyly according to a novel study which aims to help in prevention of the disorder[27].
Postaxial polydactyly type A2: It consists of Type A polydactyly phenotypes with an extra digit well-formed. A genetic study of an Indian kindred revealed disease gene locus of postaxial polydactyly type A2 (OMIM 602085) which was mapped to 13q21-q32[28]. The underlying gene for the disorder has not been identified.
Postaxial polydactyly type A3: It manifests with polydactyly phenotypes Type A/B in hands and feet. Genetic analysis of a Chinese family discovered incomplete penetrance of the phenotype and identified the disease gene locus which was mapped to 19p13.2-p13.1[29]. There is not an identified gene responsible for the disorder.
Postaxial polydactyly type A4: It is characterized by polydactyly phenotypes Type A/B in hands and feet and two to three finger/toe syndactyly. The disease locus (OMIM 608562) was mapped to 7q21-q34 by genetic analysis in a Dutch family with an autosomal dominant inheritance of the phenotype[30]. Until now there is no candidate gene for this manifestation.
Postaxial polydactyly type A5: It is characterized by polydactyly of hands and feet, minor syndactyly, and five to six metacarpal synostoses. Two Indian families and a Sicilian family were identified to have this type of autosomal recessive postaxial polydactyly[31]. Postaxial polydactyly type A5 (OMIM 263450) was mapped to 13q13.3- 13q21.2 region. The underlying gene for this phenotype has not yet been identified.
Postaxial polydactyly type A6: The phenotype is characterized by an extra functionally developed digit in hands and/or feet. Mutations in the ZNF141 gene are considered to cause postaxial polydactyly type A6 (OMIM 615226). Exome sequencing in a Pakistani family resulted in showing autosomal recessive inheritance of A6 phenotype. The ZNF141 gene consists of four exons[32]. The final protein is expressed in many different tissues and it is still unclear whether it plays a role in embryogenesis[33].
Postaxial polydactyly type B: It is the most common type of polydactyly. There is a vestigial nonfunctional, partially formed, ulnar (or fibular) digit with no bony attachments, attached by a narrow neurovascular pedicle to the lateral aspect of the hand or foot[25]. GLI3 gene mutations are associated with this often manifestation.
Central polydactyly (OMIM 174200) is a very rare phenotype which is characterized by duplication of one of the three middle digits of the hand and foot. It can be an isolated defect or can be accompanied with other anomalies. The most often manifestation of hand central polydactyly is duplication of the fourth digit[3]. Foot central polydactyly is very rare and the second toe is most commonly duplicated[34]. Central polydactyly is related to split-foot malformation with mesoaxial polydactyly and Holzgreve syndrome. CPLANE1 is the only known gene which is associated with central polydactyly.
Mirror image polydactyly: This rare non-syndromic limb malformation (OMIM 135750) presents with mirror-image hand or foot polydactyly. The malformation can be unilateral, bilateral, and very rarely tetramelic. It can be associated with other congenital anomalies or can present isolated. MIPOL1 and PITX1 gene mutations have been identified and incriminated for this disorder. A recent German study in a patient with the phenotype showed a heterozygous deletion of 4.9 Mb on 5q31 including PITX1[35].
Haas-type polysyndactyly: Haas-type polysyndactyly (OMIM 186200) is characterized by complete cutaneous syndactyly of all hand fingers and occasionally foot toes are affected. It frequently presents with polydactyly with six digits and six metacarpals. It is inherited with an autosomal dominant trait. It is usually classified as syndactyly type IV. The locus for Haas-type polysyndactyly was mapped on 7q36 by linkage and haplotype analysis of a Chinese family[36]. Mutations of the ZRS region of LMBR1 gene and other ZRS point mutations were found in families presenting with the clinical sings of Haas-type polysyndactyly according to two recent studies[37,38].
Bardet-Biedl syndrome (OMIM 209900) is an autosomal or digenic recessive disorder which can present with vision loss, obesity, hand and/or foot polydactyly, intellectual disabilities, and hypogonadism. Mutations in at least 20 genes have been identified and associated with the syndrome[39]: CCDC28B, ARL6, MKS1, BBS8, SDCCAG8, LZTFL1, WDPCP, BBS4, BBS12, TMEM67, BBS1, BBS2, BBS6, BBS10, BBS9, BBS4, BBS7, CEP290, TRIM32, BBIP1, IFT27, and IFT172genes are examples of them. A recent study in four Iranian children with a clinical diagnosis of Bardet-Biedl syndrome identified in three children one previously reported mutation in BBS12 gene (c.265-266delTT, p.L89fs) and two newly detected mutations in MKKS (c.1196T>G, p.L399X) and BBS7 gene (c.1636C>T, p.Q546X). A new mutation in ALMS1 gene was isolated in the other child[40].
McKusick-Kaufman syndrome’s phenotype (OMIM 236700) consists of the following features: Genitourinary malformations (hydrometrocolpos, glanular hypospadias, and prominent scrotal raphe), postaxial hand and/or foot polydactyly, and rarely cardiac defects. MKKS gene mutations are associated with McKusick-Kaufman syndrome and they are inherited with an autosomal recessive trait[41].
Carpenter syndrome (OMIM 201000) is characterized by craniosynostosis, involving a pointed head (acrocephaly), syndactyly of certain fingers or toes, and polydactyly. It appears most commonly with foot polydactyly, rarely hand polydactyly and hand or toe cutaneous syndactyly. RAB23 gene mutations are associated with the syndrome, which appears with autosomal recessive inheritance[42]. Recent molecular studies have identified two new mutations in RAB23 gene (NM_001278668:c.T416C:p.Leu139
Saethre-Chotzen syndrome’s phenotype (OMIM 101400) is characterized by premature closure of cranial sutures, hand syndactyly, and foot polydactyly. Foot polydactyly most often involves the first toe. TWIST1 and FGFR2 gene mutations are usually incriminated and inherited with an autosomal dominant trait[45].
Poland syndrome (OMIM 173800) involves underdeveloped pectoralis muscles on one side of chest wall and ipsilateral hand abnormalities, including short fingers and syndactyly (symbrachydactyly); however, there are rare cases of preaxial polydactyly manifestations in the literature[46]. Most cases of Poland syndrome are not related with a family history, and they are sporadic. Rarely it is inherited with an autosomal dominant trait through generations in families. There are no isolated gene mutations correlated with Poland syndrome.
Greig cephalopolysyndactyly (OMIM 175700) syndrome is an autosomal dominant syndrome, which presents with hypertelorism, macrocephaly, and polydactyly. The polydactyly is most commonly preaxial of the feet and postaxial in the hands. Greig cephalopolysyndactyly is associated with GLI3 mutations[47]. Recently, molecular studies have broadened the spectrum of known GLI3 mutations correlated with the syndrome[48,49].
Pallister-Hall syndrome (OMIM 146510) is a rare disorder which affects many parts of the body. Very often manifestation of the syndrome is postaxial polydactyly and cutaneous syndactyly of hands and toes. GLI3 gene mutations are considered responsible for this autosomal dominant disorder[50].
Jeune syndrome, Ellis-van Creveld syndrome, Saldino-Noonan syndrome, and Majewski syndrome are called short-rib polydactyly syndromes (OMIM 613091). They belong to a group of lethal congenital disorders characterized by shortening of the ribs and long bones, hand and/ or foot polydactyly, and a range of extraskeletal phenotypes. ATD1 gene is considered to be responsible for Jeune syndrome. LBN gene mutations cause Ellis-van Creveld syndrome and individuals carrying DYNC2H1 gene mutations can present with Saldino-Noonan and Majewski syndromes. Novel exome sequencing studies have isolated two new mutations in DYNC2H1 gene (c.8077G>T and c.11741_11742delTT) and a new mutation in IFT81 gene, causing malformation of the cilia[51,52]. Short-rib polydactyly syndromes are usually inherited with an autosomal recessive trait[53].
Triphalangeal thumb-polydactyly syndrome (OMIM 173800) consists of triphalangeal thumbs, pre- or post-axial polydactyly, and syndactyly. LMBR1 gene is considered to be responsible for this manifestation. It is inherited with an autosomal dominant genetic trait. Typically, the syndrome presents with duplicated triphalangeal thumbs and typical phenotypic findings include duplicated triphalangeal thumbs and syndactyly between middle, ring, or little finger[54].
Smith-Lemli-Opitz syndrome (OMIM 173800) is a multi-malformation syndrome. The responsible gene for this syndrome is considered to be DHCR7 gene and it is inherited with an autosomal recessive pattern[55]. Its phenotype contains foot syndactyly (usually of 2nd and 3rd toes) and postaxial hand polydactyly.
Genetic mechanisms which combine epigenetic and environmental factors play a significant role in foot and hand polydactyly manifestations[56]. Proper genotype-phenotype correlations might help in future genetic testing and enhance our knowledge about identified diseases and their associated genes. Recent genetic analysis techniques of extra foot or hand digit formation highlight the existence of nongradual transitions in phenotypes, suggesting a distinction between continuous and discontinuous variation in evolution. Genome sequencing will probably lead to the discovery of a number of new gene mutations responsible for non-syndromic or syndromic polydactyly. Clinical manifestation and genetic profile correlation of polydactyly types will be further established by use of bioinformatics analysis of gene mutations. Progress of prenatal diagnosis, which is still mostly postnatal, prenatal operative treatment planning, and potential future gene modification treatment will be enhanced and unknown molecular background of diseases, which is to date unclear, will be elucidated.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Faillace JJ; Liao JX, China S-Editor: Chang KL L-Editor: Wang TQ P-Editor: Chang KL
| 1. | Talamillo A, Bastida MF, Fernandez-Teran M, Ros MA. The developing limb and the control of the number of digits. Clin Genet. 2005;67:143-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (2)] |
| 2. | Kornak U, Mundlos S. Genetic disorders of the skeleton: a developmental approach. Am J Hum Genet. 2003;73:447-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 115] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 3. | Haber LL, Adams HB, Thompson GH, Duncan LS, Didomenico LA, McCluskey WP. Unique case of polydactyly and a new classification system. J Pediatr Orthop. 2007;27:326-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Gillessen-Kaesbach G, Majewski F. Bilateral complete polysyndactyly (type IV Haas). Am J Med Genet. 1991;38:29-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Malik S. Polydactyly: phenotypes, genetics and classification. Clin Genet. 2014;85:203-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 6. | Chong AK. Common congenital hand conditions. Singapore Med J. 2010;51:965-971. [PubMed] |
| 7. | Orioli IM, Castilla EE. Thumb/hallux duplication and preaxial polydactyly type I. Am J Med Genet. 1999;82:219-224. [PubMed] [DOI] [Full Text] |
| 8. | Ullah A, Umair M, Majeed AI, Abdullah, Jan A, Ahmad W. A novel homozygous sequence variant in GLI1 underlies first case of autosomal recessive pre-axial polydactyly. Clin Genet. 2019;95:540-541. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Manske MC, Kennedy CD, Huang JI. Classifications in Brief: The Wassel Classification for Radial Polydactyly. Clin Orthop Relat Res. 2017;475:1740-1746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Kyriazis Z, Kollia P, Grivea I, Varitimidis SE, Constantoulakis P, Dailiana ZH. Thumb duplication: molecular analysis of different clinical types. Eur J Orthop Surg Traumatol. 2019;29:421-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Ansar M, Meitinger T, Ahmad W. Whole‐exome sequencing revealed a nonsense mutation in STKLD1 causing non‐syndromic preaxial polydactyly type A affecting only upper limb. Clin Genet. 2019;96:134-139. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (2)] |
| 12. | Sharma D, Mirando AJ, Leinroth A, Long JT, Karner CM, Hilton MJ. HES1 is a novel downstream modifier of the SHH-GLI3 Axis in the development of preaxial polydactyly. PLoS Genet. 2021;17:e1009982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Xu C, Yang X, Zhou H, Li Y, Xing C, Zhou T, Zhong D, Lian C, Yan M, Chen T, Liao Z, Gao B, Su D, Wang T, Sharma S, Mohan C, Ahituv N, Malik S, Li QZ, Su P. A novel ZRS variant causes preaxial polydactyly type I by increased sonic hedgehog expression in the developing limb bud. Genet Med. 2020;22:189-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Wang T, Xuan Z, Dou Y, Liu Y, Fu Y, Ren J, Lu L. Identification of novel mutations in preaxial polydactyly patients through whole-exome sequencing. Mol Genet Genomic Med. 2019;7:e690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Heutink P, Zguricas J, van Oosterhout L, Breedveld GJ, Testers L, Sandkuijl LA, Snijders PJ, Weissenbach J, Lindhout D, Hovius SE. The gene for triphalangeal thumb maps to the subtelomeric region of chromosome 7q. Nat Genet. 1994;6:287-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Lettice LA, Heaney SJ, Purdie LA, Li L, de Beer P, Oostra BA, Goode D, Elgar G, Hill RE, de Graaff E. A long-range Shh enhancer regulates expression in the developing limb and fin and is associated with preaxial polydactyly. Hum Mol Genet. 2003;12:1725-1735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 878] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 17. | Gurnett CA, Bowcock AM, Dietz FR, Morcuende JA, Murray JC, Dobbs MB. Two novel point mutations in the long-range SHH enhancer in three families with triphalangeal thumb and preaxial polydactyly. Am J Med Genet A. 2007;143A:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Balci S, Demirtas M, Civelek B, Piskin M, Sensoz O, Akarsu AN. Phenotypic variability of triphalangeal thumb-polysyndactyly syndrome linked to chromosome 7q36. Am J Med Genet. 1999;87:399-406. [PubMed] |
| 19. | Klopocki E, Ott CE, Benatar N, Ullmann R, Mundlos S, Lehmann K. A microduplication of the long range SHH limb regulator (ZRS) is associated with triphalangeal thumb-polysyndactyly syndrome. J Med Genet. 2008;45:370-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 20. | Atasu M. Hereditary index finger polydactyly: phenotypic, radiological, dermatoglyphic, and genetic findings in a large family. J Med Genet. 1976;13:469-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Fujioka H, Ariga T, Horiuchi K, Otsu M, Igawa H, Kawashima K, Yamamoto Y, Sugihara T, Sakiyama Y. Molecular analysis of non-syndromic preaxial polydactyly: preaxial polydactyly type-IV and preaxial polydactyly type-I. Clin Genet. 2005;67:429-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Umm-e-Kalsoom, Basit S, Kamran-ul-Hassan Naqvi S, Ansar M, Ahmad W. Genetic mapping of an autosomal recessive postaxial polydactyly type A to chromosome 13q13.3-q21.2 and screening of the candidate genes. Hum Genet. 2012;131:415-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Jamsheer A, Sowińska A, Trzeciak T, Jamsheer-Bratkowska M, Geppert A, Latos-Bieleńska A. Expanded mutational spectrum of the GLI3 gene substantiates genotype-phenotype correlations. J Appl Genet. 2012;53:415-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Al-Qattan MM. A novel frameshift mutation of the GLI3 gene in a family with broad thumbs with/without big toes, postaxial polydactyly and variable syndactyly of the hands/feet. Clin Genet. 2012;82:502-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Ni F, Han G, Guo R, Cui H, Wang B, Li Q. A Novel Frameshift Mutation of GLI3 Causes Isolated Postaxial Polydactyly. Ann Plast Surg. 2019;82:570-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Umair M, Palander O, Bilal M, Almuzzaini B, Alam Q, Ahmad F, Younus M, Khan A, Waqas A, Rafeeq MM, Alfadhel M. Biallelic variant in DACH1, encoding Dachshund Homolog 1, defines a novel candidate locus for recessive postaxial polydactyly type A. Genomics. 2021;113:2495-2502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Bakar A, Ullah A, Bibi N, Khan H, Rahman AU, Ahmad W, Khan B. A novel homozygous variant in the GLI1 underlies postaxial polydactyly in a large consanguineous family with intra familial variable phenotypes. Eur J Med Genet. 2022;65:104599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Radhakrishna U, Blouin JL, Mehenni H, Patel UC, Patel MN, Solanki JV, Antonarakis SE. Mapping one form of autosomal dominant postaxial polydactyly type A to chromosome 7p15-q11.23 by linkage analysis. Am J Hum Genet. 1997;60:597-604. [PubMed] |
| 29. | Schrauwen I, Giese AP, Aziz A, Lafont DT, Chakchouk I, Santos-Cortez RLP, Lee K, Acharya A, Khan FS, Ullah A, Nickerson DA, Bamshad MJ, Ali G, Riazuddin S, Ansar M, Ahmad W, Ahmed ZM, Leal SM. FAM92A Underlies Nonsyndromic Postaxial Polydactyly in Humans and an Abnormal Limb and Digit Skeletal Phenotype in Mice. J Bone Miner Res. 2019;34:375-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 30. | Galjaard RJ, Smits AP, Tuerlings JH, Bais AG, Bertoli Avella AM, Breedveld G, de Graaff E, Oostra BA, Heutink P. A new locus for postaxial polydactyly type A/B on chromosome 7q21-q34. Eur J Hum Genet. 2003;11:409-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Mohan J. Postaxial polydactyly in three Indian families. J Med Genet. 1969;6:196-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Deng H, Tan T, Yuan L. Advances in the molecular genetics of non-syndromic polydactyly. Expert Rev Mol Med. 2015;17:e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Kalsoom UE, Klopocki E, Wasif N, Tariq M, Khan S, Hecht J, Krawitz P, Mundlos S, Ahmad W. Whole exome sequencing identified a novel zinc-finger gene ZNF141 associated with autosomal recessive postaxial polydactyly type A. J Med Genet. 2013;50:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 34. | Ishigaki T, Akita S, Udagawa A, Suzuki H, Mitsukawa N. Central polydactyly of the foot: An experience of a treatment of 22 patients. J Orthop Sci. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 35. | Klopocki E, Kähler C, Foulds N, Shah H, Joseph B, Vogel H, Lüttgen S, Bald R, Besoke R, Held K, Mundlos S, Kurth I. Deletions in PITX1 cause a spectrum of lower-limb malformations including mirror-image polydactyly. Eur J Hum Genet. 2012;20:705-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 36. | Malik S. Syndactyly: phenotypes, genetics and current classification. Eur J Hum Genet. 2012;20:817-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 37. | Lohan S, Spielmann M, Doelken SC, Flöttmann R, Muhammad F, Baig SM, Wajid M, Hülsemann W, Habenicht R, Kjaer KW, Patil SJ, Girisha KM, Abarca-Barriga HH, Mundlos S, Klopocki E. Microduplications encompassing the Sonic hedgehog limb enhancer ZRS are associated with Haas-type polysyndactyly and Laurin-Sandrow syndrome. Clin Genet. 2014;86:318-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 38. | Wieczorek D, Pawlik B, Li Y, Akarsu NA, Caliebe A, May KJ, Schweiger B, Vargas FR, Balci S, Gillessen-Kaesbach G, Wollnik B. A specific mutation in the distant sonic hedgehog (SHH) cis-regulator (ZRS) causes Werner mesomelic syndrome (WMS) while complete ZRS duplications underlie Haas type polysyndactyly and preaxial polydactyly (PPD) with or without triphalangeal thumb. Hum Mutat. 2010;31:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 39. | Priya S, Nampoothiri S, Sen P, Sripriya S. Bardet-Biedl syndrome: Genetics, molecular pathophysiology, and disease management. Indian J Ophthalmol. 2016;64:620-627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 40. | Dehghan R, Behnam M, Salehi M, Kelishadi R. Novel Mutations in the MKKS, BBS7, and ALMS1 Genes in Iranian Children with Clinically Suspected Bardet–Biedl Syndrome. Case Rep Ophthalmol Med. 2022;1-6. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (1)] |
| 41. | Slavotinek AM. McKusick-Kaufman Syndrome. 2002 Sep 10. In: GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993. [PubMed] |
| 42. | Prevel CD, Eppley BL, McCarty M. Acrocephalosyndactyly syndromes: a review. J Craniofac Surg. 1997;8:279-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 43. | Khairat R, Elhossini R, Sobreira N, Wohler E, Otaify G, Mohamed AM, Abdel Raouf ER, Sayed I, Aglan M, Ismail S, Temtamy SA. Expansion of the phenotypic and mutational spectrum of Carpenter syndrome. Eur J Med Genet. 2022;65:104377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 44. | Ouyang L, Yang F. Cole-Carpenter syndrome-1 with a de novo heterozygous deletion in the P4HB gene in a Chinese girl: A case report. Medicine (Baltimore). 2017;96:e9504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 45. | Alawneh RJ, Johnson AL, Hoover-Fong JE, Jackson EM, Steinberg JP, MacCarrick G. Postnatal Progressive Craniosynostosis in Syndromic Conditions: Two Patients With Saethre-Chotzen Due to TWIST1 Gene Deletions and Review of the Literature. Cleft Palate Craniofac J. 2022;10556656221090844. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 46. | Riyaz N, Riyaz A. Poland syndrome (anomaly) with congenital hemangioma: a new association. Indian J Dermatol Venereol Leprol. 2006;72:222-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 47. | Biesecker LG, Johnston JJ. Greig Cephalopolysyndactyly Syndrome. 2001 Jul 9. In: GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993. [PubMed] |
| 48. | Patel R, Singh SK, Bhattacharya V, Ali A. Novel GLI3 pathogenic variants in complex pre- and postaxial polysyndactyly and Greig cephalopolysyndactyly syndrome. Am J Med Genet A. 2021;185:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 49. | Tanteles GA, Michaelidou S, Loukianou E, Christophidou-Anastasiadou V, Kleopa KA. Novel GLI3 mutation in a Greek-Cypriot patient with Greig cephalopolysyndactyly syndrome. Clin Dysmorphol. 2015;24:102-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 50. | Mahtabfar A, Buckley N, Murphy S, Danish S, Marshall I. Pallister-Hall Syndrome Presenting in Adolescence. Case Rep Genet. 2019;2019:6845836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 51. | Xia CL, Xiao SQ, Yang X, Liu CX, Qiu H, Jiang HK, Li-Ling J, Lyu Y. Radiological and histopathological features of short ribpolydactyly syndrome type III and identification of two novel DYNC2H1 variants. Mol Med Rep. 2021;23. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 52. | Duran I, Taylor SP, Zhang W, Martin J, Forlenza KN, Spiro RP, Nickerson DA, Bamshad M, Cohn DH, Krakow D. Destabilization of the IFT-B cilia core complex due to mutations in IFT81 causes a Spectrum of Short-Rib Polydactyly Syndrome. Sci Rep. 2016;6:34232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 53. | Dagoneau N, Goulet M, Geneviève D, Sznajer Y, Martinovic J, Smithson S, Huber C, Baujat G, Flori E, Tecco L, Cavalcanti D, Delezoide AL, Serre V, Le Merrer M, Munnich A, Cormier-Daire V. DYNC2H1 mutations cause asphyxiating thoracic dystrophy and short rib-polydactyly syndrome, type III. Am J Hum Genet. 2009;84:706-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 165] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 54. | Sun M, Ma F, Zeng X, Liu Q, Zhao XL, Wu FX, Wu GP, Zhang ZF, Gu B, Zhao YF, Tian SH, Lin B, Kong XY, Zhang XL, Yang W, Lo WH, Zhang X. Triphalangeal thumb-polysyndactyly syndrome and syndactyly type IV are caused by genomic duplications involving the long range, limb-specific SHH enhancer. J Med Genet. 2008;45:589-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 55. | Porter FD. Smith-Lemli-Opitz syndrome: pathogenesis, diagnosis and management. Eur J Hum Genet. 2008;16:535-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 200] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 56. | Grzeschik KH. Human limb malformations; an approach to the molecular basis of development. Int J Dev Biol. 2002;46:983-991. [PubMed] |