Published online Sep 18, 2022. doi: 10.5312/wjo.v13.i9.791
Peer-review started: January 17, 2022
First decision: February 21, 2022
Revised: March 2, 2022
Accepted: August 24, 2022
Article in press: August 24, 2022
Published online: September 18, 2022
Processing time: 241 Days and 17.8 Hours
Ligament flavum (LF) hypertropy is the main etiopathogenesis of lumbar canal stenosis (LCS). The purely elastic LF undergoes a morphological adaptation including a reduction in the elastic fibers and a consequent increase in the collagen content, fibrosis, cicatrization, and calcification. However, the morphometric analysis can delineate the LF in patients with LCS from those without LCS, which would help in better understanding LCS pathogenesis.
To compare the histopathological changes in LF between the degenerative LCS and non-stenotic (non-LCS) group.
The present prospective study was conducted in 82 patients who were divided into two groups, namely LCS and non-LCS. Demographic details of the patients such as duration of symptoms, level of involvement, and number of segments were recorded. The LF obtained from both groups was histopathologically examined for the fibrosis score, elastic fiber degeneration, calcification, and chondroid metaplasia. Morphometrical details included a change in elastin and collagen percentages, elastin/collagen ratio, elastic fiber fragmentation, and ligamentocyte numbers. All parameters were compared between the two groups by using the independent t test, Chi-square test, and Pearson’s correlation test.
Out of 82 cases, 74 were analysed, 34 in LCS and 40 in non-LCS group. The mean ± SD age of presentation in LCS and non- LCS group was 49.2 ± 8.9 and 43.1 ± 14.3 respectively. The LCS group (n = 34) exhibited significant differences in fibrosis (P = 0.002), elastic fiber degeneration (P = 0.01), % elastic fragmentation (66.5 ± 16.3 vs 29.5 ± 16.9), % elastic, content (26.9 ± 6.7 vs 34.7 ± 8.4), % collagen content (63.6 ± 10.4 vs 54.9 ± 6.4), reduction of elastic/collagen (0.4 ± 0.1 vs 0.6 ± 0.1), and ligamentocyte number (39.1 ± 19.1 vs 53.5 ± 26.9) as compared to non-LCS group (n = 40). The calcification (P = 0.08) and Pearson’s correlation between duration and loss of elastin was not significant. The difference in LF morphology is consistent in patient’s ≥ 40 years of age among the groups as found in subgroup analysis. Similarly in the patents < 40 and > 40 in the non-LCS group.
LF is vital in the pathogenesis of LCS. The purely elastic LF undergoes a morphological adaptation that includes a reduction in the elastic fibers with a consequent increase in the collagen content, fibrosis, cicatrization, and calcification. The present study provides a detailed morphometric analysis to semiquantitatively delineate the LF changes in patients with LCS from those in patients without LCS.
Core Tip: The ligament flavum (LF) is vital in the pathogenesis of lumbar canal stenosis (LCS). The purely elastic LF undergoes a morphological adaptation that includes a reduction in the elastic fibers with a consequent increase in the collagen content, fibrosis, cicatrization, and calcification. The present study provides a detailed morphometric analysis to semiquantitatively delineate the LF changes in patients with LCS from those in patients without LCS.
- Citation: Jain M, Sable M, Tirpude AP, Sahu RN, Samanta SK, Das G. Histological difference in ligament flavum between degenerative lumbar canal stenosis and non-stenotic group: A prospective, comparative study. World J Orthop 2022; 13(9): 791-801
- URL: https://www.wjgnet.com/2218-5836/full/v13/i9/791.htm
- DOI: https://dx.doi.org/10.5312/wjo.v13.i9.791
Lumbar canal stenosis (LCS) is a common spinal disorder that affects elderly patients, leading to lower back pain, leg pain, and neurogenic claudication, which rarely ends in paresis[1,2]. Because the li
LF contains the purest form of elastic tissue among ligaments. These elastic fibers decrease with age and are replaced by collagen fibers[2]. The causes of LFH are multifactorial, including the activity levels, age, and mechanical stress[7,8]. Based on transmission electron microscopy findings, Postacchini et al[9] concluded that the reduced elasticity might cause bulging of the LF into the spinal canal even in the standing position.
Studies have exhibited a qualitative transformation in the dynamics of the LF components with degeneration[1,6,10-12]. The LFH exhibited loss of elastic fibers, increased content of collagen fibers, and chondrometaplasia, leading to calcification. A few studies have suggested this association of cal
Therefore, the present study attempted to explain the LF structure histologically in a semi-quantitative manner by using advanced imaging software.
The present prospective study was conducted following the Helsinki Declaration principles and approved by the local institutional review board (T/IM/18-19/43 dated 04/01/2019). Valid written informed consent was obtained from all participants.
The present study was conducted in 74 adult patients undergoing lumbar spine surgery between January 2019 and March 2020 in the departments of orthopedics or neurosurgery in a tertiary care center. Patients with characteristic clinical and radiological findings of spinal stenosis were grouped as the LCS study group. Simultaneously, patients with lumbar disc herniation, infective etiology (tubercular or non-tubercular), trauma, and malignancy constituted the non-LCS study group. Patients with prior lumbar surgery were excluded from the study. Clinical details such as age, sex, duration of symptoms, and level of involvement were recorded.
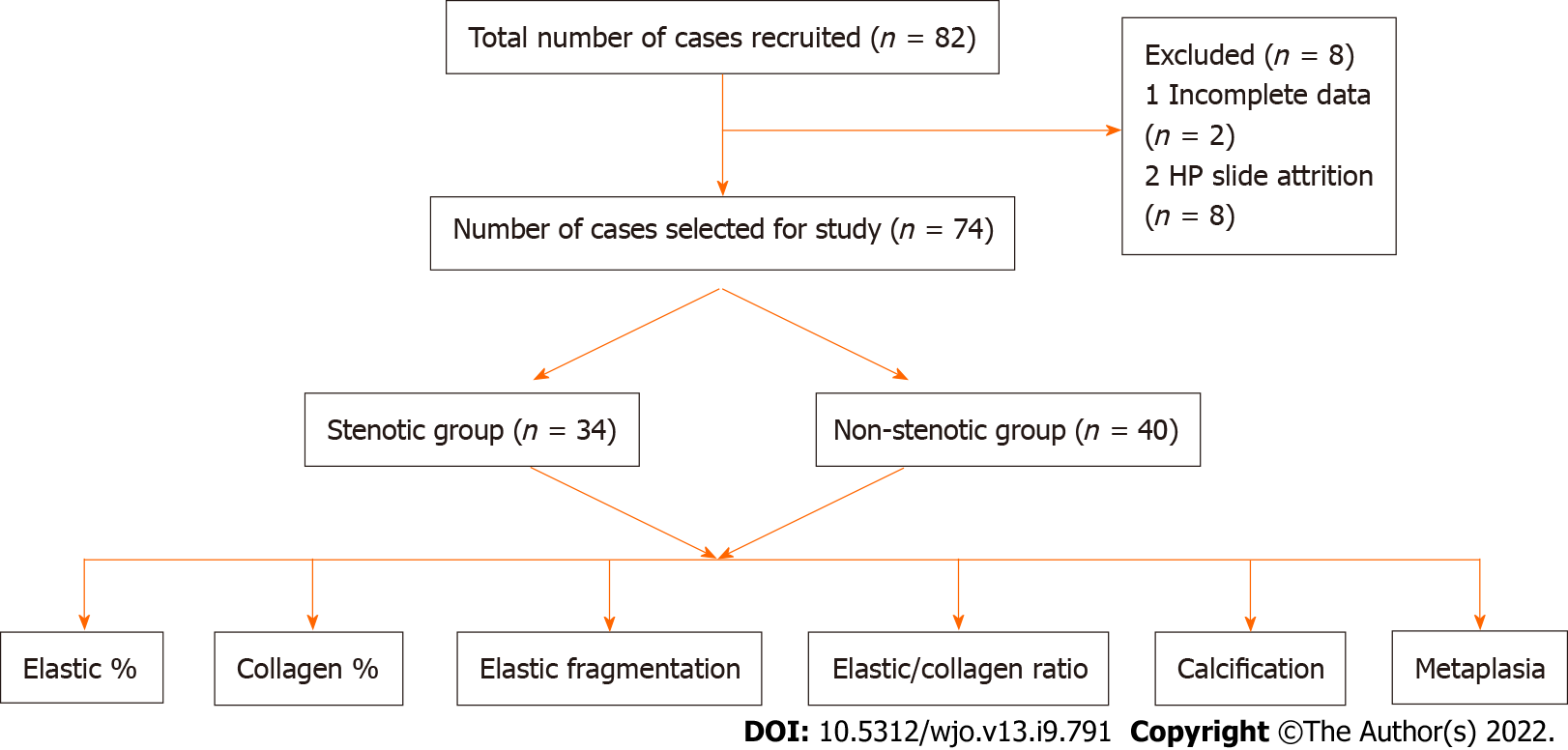
LF samples were obtained from 82 patients who underwent decompressive laminectomy in piecemeal by using a Kerrison's rounger and sent for histopathological assay in neutral buffer formalin. Of these, eight samples were excluded due to sufficient tissue availability. Thus, the final analysis was conducted in 74 patients.
The harvested LF components were kept in a solution containing 10% neutral buffered formalin for 24 h. The tissues were processed overnight on an automatic tissue processor (Leica Biosystems Ltd.) and embedded in paraffin. Multiple 4-μm thick sections were cut. All sections were stained with haematoxylin and eosin (H & E), Verhoef-Van-Gieson stains (VVG), Masson trichrome stain (MTS), reticulin, and Von-krossa stains.
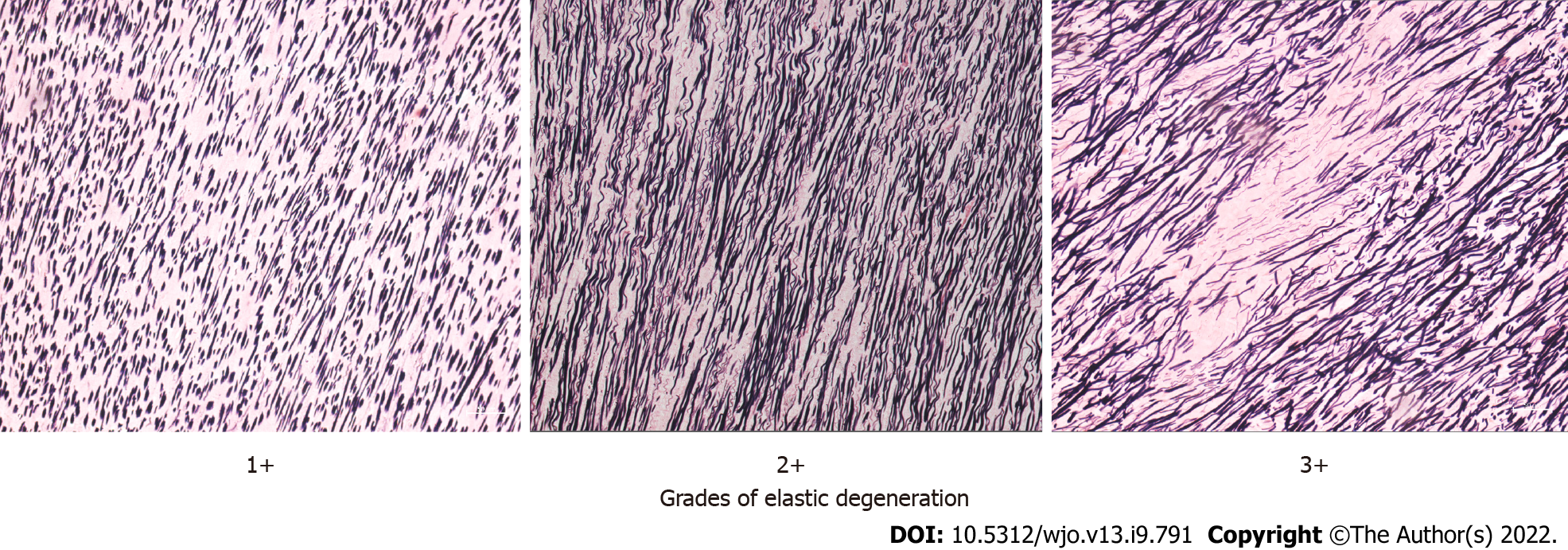
The histological evaluation was performed by two experts who were blinded to the nature of the groups. Light microscopy was used to examine LF sections for elastic degeneration, fibrosis, metaplasia (chondroid or osteoid), hemorrhage, and calcification. Elastic degeneration was graded depending upon the percentage of elastic fibers exhibiting degenerative changes and elastin fiber fragmentation (1+: 0%–33%, 2+: 33%–66%, and 3+: > 67% of elastic fibers). Fibrosis was graded as per the criterion described previously[2].
Digital images were obtained using a commercial imaging system (ZEN blue edition on ZEISS Scope A.1 microscope) at 200X magnification for H & E, VVG, and MTS stains in the tagged image file format. For each case, at least two areas from different LF regions were captured randomly. All images were imported in Image J software java windows-64 application (ImageJ bundled with 64-bit Java 1.8.0_172) for morphometric analysis.
The morphological analysis of the digital images of different stains was performed for elastin content (VVG), collagen content (MTS), and the number of ligamentocytes (H & E). The tool was used to estimate the percentage of collagen and elastic fibers, elastic to collagen fiber ratio, number of liga
Continuous data are presented as mean ± standard deviation (SD). Differences in the mean between the groups were tested using the independent t test. The Chi-square test was used to compare the categorical variables between the two groups. Correlation between various morphological parameters and duration of symptoms was determined using the Pearson's correlation test for the stenotic group. Subgroup analysis was also performed for patients aged ≥ 40 years and for patients aged < 40 and ≥ 40 years in the non-LCS group. All differences associated with a chance probability of ≤ 0.05 were considered. Data were analyzed using the IBM Statistical Package for Social Sciences ver. 17 (SPSS Inc., Chicago, IL, United States).
Of the 74 cases, 34 cases constitute the LCS group and 40 cases constituted the non-LCS group (Figure 1). The mean ± SD age of presentation in the LCS and non-LCS groups was 49.2 ± 8.9 and 43.1 ± 14.3 years, respectively. The percentage of patients in the stenotic group aged > 40 years was 91%, whereas that in the non-stenotic group was 57.5%. This difference was statistically significant (P = 0.001) (Fischer exact test). Although the non-stenotic group included permanent lumbar disc herniation, other pathologies were also observed (Table 1). Single-level involvement was observed in majority of cases. L4-5 involvement was observed in 43.5% patients, followed by L3-4 and L5-S1 involvement (23% patients each). The remaining patients exhibited involvement in L1-2 and L2-3 levels.
| Categories | LSS (n = 34) | Non-LSS (n = 40) | P value | |
| Age in years, mean (SD) | 49.2 (8.9) | 43.1 (14.3) | ||
| Sex in % | M:F – 59:41 | M:F – 60:40 | ||
| Duration in weeks, mean (SD) | 44.1 (11.6) | 9.4 (8.1) | ||
| Level | One | 30 (88.2) | 32 (80) | 0.553 |
| Two | 3 (8.8) | 7 (17.5) | ||
| Three | 1 (2.9) | 1 (2.5) | ||
| Diagnosis | Lumbar canal stenosis | PIVD – 18 | ||
| Trauma – 8 | ||||
| Potts spine – 7 | ||||
| Tumour – 5 | ||||
| Epidural abscess (non-TB) – 2 | ||||
The histological differences in elastin fibers, collagen content, and ligamentocytes were compared between the two groups. The LCS group exhibited higher elastic degeneration and fibrosis than the non-LCS group (P = 0.01 and 0.002, respectively) (Table 2; Figures 2-4). On the other hand, the extent of calcification, chondroid metaplasia, and hemorrhage was statistically nonsignificant between the groups.
| Variable | Histological features | Stenotic (n = 34) | Non stenotic (n = 40) | P value (Chi square test) |
| Elastin degeneration | 1+ and 2+ | 9 (26.5) | 22 (55) | 0.010 |
| 3+ | 25 (73.5) | 18 (45) | ||
| Fibrosis | 1+ and 2+ | 10 | 17 | 0.002 |
| 3+ | 24 | 13 | ||
| Calcification | Absent | 28 | 38 | 0.081 |
| Present | 6 | 2 | ||
| Chondroid metaplasia | Absent | 22 | 26 | 0.979 |
| Present | 12 | 14 | ||
| Haemorrhage | Absent | 17 | 23 | 0.519 |
| Present | 17 | 17 |
LF exhibited a significant reduction in the elastin content in the LCS group (P < 0.0001, independent t test) and an increase in the collagen content (P < 0.0001, independent t test) compared with those in the non-LCS group. The elastin/collagen ratio, width of elastic fibers, and ligamentocyte number were also significantly lower in the LCS group than in the non-LCS group (Table 3).
| Factor | mean | SD | t | df | P value | |
| % Elastin fragmentation | NS (40) | 29.50 | 16.9 | -9.51 | 71 | < 0.0001 |
| S (34) | 66.47 | 16.3 | ||||
| Collagen content (area %) | NS (40) | 54.94 | 6.41 | -4.38 | 53.2 | < 0.0001 |
| S (34) | 63.59 | 10.36 | ||||
| Elastic content (area %) | NS (40) | 34.73 | 8.36 | 4.36 | 72 | < 0.0001 |
| S (34) | 26.94 | 6.71 | ||||
| Elastin/Collagen ratio | NS (40) | 0.63 | 0.14 | 6.01 | 71.27 | < 0.0001 |
| S (34) | 0.43 | 0.13 | ||||
| Width of elastin fibers | NS (39) | 4.37 | 1.07 | 5.44 | 71 | < 0.0001 |
| S (34) | 3.27 | 0.54 | ||||
| Ligamentocyte numbers | NS (40) | 53.45 | 26.85 | 2.6 | 72 | < 0.0001 |
| S (34) | 39.11 | 19.05 | ||||
The Pearson’s correlation test indicated a moderate correlation between decrease in the number of ligamentocytes and age in both groups (non-LCS-R: −0.52, P < 0.001 and LCS-R: −0.578, P < 0.001). All other morphological changes were statistically nonsignificant. The duration of symptoms in the LCS group was not significantly correlated with morphological changes in both groups.
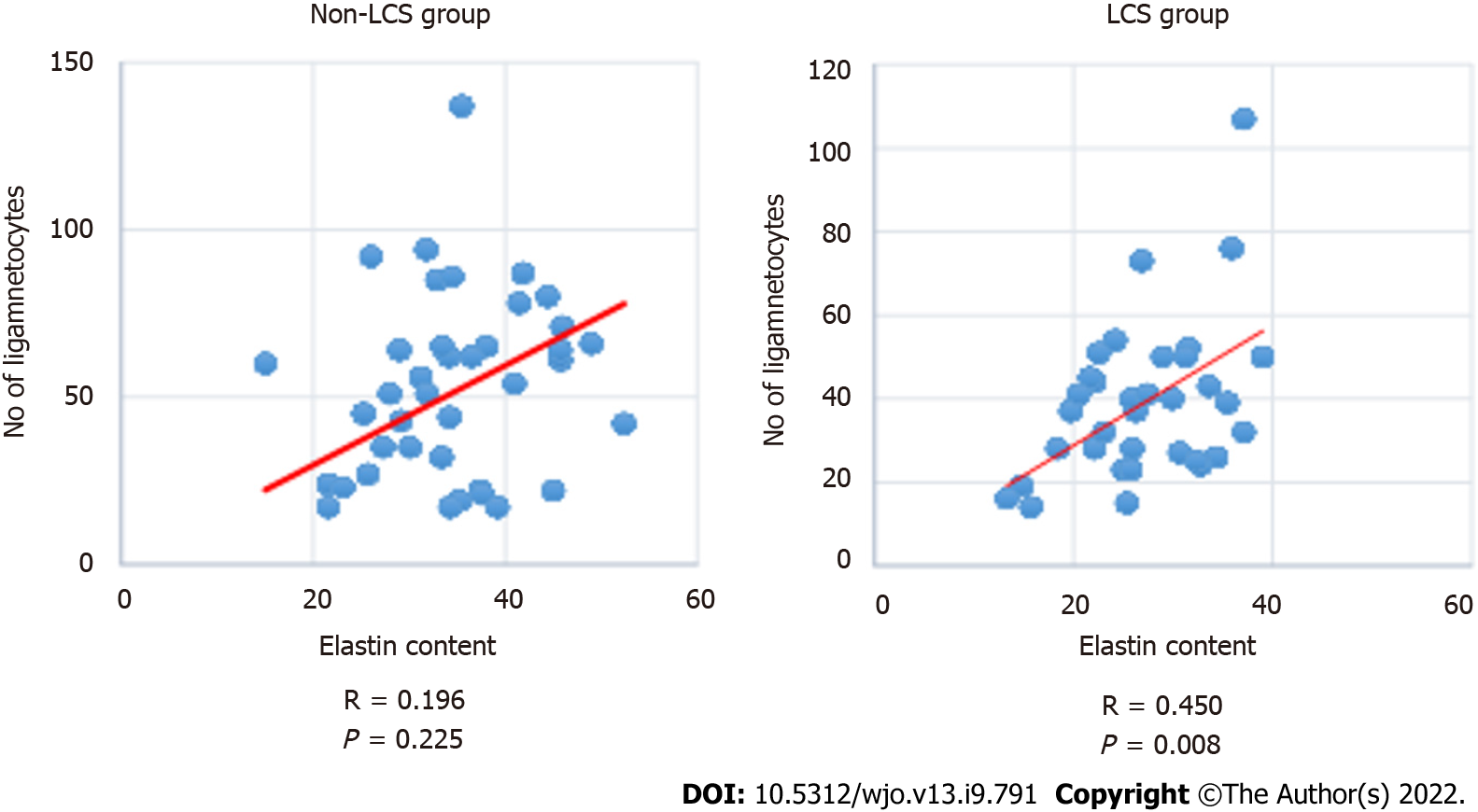
The ligamentocyte number was moderately correlated with the elastin content in the LCS group (R: −0.450, P = 0.008), (Figure 5). On the other hand, the collagen content was moderately correlated with the elastic content in the non-LCS group (R: −504, P: 0.001). The LCS group exhibited an inverse correlation between the elastin and collagen contents. However, this difference was statistically nonsignificant.
Subgroup analysis exhibited a statistically nonsignificant difference between the patients aged < 40 years and those aged > 40 years in the LCS group. Additionally, the difference between the LCS and non-LCS groups in the percentage of patients aged > 40 years was statistically significant (Table 4).
| Factor | mean | SD | t | df | P value | |
| % Elastin fragmentation | NS (25) | 31.80 | 18.123 | -7.4 | 43.93 | < 0.0001 |
| S (31) | 67.10 | 16.96 | ||||
| Collagen content (area %) | NS (25) | 55.12 | 7.62 | -3.4 | 53.19 | 0.001 |
| S (31) | 63.58 | 10.75 | ||||
| Elastic content (area %) | NS (25) | 34.898 | 7.92 | 4.20 | 46.6 | < 0.0001 |
| S (31) | 26.57 | 6.60 | ||||
| Elastin/Collagen ratio | NS (25) | 0.63 | 0.14 | 5.54 | 50.09 | < 0.0001 |
| S (31) | 0.43 | 0.13 | ||||
| Width of elastin fibers | NS (25) | 3.75 | 0.98 | 4.42 | 31.94 | < 0.0001 |
| S (31) | 2.77 | 0.49 | ||||
| Ligamentocyte numbers | NS (25) | 46.56 | 22.4.0 | 1.88 | 41.03 | 0.66 |
| S (31) | 36.612 | 15.41 | ||||
The LF, which envelops the spinal canal, is a highly elastic structure that contains four times pure elastin than collagen[6]. If the LF surrounding the spinal canal becomes hypertrophic, it will compress the dural sac containing the cauda equina or the nerve root. Elsberg[13] first reported a case of LFH causing sciatica. Several clinical studies have reported that LFH is the primary pathology in LCS[1,2,4,6-10]. Although surgical excision is the only therapeutic management for patients with LFH in LCS, a deeper understanding of the pathophysiology can encourage future nonsurgical or prophylactic treatment modalities. Thus, the present study attempted to study the pathological changes in LF and compare the LCS and non-LCS study groups.
Sairyo et al[1] had proposed that LFH occurs due to degenerative changes with aging process, and also due to increased mechanical stress occurring in instability. Wang et al[14] have experimentally demonstrated increased motion in lumbar spine induced LFH. Chuang et al[15] have found that age-related LFH occurred due to activation of the Akt and MAPK (apoptotic) pathways. The authors also postulated that hypertrophy is initiated in all subjects after the second decade of life. Zaki et al[16] also found that older individuals had some loss and rupture of elastic fibres with abnormal collagen, increase in vascularity and ossification particularly in the lumbar region as compared to the thoracic and cervical spine. Postacchini et al[9] observed that although older individuals with disc herniation exhibited some elastic fiber loss, the stenotic group of similar age exhibited more collagen and chondroid metaplasia and were strikingly different. However, the authors noted that there was no difference was observed in stenotic changes related to age and listhesis (degenerative), implying that instability does not accelerate hypertrophic changes[9]. The present study also exhibited no LCS-induced morphological changes in the non-LCS group of similar age. Similarly, no difference was observed in the non-LCS group with age < 40 years, whereas a statistically significant difference was observed from age > 40 years.
Sairyo et al[1] exhibited that LF thickness increased with age; however, the changes with age exhibited spinal level dependence. The increment at L4/5 and L3/4 levels was more extensive than that at L2/3 and L5/S1 levels. Similar changes in magnetic resonance imaging (MRI) were also reported by Kolte et al[7]. Okuda et al[10] exhibited that thickening was correlated to calcification, which was prime in LCS. A positive correlation was also observed by authors between calcification and clinical scoring (Japanese orthopedic association scores). The present study did not measure the thickness, either grossly, histologically, or radiologically. The present understanding of LCS has evolved from a pure 'static com
Schräder et al[12] also studied 41 Ligaments in 21 patients and reported single-level stenosis in five patients, bi-segmental stenosis in 24 patients, and stenosis on three levels in 12 patients. Additionally, Hulmani et al[4] exhibited more double-level stenosis in their series. However, the present study exhibited more single-level involvement (n = 32) than double-level (n = 7) or multilevel (n = 1) involvement. This may be due to the higher age group cohort in the study by Hulmani et al[4] compared to that in the present study (72 vs 49) due to the preferential selection. The present study exhibited that L4-5 was the most common involvement, followed by L3-4. Sairyo et al[1] proposed that high mechanical stress might be responsible for the preferential increase in thickness at the L4-5 level.
Okuda et al[10] and Elsberg et al[13] observed that nearly all ligaments were calcified in LCS. Calcium deposition within the ligament significantly aggravated the symptoms, and this process increased with age. The increase in the formation of calcium crystals is a significant factor for LF thickening. Okuda et al[10] observed that the mean age of patients with calcification and those without calcification was 74 ± 2.0 and 68 ± 1.4 years, respectively. Therefore, patients with calcification were significantly older than those in the LCS group. No reactive granulomatous tissue formation was noticed in the calcification focus. Other researchers have reiterated a smooth transition between calcific zones and surroundings[10,17]. Okuda et al[10] reported focal and dispersion-type calcification in their patients with LCS and correlated calcification with a low clinical score.
Altun et al[2], Hulmani et al[4], and Reyes-Sánchez et al[18] exhibited contrasting findings regarding calcification. No statistical difference was observed in calcification between the LCS group and the lumbar disc herniation (LDH) group in these studies.
Schräder et al[12] exhibited calcification of all the ligaments, and the patients also exhibited relevant fibrosis with decrease in the elastic/collagenous fiber ratio. Additionally, Sairyo et al[1] reported that the LCS group exhibited increased LF thickness and fibrosis with reduced elastic fibers. These transformations were more predominant along the dorsal side than those along the middle of the dural side. Sato tel. also found that dorsal side is affected 30 more than the dural side[19] Peng et al[20] revealed that the dorsal fibers of the LF were subjected to higher stress than the dural fibers that have a fluid-filled tube, which keeps it smooth. Hamdan et al[11] exhibited that the LCS changes were more in the central portion than in the attachments. Yabe et al[21] reported a severe reduction in elastic fibres on the dorsal hypertrophied LF. We did not differentiate among the sides as the stenotic effect was due to in-toto changes in LF. Moreover, the LF was removed piecemeal in most of our cases. Hence, such differences were not observed in the present study.
Fuertes et al[22] were first to comment on the anatomical and fiber arrangements on different layers. The authors realized that aging and repetitive stress altered the elastic fiber organization, causing disarray, derangement, and even complete transformation with no elastic fibers. In the present study, morphologic changes such as diameter irregularity, orientation turbulence, and extent of fragmentation were observed in elastic fibers. A similar finding was observed though electron microscopy in the study by Hulmani et al[4]. The image software was used to perform the morphometric study based on light microscopy. The present study did not exhibit significant ganglionic cystic changes or chondroid metaplasia despite substantial fibrosis. The present study noted a statistically significant reduction in the elastin percentage, increase in collagen, and reduction in the elastin/collagen ratio. This finding is in contrast to that of other studies. Additionally, a significant change was observed in the elastic fibers (4.37 ± 1.07 vs 3.27 ± 0.54; P < 0.001), fragmentation, and decrease in the number of ligamentocytes. Altun et al[2] reported that elastic fiber reduction or collagen increase was not significant, except for calcification. Okuda et al[10] first described elastic fiber degeneration, and it was accompanied by a proliferation of collagen fibers among elastic fibers. Additionally, Hulmani et al[4] reported findings such as ganglion-like cystic lesion, mucinous degeneration, and vascularization that were confirmed through electron microscopy. Reyes-Sánchez et al[18] exhibited more cystic degeneration, fibrillar appearance, and hypercellularity in the degenerative listhetic group than in the degenerative stenotic group. These results could be caused by instability rather than a degenerative disease in the spine. Schräder et al[12] noted that the parallel arrangement of LF elastic fibers was lost in degenerative LCS. This finding is concurrent with that of the present study and the study by Altun et al[2].
Significant fibrosis was observed in the LCS group in the present study[2,4]. This finding is in contrast to that of Hulmani et al[4] or Altun et al[2], and Cheung et al[23] exhibited a positive correlation between fibrosis and LFH in the LCS group. On the other hand, the developmental stenotic group exhibited paradoxically less fibrosis[23]. Okuda et al[10] also exhibited graded fibrosis in their LCS patients. However, the correlation with the clinical symptoms of patients was not significant. Additionally, the authors exhibited a large number of chondroid cells in patients with spondylolisthesis[10]. These findings are similar to those of Fukuyama et al[8], who postulated that unstable lumbar spine accelerates LF degeneration and chondrometaplasia.
LFH etiology is multifactorial. The morphological transformation that includes reduction in the elastin/collagen ratio, degeneration, fragmentation of the residual elastic fibers, fibrosis, cicatrization, and calcification leads to a loss in elasticity that can infold into the spinal canal, causing narrowing. Future studies can evaluate the correlation between symptom duration and progression of specific changes.
Several inflammatory cytokines have been studied which are responsible for the growth and reproduction and some of these plays’ crucial role in inflammatory response and progressive LF fibrosis[24]. However, we have not studied any such markers.
The present study exhibited that morphometric findings can be studied satisfactorily even in the absence of an electron microscope, which can be reproduced even in less sophisticated setups. However, it could not differentiate between the dorsal and dural aspects as we removed the LF piecemeal and not as a whole. The central and peripheral parts could not be segregated, which could allow more in-depth understanding, particularly of chondroid metaplasia. Clinical scoring, occupational activity, and MRI measurement were also ignored to keep the study simple. Additionally, gene expression was not studied in the present study.
The LCS and non-LCS groups differ in clinical parameters, mainly symptom duration. Histopathologically, the two groups exhibited significant differences in elastin degeneration, fragmentation, elastic/collagen ratio, fibrosis, and number of ligamentocytes. However, calcification was not significant between the groups.
Ligament flavum (LF) hypertropy is the main etiopathogenesis of lumbar canal stenosis (LCS). The purely elastic LF undergoes a morphological adaptation including a reduction in the elastic fibers and a consequent increase in the collagen content, fibrosis, cicatrization, and calcification. However, the morphometric analysis can delineate the LF in patients with LCS from those without LCS, which would help in better understanding LCS pathogenesis.
The research is motivated due to high footfall of these patient on Orthopedic outpatient department. An interdepartmental meeting was made to analyze these patients and funds were provided by the institute.
To compare the histopathological changes in LF between the degenerative LCS and non-stenotic (non-LCS) group.
The present prospective study was conducted in 82 patients who were divided into two groups, namely LCS and non-LCS. Demographic details of the patients such as duration of symptoms, level of involvement, and number of segments were recorded. The LF obtained from both groups was histopathologically examined for the fibrosis score, elastic fiber degeneration, calcification, and chondroid metaplasia. Morphometrical details included a change in elastin and collagen percentages, elastin/collagen ratio, elastic fiber fragmentation, and ligamentocyte numbers. All parameters were compared between the two groups by using the independent t test, Chi-square test, and Pearson’s correlation test.
Of the total, we selected 74 patients. The number of patients in the LCS and non-LCS groups was 34 and 40, respectively. The mean ± standard deviation of age of presentation in the LCS and non-LCS groups was 49.2 ± 8.9 and 43.1 ± 14.3 years, respectively. The difference in fibrosis (P = 0.002), elastic fiber degeneration (P = 0.01), elastic fragmentation percentage (66.5% ± 16.3% vs 29.5% ± 16.9%), elastic content percentage (26.9% ± 6.7% vs 34.7% ± 8.4%), collagen content percentage (63.6% ± 10.4% vs 54.9% ± 6.4%), reduction of elastic/collagen ratio (0.4 ± 0.1 vs 0.6 ± 0.1), and ligamentocyte number (39.1 ± 19.1 vs 53.5 ± 26.9) between the LCS and non-LCS groups was statistically significant. The difference in calcification (P = 0.08) and Pearson’s correlation between duration and loss of elastin was statistically nonsignificant. Subgroup analysis exhibited a consistent difference in LF morphology in patients aged ≥ 40 years between the two groups. A similar finding was observed in patients aged < 40 and > 40 years in the non-LCS group.
The quality change in elastin fibers and an increase in the collagen content and fibrosis cause loss of elasticity in LF, contributing to LCS pathogenesis. However, calcification did not play a significant role in LCS pathogenesis.
Tne study compare the histopathological changes in LF between the degenerative LCS and non-stenotic (non-LCS) group.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Elgafy H, United States; Vahedi P, Iran S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Sairyo K, Biyani A, Goel V, Leaman D, Booth R Jr, Thomas J, Gehling D, Vishnubhotla L, Long R, Ebraheim N. Pathomechanism of ligamentum flavum hypertrophy: a multidisciplinary investigation based on clinical, biomechanical, histologic, and biologic assessments. Spine (Phila Pa 1976). 2005;30:2649-2656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 182] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 2. | Altun I, Yüksel KZ. Histopathological Analysis of Ligamentum Flavum in Lumbar Spinal Stenosis and Disc Herniation. Asian Spine J. 2017;11:71-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Hansson T, Suzuki N, Hebelka H, Gaulitz A. The narrowing of the lumbar spinal canal during loaded MRI: the effects of the disc and ligamentum flavum. Eur Spine J. 2009;18:679-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 4. | Hulmani D, Garg B, Mehta N, Mridha AR, Nag TC, Farooque K. Morphological Changes in the Ligamentum Flavum in Degenerative Lumbar Canal Stenosis: A Prospective, Comparative Study. Asian Spine J. 2020;14:773-781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Giles LG, Kaveri MJ. Some osseous and soft tissue causes of human intervertebral canal (foramen) stenosis. J Rheumatol. 1990;17:1474-1481. [PubMed] |
| 6. | Zhong ZM, Zha DS, Xiao WD, Wu SH, Wu Q, Zhang Y, Liu FQ, Chen JT. Hypertrophy of ligamentum flavum in lumbar spine stenosis associated with the increased expression of connective tissue growth factor. J Orthop Res. 2011;29:1592-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Kolte VS, Khambatta S, Ambiye MV. Thickness of the ligamentum flavum: correlation with age and its asymmetry-an magnetic resonance imaging study. Asian Spine J. 2015;9:245-253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Fukuyama S, Nakamura T, Ikeda T, Takagi K. The effect of mechanical stress on hypertrophy of the lumbar ligamentum flavum. J Spinal Disord. 1995;8:126-130. [PubMed] |
| 9. | Postacchini F, Gumina S, Cinotti G, Perugia D, DeMartino C. Ligamenta flava in lumbar disc herniation and spinal stenosis. Light and electron microscopic morphology. Spine (Phila Pa 1976). 1994;19:917-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 69] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Okuda T, Baba I, Fujimoto Y, Tanaka N, Sumida T, Manabe H, Hayashi Y, Ochi M. The pathology of ligamentum flavum in degenerative lumbar disease. Spine (Phila Pa 1976). 2004;29:1689-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Hamdan TA, Jbara KK, Hatem HA. Histological Changes of Ligamenta Flava in Lumbar Disc Herniation and Spinal Canal Stenosis. Basrah J Surg. 2005;11:1-14 Available from: https://www.semanticscholar.org/paper/HISTOLOGICAL-CHANGES-OF-LIGAMENTA-FLAVA-IN-LUMBAR-Hatem-Jbara/9df6335c9351393e307b723bf70dea3a7980d2c2. |
| 12. | Schräder PK, Grob D, Rahn BA, Cordey J, Dvorak J. Histology of the ligamentum flavum in patients with degenerative lumbar spinal stenosis. Eur Spine J. 1999;8:323-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 82] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Elsberg C. Experiences in spinal surgery: observations upon 60 Laminectomies for spinal disease. Surg Gynecol Obs. 1913;16:117-132. |
| 14. | Wang B, Gao C, Zhang P, Sun W, Zhang J, Gao J. The increased motion of lumbar induces ligamentum flavum hypertrophy in a rat model. BMC Musculoskelet Disord. 2021;22:334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Chuang HC, Tsai KL, Tsai KJ, Tu TY, Shyong YJ, Jou IM, Hsu CC, Shih SS, Liu YF, Lin CL. Oxidative stress mediates age-related hypertrophy of ligamentum flavum by inducing inflammation, fibrosis, and apoptosis through activating Akt and MAPK pathways. Aging (Albany NY). 2020;12:24168-24183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Zaki SM. Study of the human ligamentum flavum in old age: a histological and morphometric study. Folia Morphol (Warsz). 2014;73:492-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Baba H, Maezawa Y, Furusawa N, Imura S, Tomita K. The role of calcium deposition in the ligamentum flavum causing a cauda equina syndrome and lumbar radiculopathy. Paraplegia. 1995;33:219-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Reyes-Sánchez A, García-Ramos CL, Deras-Barrientos CM, Alpizar-Aguirre A, Rosales-Olivarez LM, Pichardo-Bahena R. Ligamentum flavum in lumbar spinal stenosis, disc herniation and degenerative spondylolisthesis. An histopathological description. Acta Ortop Mex. 2019;33:308-313. [PubMed] |
| 19. | Sato N, Higashino K, Sakai T, Terai T, Goel VK, Biyani A, Ebraheim N, Takata Y, Hayashi F, Yamashita K, Morimoto M, Manabe H, Sairyo K. Role of Epiligament in Ligamentum Flavum Hypertrophy in Patients with Lumbar Spinal Canal Stenosis:a Pilot Study. J Med Invest. 2018;65:85-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Peng YX, Zheng ZY, Wang Md WG, Liu L, Chen Md F, Xu Md HT, Zhang ZM. Relationship between the location of ligamentum flavum hypertrophy and its stress in finite element analysis. Orthop Surg. 2020;12:974-982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 21. | Yabe Y, Hagiwara Y, Tsuchiya M, Honda M, Hatori K, Sonofuchi K, Kanazawa K, Koide M, Sekiguchi T, Itaya N, Itoi E. Decreased elastic fibers and increased proteoglycans in the ligamentum flavum of patients with lumbar spinal canal stenosis. J Orthop Res. 2016;34:1241-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Viejo-Fuertes D, Liguoro D, Rivel J, Midy D, Guerin J. Morphologic and histologic study of the ligamentum flavum in the thoraco-lumbar region. Surg Radiol Anat. 1998;20:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Cheung PWH, Tam V, Leung VYL, Samartzis D, Cheung KM, Luk KD, Cheung JPY. The paradoxical relationship between ligamentum flavum hypertrophy and developmental lumbar spinal stenosis. Scoliosis Spinal Disord. 2016;11:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Sun C, Zhang H, Wang X, Liu X. Ligamentum flavum fibrosis and hypertrophy: Molecular pathways, cellular mechanisms, and future directions. FASEB J. 2020;34:9854-9868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |