Published online Apr 18, 2021. doi: 10.5312/wjo.v12.i4.214
Peer-review started: September 10, 2020
First decision: January 27, 2021
Revised: February 1, 2021
Accepted: March 11, 2021
Article in press: March 11, 2021
Published online: April 18, 2021
Processing time: 213 Days and 20.8 Hours
Today, biological fixation of uncemented press-fit acetabular components plays an important role in total hip arthroplasty. Long-term stable fixation of these implants depends on the osseointegration of the acetabular cup bone tissue into the acetabular cup implant, and their ability to withstand functional loads.
To compare the strength of bone-implant osseointegration of four types of porous metal implants in normal and osteoporotic bone in rabbits.
The study was performed in 50 female California rabbits divided into non-ovariectomized (non-OVX) and ovariectomized groups (OVX) at 6 mo of age. Rabbits were sacrificed 8 wk after the implantation of four biomaterials [TTM, CONCELOC, Zimmer Biomet's Trabecular Metal (TANTALUM), and ATLANT] in a 5-mm diameter defect created in the left femur. A biomechanical evaluation of the femur was carried out by testing implant breakout force. The force was gradually increased until complete detachment of the implant from the bone occurred.
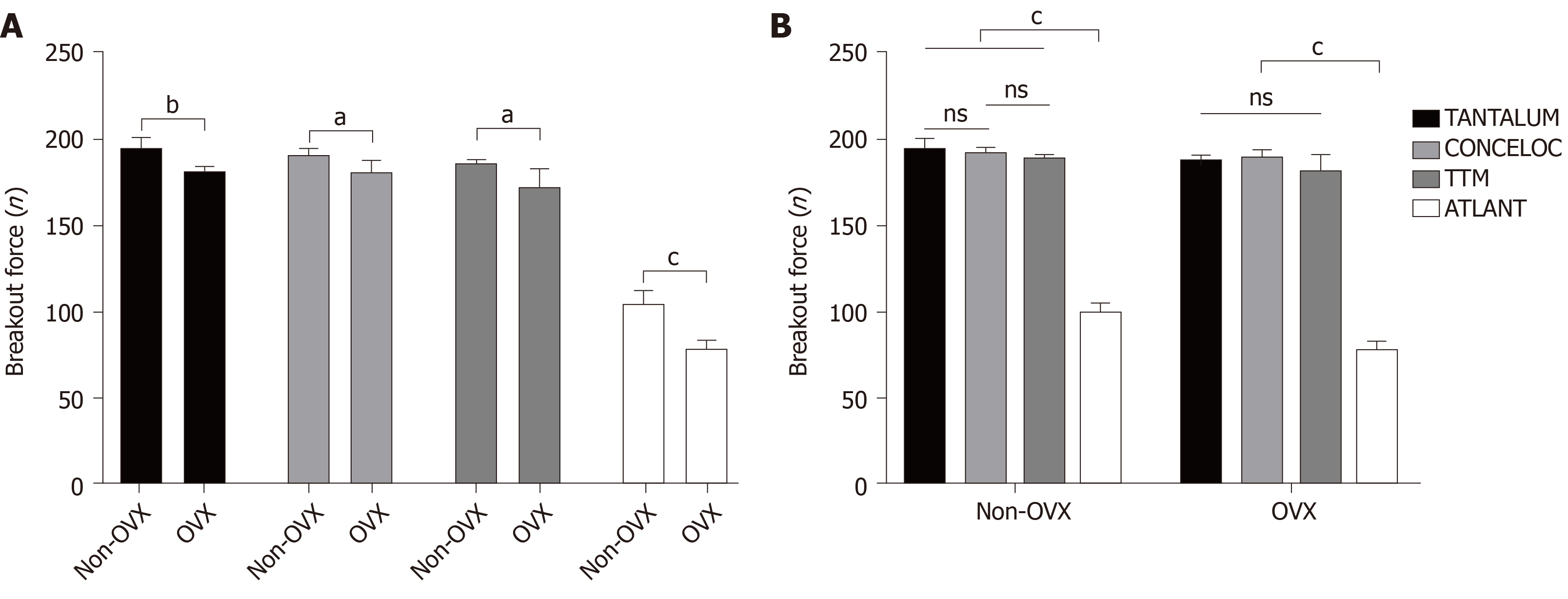
The breakout force needed for implant detachment was significantly higher in the non-OVX group, compared with the OVX group for all implants (TANTALUM, 194.7 ± 6.1 N vs 181.3 ± 2.8 N; P = 0.005; CONCELOC, 190.8 ± 3.6 N vs 180.9 ± 6.6 N; P = 0.019; TTM, 186.3 ± 1.8 N vs 172.0 N ± 11.0 N; P = 0.043; and ATLANT, 104.9 ± 7.0 N vs 78.9 N ± 4.5 N; P = 0.001). In the OVX group, The breakout forces in TANTALUM, TTM, and CONCELOC did not differ significantly (P = 0.066). The breakout force for ATLANT in the OVX group was lower by a factor of 2.3 compared with TANTALUM and CONCELOC, and by 2.2 compared with TTM (P = 0.001). In the non-OVX group, the breakout force for ATLANT was significantly different from all other implants, with a reduction in fixation strength by a factor of 1.9 (P = 0.001).
TANTALUM, TTM, and CONCELOC had equal bone-implant osseointegration in healthy and in osteoporotic bone. ATLANT had significantly decreased osseointegration (P = 0.001) in healthy and in osteoporotic bone.
Core Tip: In an in vivo model of osteoporosis, it was found that some types of porous acetabular components are more compatible with osteoporotic bone. The study results can help to select the right choice of acetabular components for hip replacement in patients with low bone mass.
- Citation: Bondarenko S, Filipenko V, Karpinsky M, Karpinska O, Ivanov G, Maltseva V, Badnaoui AA, Schwarzkopf R. Osseointegration of porous titanium and tantalum implants in ovariectomized rabbits: A biomechanical study. World J Orthop 2021; 12(4): 214-222
- URL: https://www.wjgnet.com/2218-5836/full/v12/i4/214.htm
- DOI: https://dx.doi.org/10.5312/wjo.v12.i4.214
Today, biological fixation of uncemented press-fit acetabular components plays an important role in total hip arthroplasty. Long-term stable fixation of these implants largely depends on the osseointegration of bone tissue into the acetabular cup implant and their ability to withstand functional loads[1,2].
It has been established that both the implant surface and the condition of bone tissue are of great importance in the process of osseointegration[3]. Implant qualities including structure, strength, stiffness, porosity, coefficient of friction, and surface roughness have significant impact on the quality and quantity of bone osseo
In a previous experimental animal study utilizing a rat model, it was shown that osseointegration of current porous acetabular implant materials, as well as the possibility of successful implantation and osseointegration in osteoporotic bone, directly depends on the structural characteristics of the porous implant[9]. However, there is a lack of comparative data of the strength and stability of the bonе-metal osseointegration of current porous materials used in acetabular components in both normal and in osteoporotic bones.
The study aim was to carry out a comparative analysis of the strength of the formed bonе-metal osseointegration among four types of porous metal implants in an in vivo animal model with both normal bone and after the simulation of osteoporosis. Our hypothesis was that there will be a difference in the strength between the formed bone metal osseointegration between normal and osteoporotic bone models.
Fifty female California rabbits 6 mo of age and weighing 4.5-5.0 kg) were kept in conditions of 24 °C, 12/12 h light/dark, and 60% humidity, with ad libitum access to food and water, and a standard diet. All surgeries (ovariectomy and implantation of materials) were performed under general intramuscular anesthesia (xylazine hydrochloride 15 mg/kg and ketamine 50 mg/kg). Euthanasia was carried out by overdosing of ketamine (50 mg/kg) and subsequent air embolism. All experiments were performed according to the national guidelines and all appropriate measures were taken to minimize pain or discomfort to the animals. The study design was approved by the local Bioethics Committee.
Rabbits were randomly divided into healthy control non-ovariectomized (non-OVX) and ovariectomized (OVX) groups of 25 each. To simulate osteoporosis, ovariectomy was performed in the OVX group. After 3 mo, 10 rabbits (5 non-OVX and 5 OVX) were sacrificed to confirm of osteoporosis development. For the remaining rabbits, (n = 40) one of the four types of porous materials were implanted. All rabbits with implants were sacrificed 8 wk after implantation.
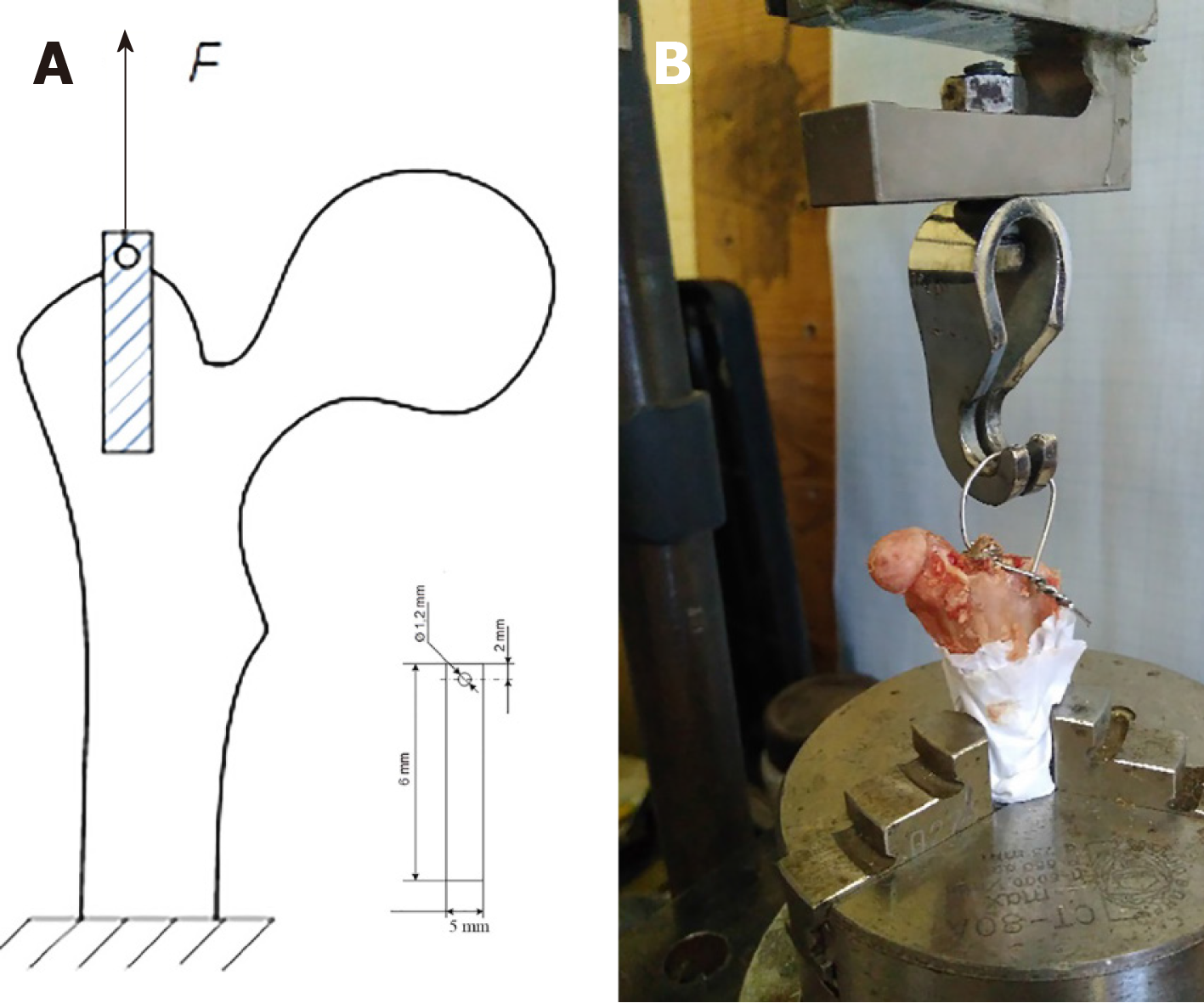
The four types of implants used in this study were of comparable 80% or greater porosity. Three were Ti6-Al4-V titanium alloys: TTM (AK Medical, Beijing, China), CONCELOC (Smith & Nephew, Memphis, TN, United States), ATLANT (TITAN-MED, Kyiv, Ukraine). The fourth was porous Zimmer Biomet's Trabecular Metal (TANTALUM) (Zimmer, Warsaw, United States). The elastic moduli are 3 GPa for TANTALUM[10], 12.9 for GPa[11], 4.3 GPa for CONCELOC[12], and 113 GPa for ATLANT. A 1.2 mm diameter hole was drilled on one side of the implants to allow mounting of the testing jig. The testing jig that was attached to the implant was used to test breaking strength during the study (Figure 1A). Prior to implantation, the biomaterials were sterilized by autoclaving at 132 °C for 20 min.
Ovariectomy (n = 25) was performed under general anesthesia by two dorsolateral 2.5 cm incisions of the skin and muscles following the method described by Wanderman et al[13].
Implantation of materials: Each type of porous material was implanted in 5 healthy and 5 ovariectomized rabbits (n = 40) under general anesthesia under sterile conditions. The surgical field in the proximal part of the left femur was treated with Betadine antiseptic solution, after which the skin was incised from the lateral approach along the anterior femoral region above the greater trochanter. The musculus tensor fasciae latae and musculus quadriceps femoris were bluntly dissected and sequentially fixed. The greater trochanteric area was perforated by a burr to create a bone defect to match the biomaterial samples (diameter of 5 mm, length of 6 mm). After that, the wound was sutured in layers and the skin was treated with Betadine antiseptic. As postoperative pharmacological therapy, benzylpenicillin, dihydrostreptomycin (combikel 40) and meloxicam were administered.
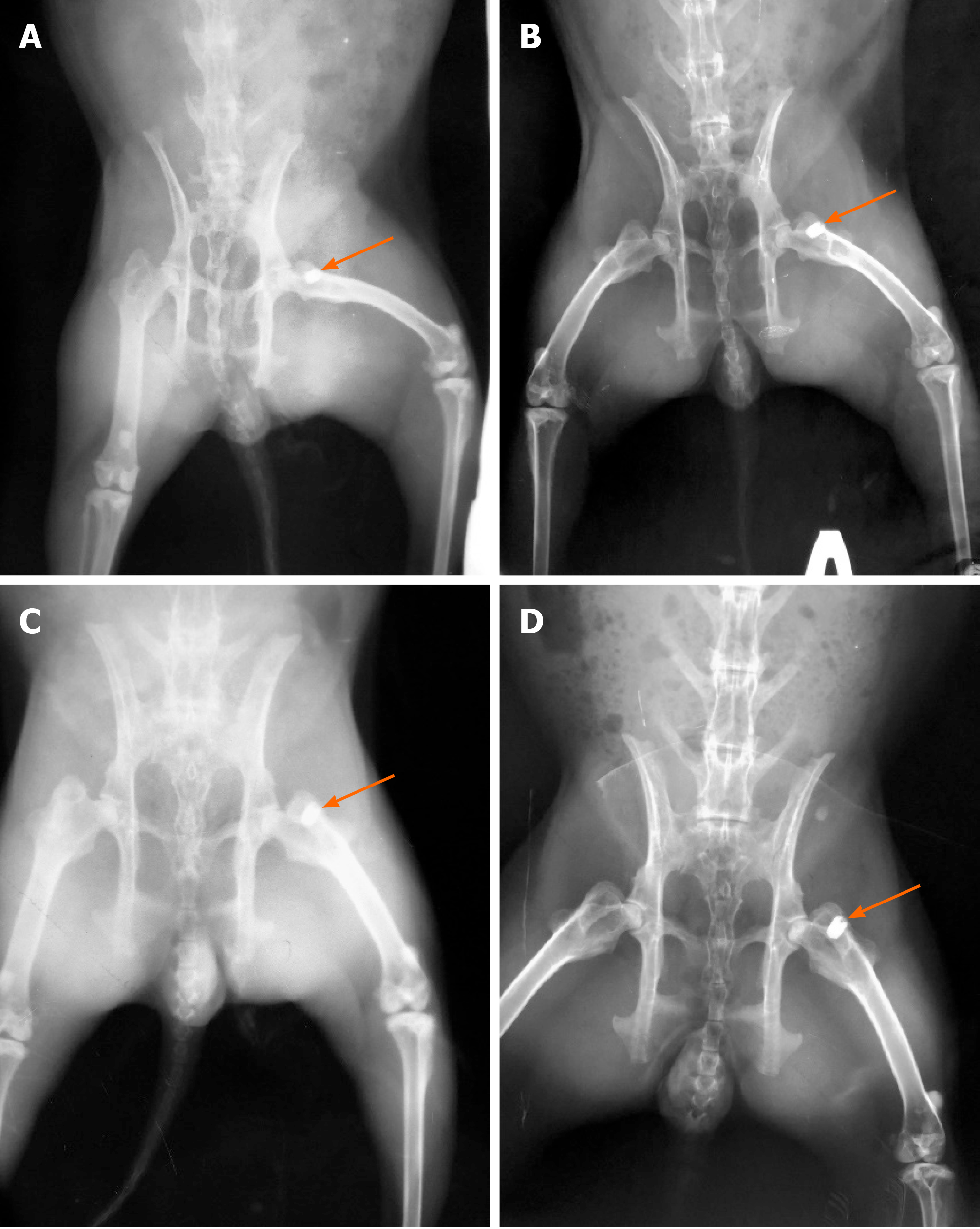
Radiographic evaluation was performed three times. Three months after ovariectomy to control osteoporosis development in 5 OVX and 5 non-OVX rabbits, immediately after implantation to evaluate the position of implants, and at 8 wk after implantation for all 20 OVX and 20 non-OVX rabbits (Figure 2). In all cases, a digital X-ray diagnostic system (OPERA T90cex; General Medical Merate S.p.A., Italy) was used.
Radiographs of the femur was obtained in 10 rabbits (5 OVX and 5 non-OVX) before implantation of materials to verify the osteoporosis model (Figure 3)[14]. This method is used as an alternative to measurement of bone mineral density in diagnosis of osteoporosis[14]. Using “X-Rays” software (Kharkiv National University of Radioelectronics, Ukraine)[15,16], the cortical thickness index was automatically calculated based on the measurement of the thickness of the cortical layer of the femur under the lesser trochanter in 10 rabbits. This software allows performing a coordinate-brightness analysis of digital radiographs, and provides spatial sampling with 0.042 mm elements and brightness quantization with a grayscale of 256 intensities. The analysis was performed by two independent experts.
The implanted materials were rigidly fixed to the testing jig and breakout force testing was performed to detach the implant from the bone tissue (Figure 1B). We applied a breakout force to the implant at a constant speed of 1 mm per minute, which was gradually increased until complete detachment of the implant from the bone. The maximum value of the breakout force (N) was recorded with a strain gauge (SBA-100L) and a CAS type CI-2001A registration device (South Korea) (Figure 4).
Data were reported as means ± SD. Unpaired t-tests were used to evaluate the effect of osteoporosis on the stability of the same type of implant. Unpaired t-tests were performed for verification of osteoporosis model. To analyze the effect of the type of material on the strength of osseointegration in the non-OVX and OVX groups, one-way analysis of variance (ANOVA) was carried out with the post-hoc Duncan test. The critical level of significance was accepted as 0.05. The analysis was performed with IBM SPSS Statistics 19.0 software. The statistical methods were reviewed by Olena Karpinska and Michael Karpinsky of the Department of Biomechanics, Sytenko Institute of Spine and Joint Pathology, National Academy of Medical Sciences of Ukraine.
In the OVX group (n = 5), the cortical thickness index of the proximal femur was 1.4 times lower (P = 0.001) than that in the non-OVX group (n = 5) (0.482 ± 0.033 vs 0.660 ± 0.007, unpaired t-test).
Data were obtained on the maximum breakout force that led to detachment of the implant from the femoral bone in both normal and osteoporotic bone tissue. The strength of the implant attachment in the femoral bone tissue was significantly higher in non-OVX group (n = 20), compared with the OVX group (n = 20) for all materials (Figure 5, unpaired t-test). When evaluating each implanted material, the breakout force was higher in the non-OVX group by a factor of 1.1 for TANTALUM (194.7 ± 6.1 N vs 181.3 ± 2.8; P = 0.005); CONCELOC (190.8 ± 3.6 N vs 180.9 ± 6.6 N; P = 0.019); and TTM (186.3 ± 1.8 N vs 172.0 ± 11.0 N; P = 0.043), and by a factor of 1.3 (104.9 ± 7.0 N vs 78.9 ± 4.5 N; P = 0.001) for ATLANT, compared with the OVX group.
TANTALUM implants had the highest breakout strength in osteoporotic bone tissue at a load of 181.3 ± 2.8 N and in normal bone tissue at a load of 194.7 ± 6.1 N (Figure 5). The lowest breakout strength was shown in ATLANT implants. Failure was observed in normal bone tissue at a load of 104.9 N ± 7.0 N and in osteoporotic bone at 78.9 ± 4.5 N. In the OVX group (osteoporotic bone), the breakout forces of TANTALUM, TTM, and CONCELOC did not differ significantly (P = 0.066, Figure 5, one-way ANOVA). On the contrary, The breakout force of ATLANT implants was lower by a factor of 2.3 compared with TANTALUM and CONCELOC and by a factor of 2.2 compared with TTM (P = 0.001). Results in the non-OVX group (normal bone) were similar to those in the OVX group with minor differences (Figure 5, one-way ANOVA). The breakout force for TANTALUM and CONCELOC implants did not differ significantly (P = 0.239). ATLANT implants were significantly different from all other implants, with a reduction in fixation strength of approximately 1.9 times (P = 0.001).
In this biomechanical study, we examined the strength of osseointegration of three porous titanium and one porous tantalum materials. We studied the breakout strength of the implanted materials 8 wk after their implantation in the metaphysis of the greater trochanter of the femur in an in vivo rabbit model. Three of the studied materials in this study (TANTALUM, CONCELOC, TTM) are used in the manufacture of acetabular components for total hip replacement and are currently used in clinical practice. The fourth studied sample (ATLANT) is a new material used in the manufacture of acetabular cups. A unique aspect of our study is the comparison of the breakout strength among the four studied materials in both normal and osteoporotic bone in a rabbit ovariectomy model.
The importance of bone quality for osseointegration of porous implants has been shown both in cadaver studies[17,18] and in an animal model[19]. Beckmann et al[18] reported the results of a biomechanical study (multi-axial testing machine) that examined the mobility of an acetabular titanium cup with a porous surface in 10 cadaveric pelvises. They found an inverse relationship between the bone mineral density (BMD) of the femoral neck and the mobility of the acetabular cup. Similar data were obtained when using an additional acetabular porous augment[18]. The differences in osseointegration and breakout strength between different commercial acetabular cup materials are especially important in patients with low BMD. It has been found that patients with low BMD have an increased risk of migration of uncemented hydroxyapatite-coated titanium alloy acetabular cups 3–12 mo after total hip arthroplasty (THA) compared with patients with normal BMD[8]. According to a clinical study of 283 patients evaluated 2 years after revision THA, it was found that porous tantalum acetabular cups were more stable than porous titanium cups in patients with low BMD[20]. However, the long-term survivorship of acetabular cups in patients with osteoporosis is poorly understood in comparison with patients with normal BMD[21].
According to our data, the stability of implants in the OVX group was lower for all materials studied compared with the non-OVX group. Similar results were obtained by Fujimoto et al[19] when evaluating titanium implants in an experimental model of glucocorticoid-induced osteoporosis in rabbits. Similar to our results of the non-OVX group, Duan et al[22] presented biomechanical testing (push-out test) of medical Ti-6Al-4V substrates with titanium and tantalum coated implants. Their results showed that at 9 wk after implantation, the titanium and tantalum implants had similar push-out strengths when evaluated in New Zealand white rabbit femurs.
Our findings of similar breakout forces in tantalum (TANTALUM) and titanium implants (TTM and CONCELOC) in the OVX group may have occurred because the evaluated titanium implants had similar porosity, highlighting the importance of high porosity percentage in these implants. It has been shown that high porosity improves osseointegration compared to nonporous implants[23,24]. Pore size is also an important factor affecting osseointegration of the implant[23,24]. These variables probably explain the lower values of breakout force exhibited by the ATLANT component material compared with the other implants in both OVX and non-OVX models. A recent study in rabbits that compared titanium implants manufactured by additive technology and with three different pore sizes (500 μm, 700 μm and 900 μm)[25] showed that the best interfacial strength was achieved when the pore size was 700 μm, when evaluated by a push-out test at weeks 4 and 12 after implantation. This emphasizes the importance of the material pore size for its osseointegrative qualities. This knowledge may help manufacturers design materials made with similar technology and from different alloy materials. Nevertheless, when comparing tantalum and titanium implants with the same 500 µM pore size and 70% porosity in a rabbit model, the authors did not find any differences in the push-out test indices at 2 wk, 4 wk, and 8 wk after implantation[26]. The same results were reported in a similar study by Su et al[27] when comparing tantalum and titanium implants with the same pore size.
A limitation of our study was the use of one type of test to evaluate implant stability. However, our study is one of the few studies comparing tantalum and titanium materials in a low bone-mass model. Thus, these results will help expand the current knowledge of the stability of the studied materials in cases of osteoporosis.
TANTALUM, TTM and CONCELOC had equal bone-implant osseointegration in both healthy and osteoporotic bones. ATLANT showed a significant decrease in osseointegration (P = 0.001) in both healthy and osteoporotic bone.
Highly porous metal acetabular components are widely used in patients with low bone mass, but the strength of osseointegration may differ.
There is a need to perform studies to compare the strength of osseintegration of new porous metal biomaterials used in total hip arthroplasty of patients with low bone mass.
The objective of this study was to compare the strength of the formed bonе-metal osseointegration among four types of porous metal biomaterials in an in vivo animal model with both normal bone and after simulation of osteoporosis
The experimental study was performed in a rabbit model of postmenopausal osteoporosis. Biomechanical evaluation of the femur was carried out by testing the implant breakout force 8 wk after implantation of four types of biomaterials: TTM, CONCELOC, Zimmer Biomet's Trabecular Metal (TANTALUM), and ATLANT. The force was gradually increased until complete detachment of the implant from the bone.
The breakout force needed for implant detachment was significantly higher in healthy controls, compared with the ovariectomized group for all implants. The breakout force for ATLANT in the ovariectomized group was lower than that observed with TANTALUM, CONCELOC’ and TTM.
TANTALUM, TTM and CONCELOC had equal bone-implant osseointegration in healthy and osteoporotic bones. ATLANT showed a significant decrease in osseointegration in healthy and osteoporotic bone.
Further studies on the use of other biomechanical methods will expand the knowledge of the strength of osseointegration of modern porous materials, which will help in choosing optimal materials for acetabular implants when performing total hip arthroplasty in patients with osteoporosis.
Manuscript source: Unsolicited manuscript
Specialty type: Biochemical Research Methods
Country/Territory of origin: Ukraine
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jennane R S-Editor: Liu M L-Editor: Filipodia P-Editor: Xing YX
| 1. | Gruen TA, Poggie RA, Lewallen DG, Hanssen AD, Lewis RJ, O'Keefe TJ, Stulberg SD, Sutherland CJ. Radiographic evaluation of a monoblock acetabular component: a multicenter study with 2- to 5-year results. J Arthroplasty. 2005;20:369-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 91] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Yuan BJ, Lewallen DG, Hanssen AD. Porous metal acetabular components have a low rate of mechanical failure in THA after operatively treated acetabular fracture. Clin Orthop Relat Res. 2015;473:536-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Karachalios T. Bone-implant interface in orthopedic surgery: basic science to clinical applications. London: Springer-Verlag, 2014: 13-26. [DOI] [Full Text] |
| 4. | Naziri Q, Issa K, Pivec R, Harwin SF, Delanois RE, Mont MA. Excellent results of primary THA using a highly porous titanium cup. Orthopedics. 2013;36:e390-e394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Marin E, Fedrizzi L, Zagra L. Porous metallic structures for orthopaedic applications: a short review of materials and technologies. Eur Orthop Traumatol. 2010;1:103109. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Fini M, Giavaresi G, Torricelli P, Borsari V, Giardino R, Nicolini A, Carpi A. Osteoporosis and biomaterial osteointegration. Biomed Pharmacother. 2004;58:487-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Anderson KD, Ko FC, Virdi AS, Sumner DR, Ross RD. Biomechanics of Implant Fixation in Osteoporotic Bone. Curr Osteoporos Rep. 2020;18:577-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Finnilä S, Moritz N, SvedströM E, Alm JJ, Aro HT. Increased migration of uncemented acetabular cups in female total hip arthroplasty patients with low systemic bone mineral density. A 2-year RSA and 8-year radiographic follow-up study of 34 patients. Acta Orthop. 2016;87:48-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 9. | Bondarenko S, Ashukina N, Maltseva V, Ivanov G, Badnaoui AA, Schwarzkopf R. Evaluation of the bone morphology around four types of porous metal implants placed in distal femur of ovariectomized rats. J Orthop Surg Res. 2020;15:296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Yang J, Cai H, Lv J, Zhang K, Leng H, Sun C, Wang Z, Liu Z. In vivo study of a self-stabilizing artificial vertebral body fabricated by electron beam melting. Spine (Phila Pa 1976). 2014;39:E486-E492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Smith & Nephew Material specifications. Research report OR-14-106; 2016. [accessed February 24, 2021]. In smith-nephew.com [Internet]. Available from: https://www.smith-nephew.com/global/assets/pdf/products/conceloc-material-data-sheet-03955.pdf. |
| 12. | Beckmann NA, Jaeger S, Janoszka MB, Klotz MC, Bruckner T, Bitsch RG. Comparison of the Primary Stability of a Porous Coated Acetabular Revision Cup With a Standard Cup. J Arthroplasty. 2018;33:580-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Wanderman NR, Mallet C, Giambini H, Bao N, Zhao C, An KN, Freedman BA, Nassr A. An Ovariectomy-Induced Rabbit Osteoporotic Model: A New Perspective. Asian Spine J. 2018;12:12-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Nguyen BN, Hoshino H, Togawa D, Matsuyama Y. Cortical Thickness Index of the Proximal Femur: A Radiographic Parameter for Preliminary Assessment of Bone Mineral Density and Osteoporosis Status in the Age 50 Years and Over Population. Clin Orthop Surg. 2018;10:149-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 15. | Arsenidze TO, Sharmazanova OP, Avrunin OH, inventors; Kharkiv Medical Academy of Postgraduate Education; D Inc. , assignee. Method for Diagnosing Osteoporosis. Ukraine Patent UA 105663. 2016 Mar 25. [accessed February 24, 2021]. In Specialized Data Base: Inventions (Utility Models) in Ukraine [Internet]. Available from: https://base.uipv.org/searchINV/search.php?action=viewdetails&IdClaim=221648&chapter=biblio. |
| 16. | Arsenіdze TO, Avrunіn OG, Aver'yanova LO. [Comparative Analysis of Automated and Manual Definition of Cortical Index for Femur in Infants by X-Ray Data]. Radiation diagnostics, radiation therapy 2016; 3-4: 121-124. [Russian] [accessed February 24, 2021]. In Open Electronic Archive of Kharkov National University of Radio Electronics [Internet]. Available from: https://openarchive.nure.ua/handle/document/4012. |
| 17. | Zhang Y, Ahn PB, Fitzpatrick DC, Heiner AD, Poggie RA, Brown TD. Interfacial frictional behavior: cancellous bone, cortical bone, and a novel porous tantalum biomaterial. J Musculoskelet Res. 1999;3:245-251. [RCA] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 18. | Beckmann NA, Bitsch RG, Janoszka MB, Klotz MC, Bruckner T, Jaeger S. Treatment of High-Grade Acetabular Defects: Do Porous Titanium Cups Provide Better Stability Than Traditional Titanium Cups When Combined With an Augment? J Arthroplasty. 2018;33:1838-1843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Fujimoto T, Niimi A, Sawai T, Ueda M. Effects of steroid-induced osteoporosis on osseointegration of titanium implants. Int J Oral Maxillofac Implants. 1998;13:183-189. [PubMed] |
| 20. | Jafari SM, Bender B, Coyle C, Parvizi J, Sharkey PF, Hozack WJ. Do tantalum and titanium cups show similar results in revision hip arthroplasty? Clin Orthop Relat Res. 2010;468:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Karachalios TS, Koutalos AA, Komnos GA. Total hip arthroplasty in patients with osteoporosis. Hip Int. 2020;370-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 22. | Duan Y, Liu L, Wang L, Guo F, Li H, Shi L, Li M, Yin D, Jiang C, Zhu Q. Preliminary study of the biomechanical behavior and physical characteristics of tantalum (Ta)-coated prostheses. J Orthop Sci. 2012;17:173-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Bandyopadhyay A, Shivaram A, Tarafder S, Sahasrabudhe H, Banerjee D, Bose S. In Vivo Response of Laser Processed Porous Titanium Implants for Load-Bearing Implants. Ann Biomed Eng. 2017;45:249-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 24. | Cohen DJ, Cheng A, Sahingur K, Clohessy RM, Hopkins LB, Boyan BD, Schwartz Z. Performance of laser sintered Ti-6Al-4V implants with bone-inspired porosity and micro/nanoscale surface roughness in the rabbit femur. Biomed Mater. 2017;12:025021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 25. | Ran Q, Yang W, Hu Y, Shen X, Yu Y, Xiang Y, Cai K. Osteogenesis of 3D printed porous Ti6Al4V implants with different pore sizes. J Mech Behav Biomed Mater. 2018;84:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 148] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 26. | Wang H, Su K, Su L, Liang P, Ji P, Wang C. Comparison of 3D-printed porous tantalum and titanium scaffolds on osteointegration and osteogenesis. Mater Sci Eng C Mater Biol Appl. 2019;104:109908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 27. | Su KX, Ji P, Wang H, Li LL, Su LZ, Wang C. [In vivo study of 3D printed porous tantalum implant on osseointegration]. Hua Xi Kou Qiang Yi Xue Za Zhi. 2018;36:291-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |