Published online Oct 18, 2021. doi: 10.5312/wjo.v12.i10.743
Peer-review started: February 25, 2021
First decision: May 3, 2021
Revised: May 16, 2021
Accepted: August 6, 2021
Article in press: August 6, 2021
Published online: October 18, 2021
Processing time: 230 Days and 8.7 Hours
Metastatic bone disease of the distal extremities, also known as acrometastasis, is very rare. Thus, there is very limited information regarding the clinical manifestations and methods of surgical treatment. The current available literature shows that acrometastases are often encountered in the context of advanced disease and are thus associated with poor patient survival. As metastatic bone disease is generally uncurable, the goal of surgical treatment is to provide the patient with good function with as few complications as possible. In this article, we discuss the clinical manifestation of acrometastases, the methods of surgical intervention, and the expected clinical outcome. Non-surgically managed pathological fractures generally remain ununited; therefore, conservative treatment is reserved for patients with poor general condition or dismal prognosis. The current evidence suggests that in lesions of the lower arm and leg, osteosynthesis (plate and screw fixation or intramedullary nail) is the most common method of reconstruction, whereas local excision or amputation are more commonly used in cases of more distal lesions (such as ankle, foot and hand). Following surgery most patients receive adjuvant radiotherapy, even though its role is poorly documented. Close collaboration between orthopedic surgeons and medical oncologists is necessary to improve patient care and treatment outcome. Further studies are needed in order to provide stronger clinical evidence and improve decision-making, in an effort to optimize the patients’ quality of life and avoid the need for revision surgery.
Core Tip: Metastatic bone disease distal to the elbow and knee is rare, often encountered in patients with spread cancer. Limb-preserving surgery is often possible in the lower arm and leg, and osteosynthesis with plate and screws or intramedullary nails are the most common surgical methods. In the lesions of the ankle, hand and foot, amputation is often utilized.
- Citation: Sebghati J, Khalili P, Tsagkozis P. Surgical treatment of metastatic bone disease of the distal extremities. World J Orthop 2021; 12(10): 743-750
- URL: https://www.wjgnet.com/2218-5836/full/v12/i10/743.htm
- DOI: https://dx.doi.org/10.5312/wjo.v12.i10.743
Bone is one of the most common organs affected by cancer metastases[1]. Metastatic bone disease (MBD) can be caused by different primary tumors, with the highest prevalence being from breast and prostate cancer[2]. It has been described that the incidence of bone metastases depends on the origin of the primary tumor, although it increases primarily with more advanced disease, regardless of the tumor origin[3,4]. MBD causes a disruption of the bone’s normal metabolism and physiology, which eventually could lead to hypercalcemia and bone pain[1,2]. Moreover, the disruption of bone architecture can lead to reduced bearing capacity and microfractures. In turn, this can cause a total loss of the bony integrity and result in bone fractures[1]. The skeleton is not equally affected by MBD, which is more common with metastases in bones consisting of a larger amount red marrow and trabecular bone. Metastatic tumors are also more abundant in the axial skeleton[2]. The most frequently affected long bone is femur, followed by humerus[5].
It is important to diagnose MBD in its early stages so that mortality and morbiditiy can be reduced[6]. Generally, the diagnosis of MBD often starts with conventional radiography (X-ray) or computed tomography (CT), magnetic resonance imaging (MRI) and/or bone scans, depending on the clinical suspicion and origin of the metastasis. Conventional plain radiographs can detect and localize bone lesions, their frequency and size, the occurrence of pathological fractures, and potential soft tissue involvement. Moreover, X-rays can determine whether the bone lesions are sclerotic or lytic. CT scans could be helpful in situations when there is cortical destruction and mineralization[7], and they support the diagnosis process by differentiating benign and malignant tumors[6]. Moreover, CT scanning with 2D frontal-sagittal recon
Isotope bone scans are another method that is helpful in the diagnosis of MBD. These scans are useful when detecting obscured bone lesions and mapping tissue characterization. Positron emission tomography (PET) scans are superior to all other imaging techniques in cases of detecting primary lesion sites in the earlier stages of disease. PET scans are highly accurate and can also detect lesions in the distal extremities[10]. Moreover, fine needle aspiration cytology or core-needle biopsy is necessary and should take place in tissue diagnosis[9].
MBD is associated with poor survival, which is mainly due to the primary tumors’ type and origin[10]. Generally, MBD is incurable and the treatment options aim to reduce symptoms[11]. Currently, the common treatments include osteoclast inhibition, chemotherapy, radiotherapy, and surgery[11]. Bisphosphonates and denosumab aim to inhibit bone resorption and thereby bone destruction[12-14], while radiation and chemotherapy help to ease pain and control tumor growth. Surgical treatment is needed when the MBD results in impending or pathological fractures[3,11]. However, surgery does not have a cancer-reducing effect nor improve survival[15,16]. Instead, it is the treatment of choice when it comes to stabilizing the bone structure and reducing pain[15].
Pathological fractures managed non-surgically will generally remain ununited[1,17,18]. Therefore, conservative treatment is an option only when the patient is inoperable[18]. Most patients receive radiation therapy following surgery[3], due to its counteractive effects towards pain, local recurrence, and tumor growth[3,19]. However, adjuvant radiotherapy could potentially cause surgical failures when some surgical techniques are used, such as osteosynthesis and un-cemented implants, and lead to wound healing problems and infection[20,21]. Generally, the role of adjuvant radiotherapy is poorly documented[19,22].
As previously mentioned, the most frequently affected long bone is femur, followed by humerus. Other long bones, such as the ones in hands and feet, rarely harbor metastases[5]. The term acrometastases is used inconsistently[23]; sometimes, it is defined as metastatic lesions distal to the elbow and knee[16] and, other times as lesions distal to the ankle and wrist[23]. Acrometastases are a rare occurrence and the incidence is reported as 7%[17]. Approximately 0.1% of all acrometastases are located distal to the ankle and wrist[16]. The hands are more often affected by osseous metastases compared to the feet[9], with a ratio of 3:1[24]. Significant delay of the diagnosis is common[23], due to its rarity in combination with unspecific symptoms[16]. Associated signs and symptoms are, in general, soft tissue swelling, pain and functional impairment, steering the clinicians’ ideas towards more benign conditions, such as gout, ligamentous sprains, osteoarthritis and more[16,25]. In addition, the common practice of metastatic skeletal surveys where whole body CT is used is to exclude the distal extremities, leading to possible under-reporting of these cases[26]. Considering metastatic disease as a differential diagnosis can therefore prevent late diagnosis and delayed treatment. For acrometastases in general, histological examinations have shown that the main tumor type is lung cancer, followed by gastrointestinal tract and genitourinary tract tumors[9].
Complete staging must be performed in order to determine the primary lesion, extension and metastatic count. This information is necessary in order to evaluate prognosis and provide input in determination of the treatment approach[16]. Staging often includes radiology of the lesion, preferably MRI, CT of the chest and abdomen, and bone scans. Tissue diagnosis is of importance. Some authors have recommended fine needle aspiration over incisional biopsies because of the risk of making the lesion extracompartmental[9,27]. When staging has been performed and the patients’ prognosis and overall functional status is assessed as sufficient, surgical treatment can be considered[16].
Important elements exist that should be taken into consideration in the surgical planning and reconstruction decision-making. For example, renal metastases are relatively resistant to photon beam radiation therapy, explaining why total resection is recommended. This is in comparison to radiosensitive tumors for which intralesional curettage and stabilization is fitting[28]. Studies have also shown that certain tumor types, such as metastases from renal and thyroid origin, have the best prognosis when totally resected, when local recurrence and disease-free survival are the primary outcome measurements[29]. Nonetheless, prognosis of patients with acrometastatic cancer is poor[30], since most have widespread disease, and their mean survival time has been reported to be less than 6 mo[31].
Acrometastases in the hands are mostly originated from lung cancer[9,32]. The phalanges are the most commonly affected location[10,33], followed by the metacarpal bones[33]. The mean survival for patients suffering from hand metastases have been reported to range from 5 mo to over 1 year[10,24,33]. Although there is no standardized treatment for hand metastases, a variety of treatment options, including radiotherapy, curettage, resection, radical disarticulation of the ray and amputation is available[9,16,33,34]. Most commonly limb salvage surgery is used, including resection and curettage[32]. As the prognosis of acrometastases depends on the characteristics of the primary tumor[10], the approach differs. In cases with poor response to cancer treatments, including radiation and chemotherapy, amputation is a more appropriate method[35]. Moreover, surgical methods, such as local excisions and curettage, could be hard to conduct due to the limited amount of soft tissue in the hands[16,35].
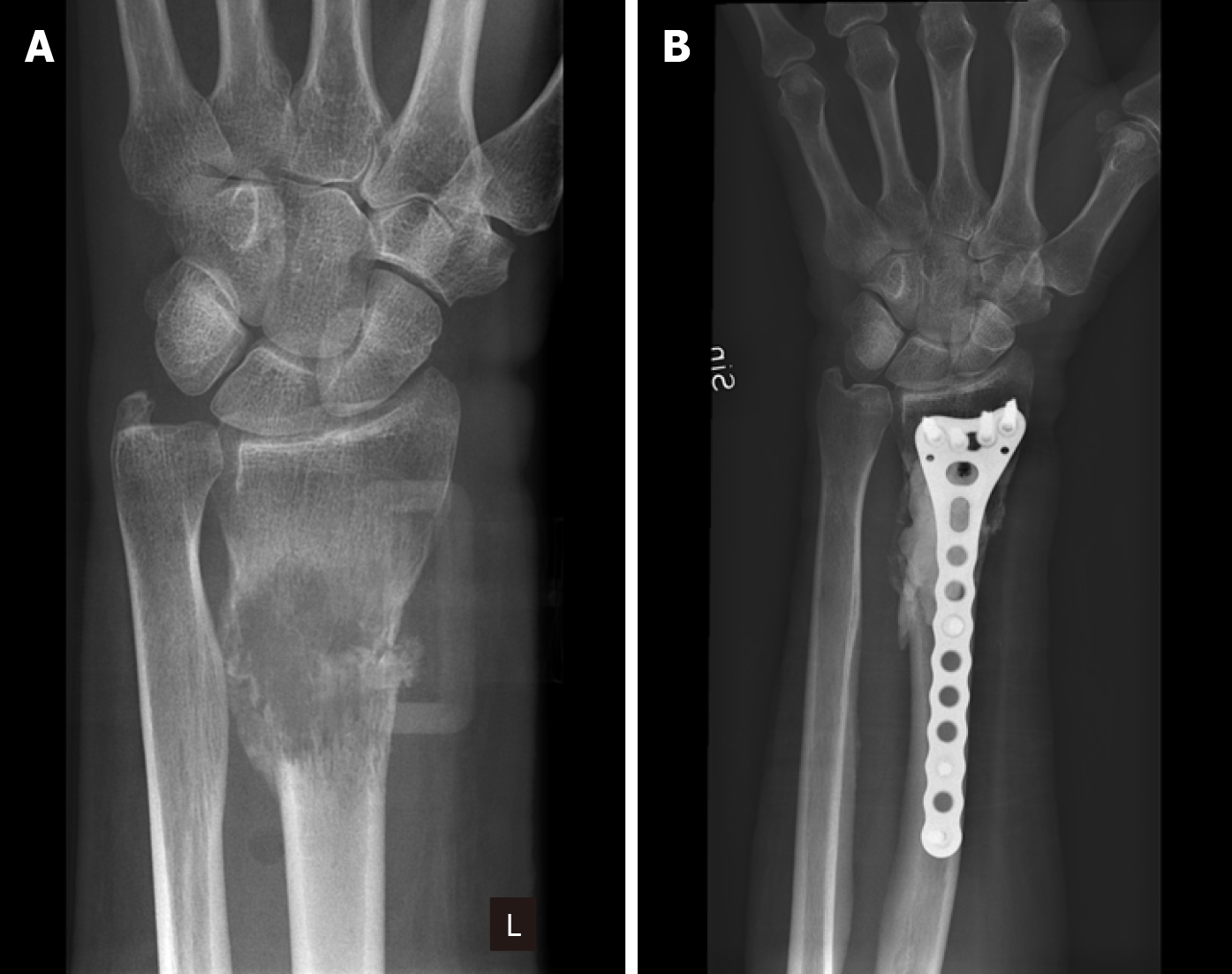
MBD in the lower arm is extremely rare. The incidence of metastases involving ulna has been reported as low as 1%[36]. In another small cohort study, the incidence of lower arm metastases was reported as 2 out of 34 fractures (6%)[37]. Because of the rare occurrence of MBD in the lower arm, the literature is sparse regarding these locations. To our knowledge, metastatic bone lesions in ulna and radius are very rarely described in the literature. Our experience is that most lesions can be managed with local excision and osteosynthesis with plate and screws (Figure 1).
Studies suggest that less than 50% of acrometastases involve the feet[38,39]. MBD of the foot occurs in the context of widespread dissemination, which is the main reason why it has a poor prognosis[24]. Lung cancer is the most common primary tumor, followed by breast, kidney and colon/rectum, respectively. The hindfoot (calcaneus and talus) is the most common site, followed by the forefoot (metatarsal bones and phalanxes) and mid foot (cuniforme-, navicular- and cuboid bones)[16]. An average survival of 15 mo for patients with acrometastases of the foot has been reported[30].
Treatments of MBD of the foot vary from simple palliative care or pharmacological treatment, radiation therapy, chemotherapy, and surgery[30,35]. No standard treatment protocols exist because of the rarity of the condition and therefore are often approached on a case-by-case basis. The main goal however should be focused on palliation and improving quality of life[35]. Amputation and local curettage is common practice for these cases, with the former being the most frequent surgical option[16,35]. Midfoot or, more commonly, transtibial amputation can be performed depending on the location and spread of the tumor. If amputation is not acceptable curettage can be an alternative[24]. However, the recurrence rate after curettage has been reported to be approximately 20%[9]. Adjuvant radiation therapy or marginal excision are other options[24].
MBD of the tibia has been reported to account for 3%-4% of MBD, thus being more frequent than metastases in the foot[17,40]. Fibular metastases are very rare and the representation in the literature mainly consists of case reports[41-43]. Due to its rarity, published cohorts of cases are small[17,44]. The somewhat higher occurrence of MBD involving the tibia has resulted in better data. The primary tumors giving rise to bone metastases in the tibia are breast and prostate cancer in women and men respectively, with lung, kidney and colorectal cancers also being represented independent of sex[8,23,45]. The most common site of metastases in the tibia is the proximal metaphyseal region, followed by the diaphysis[21,23].
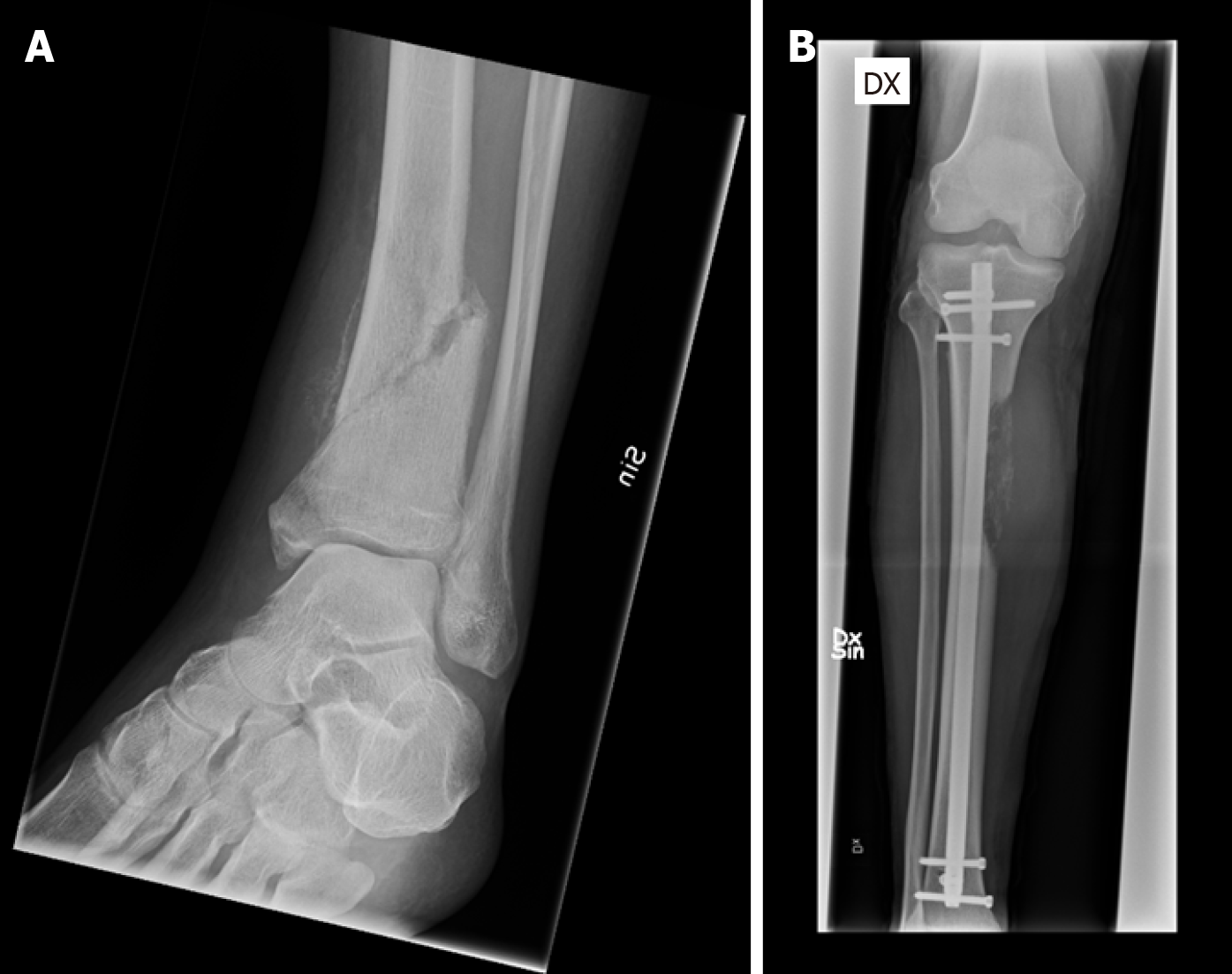
Surgery is the primary treatment choice, since the tibia is a major weight-bearing bone. The loading forces affecting the knee and ankle are mainly compressive, and the tensile forces are lower than in the proximal femur, hence decreasing the risk of mechanical failure[20]. The three main surgical management principles for MBD in the lower leg are stabilization after curettage with or without cement or bone graft, endoprosthetic reconstruction after complete resection, and amputation[28]. For reconstruction, intramedullary (IM) nails, plate and screws with or without cement, and endoprostheses are suitable options[28]. In the cases of proximally located tumors, especially the tibial metaphysis, curettage followed by cementation and stabilization with plate and screws is a good alternative. However, if the lesion is highly destructive and affecting the joint, endoprosthetic reconstruction with a medial gastrocnemius flap can be considered in patients with good prognosis[28]. Closed IM nailing, sometimes with the addition of curettage, and generally followed by radiation therapy, is the preferred method of choice when metastases are located in the diaphysis[8]. IM nail devices typically allow immediate weight bearing, and postoperative radiation therapy is not contraindicated, which is of great importance to the patient (Figure 2).
Metastatic lesions in the distal parts of the tibia are rare[21]. If reconstruction is an alternative, plate and screw fixation could be implemented with or without curettage and bone cement. Since there are no suitable prosthetic devices available after resection of the distal tibia, in cases of large destruction a retrograde IM nail through the calcaneus and talus into the tibia is the main option. Postoperative radiation therapy jeopardizes the fusion of the arthrodesis in these cases[20]. However, due to the poor functional performance of limb-sparing surgery, below knee amputation is a good alternative[28]. This procedure is also suitable in cases in which tumor growth is not controllable by adjuvant therapy, if soft-tissue quality is poor, or if previous surgery or reconstructions have failed[45].
As for the even rarer occurrence of fibular metastases, resection without reconstruction is the most reported procedure in the literature by far[20,28,44]. The fibula, as well as the ribs and clavicle, is an expandable bone, and resection can be performed without any functional impairment[20].
MBD in the distal extremities (acrometastases) is a rare condition and very poorly investigated in the medical literature. Patients with acrometastases generally have advanced disease and poor survival. Choosing the proper surgical treatment is important in order to improve the patient’s quality of life and avoid implant failure and the need for revision surgery. Osteosynthesis is the most common treatment method in proximal acrometastases (lower arm and leg), with amputation being more common for distal lesions.
The authors would like to express their gratitude to Mr. Helmuts Kleins for linguistic review of the manuscript.
Manuscript source: Invited manuscript
Specialty type: Orthopedics
Country/Territory of origin: Sweden
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chisthi MM S-Editor: Wang JL L-Editor: Filipodia P-Editor: Wang LYT
| 1. | Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27:165-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1313] [Cited by in RCA: 1337] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 2. | Fornetti J, Welm AL, Stewart SA. Understanding the Bone in Cancer Metastasis. J Bone Miner Res. 2018;33:2099-2113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 311] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 3. | Guzik G. Oncological and functional results after surgical treatment of bone metastases at the proximal femur. BMC Surg. 2018;18:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 4. | Hernandez RK, Wade SW, Reich A, Pirolli M, Liede A, Lyman GH. Incidence of bone metastases in patients with solid tumors: analysis of oncology electronic medical records in the United States. BMC Cancer. 2018;18:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 236] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 5. | Casadei R, Drago G, Di Pressa F, Donati D. Humeral metastasis of renal cancer: Surgical options and review of literature. Orthop Traumatol Surg Res. 2018;104:533-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Isaac A, Dalili D, Weber MA. State-of-the-art imaging for diagnosis of metastatic bone disease. Radiologe. 2020;60:1-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 7. | Zimmer WD, Berquist TH, McLeod RA, Sim FH, Pritchard DJ, Shives TC, Wold LE, May GR. Bone tumors: magnetic resonance imaging vs computed tomography. Radiology. 1985;155:709-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 159] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Bonnevialle P, Descamps J, Niglis L, Lebaron M, Falguieres J, Mericq O, Fabre T, Reina N, Sailhan F; members of the SoFCOT. Surgical treatment of tibial metastases: Retrospective, multicenter, observational study of 25 patients. Orthop Traumatol Surg Res. 2020;106:1039-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Spiteri V, Bibra A, Ashwood N, Cobb J. Managing acrometastases treatment strategy with a case illustration. Ann R Coll Surg Engl. 2008;90:W8-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Afshar A, Farhadnia P, Khalkhali H. Metastases to the hand and wrist: an analysis of 221 cases. J Hand Surg Am. 2014;39:923-32.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Wood TJ, Racano A, Yeung H, Farrokhyar F, Ghert M, Deheshi BM. Surgical management of bone metastases: quality of evidence and systematic review. Ann Surg Oncol. 2014;21:4081-4089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Sousa S, Clézardin P. Bone-Targeted Therapies in Cancer-Induced Bone Disease. Calcif Tissue Int. 2018;102:227-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (1)] |
| 13. | Kurata T, Nakagawa K. Efficacy and safety of denosumab for the treatment of bone metastases in patients with advanced cancer. Jpn J Clin Oncol. 2012;42:663-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Fizazi K, Carducci M, Smith M, Damião R, Brown J, Karsh L, Milecki P, Shore N, Rader M, Wang H, Jiang Q, Tadros S, Dansey R, Goessl C. Denosumab vs zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377:813-822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1573] [Cited by in RCA: 1384] [Article Influence: 98.9] [Reference Citation Analysis (0)] |
| 15. | Cai L, Dong S, Chen H. Olecranon metastases from primary lung cancer: case report and review of the literature. J Int Med Res. 2019;47:5312-5317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Stomeo D, Tulli A, Ziranu A, Perisano C, De Santis V, Maccauro G. Acrometastasis: a literature review. Eur Rev Med Pharmacol Sci. 2015;19:2906-2915. [PubMed] |
| 17. | Leeson MC, Makley JT, Carter JR. Metastatic skeletal disease distal to the elbow and knee. Clin Orthop Relat Res. 1986;94-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | De Geeter K, Reynders P, Samson I, Broos PL. Metastatic fractures of the tibia. Acta Orthop Belg. 2001;67:54-59. [PubMed] |
| 19. | Willeumier JJ, van der Linden YM, van de Sande MAJ, Dijkstra PDS. Treatment of pathological fractures of the long bones. EFORT Open Rev. 2016;1:136-145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 20. | Capanna R, Campanacci DA. The treatment of metastases in the appendicular skeleton. J Bone Joint Surg Br. 2001;83:471-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Kelly CM, Wilkins RM, Eckardt JJ, Ward WG. Treatment of metastatic disease of the tibia. Clin Orthop Relat Res. 2003;S219-S229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 22. | Willeumier JJ, van der Linden YM, Dijkstra PD. Lack of clinical evidence for postoperative radiotherapy after surgical fixation of impending or actual pathologic fractures in the long bones in patients with cancer; a systematic review. Radiother Oncol. 2016;121:138-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Greenbaum SL, Thornhill BA, Geller DS. Characterization and Surgical Management of Metastatic Disease of the Tibia. Am J Orthop (Belle Mead NJ). 2017;46:E423-E428. [PubMed] |
| 24. | Healey JH, Turnbull AD, Miedema B, Lane JM. Acrometastases. A study of twenty-nine patients with osseous involvement of the hands and feet. J Bone Joint Surg Am. 1986;68:743-746. [PubMed] |
| 25. | Evans S, Ramasamy A, Jeys L, Grimer R. Delayed diagnosis in metastatic lesions of the foot. Ann R Coll Surg Engl. 2014;96:536-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Hattrup SJ, Amadio PC, Sim FH, Lombardi RM. Metastatic tumors of the foot and ankle. Foot Ankle. 1988;8:243-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Knapp D, Abdul-Karim FW. Fine needle aspiration cytology of acrometastasis. A report of two cases. Acta Cytol. 1994;38:589-591. [PubMed] |
| 28. |
Oskouei SV, Walton ZJ.
Treatment of metastatic disease to the tibia, ankle, and foot: |
| 29. | Ljungberg B. The role of metastasectomy in renal cell carcinoma in the era of targeted therapy. Curr Urol Rep. 2013;14:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 30. | Greco T, Cianni L, De Mauro D, Dughiero G, Bocchi MB, Cazzato G, Ragonesi G, Liuzza F, Maccauro G, Perisano C. Foot metastasis: Current knowledge. Orthop Rev (Pavia). 2020;12:8671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 31. | Flynn CJ, Danjoux C, Wong J, Christakis M, Rubenstein J, Yee A, Yip D, Chow E. Two cases of acrometastasis to the hands and review of the literature. Curr Oncol. 2008;15:51-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 32. | El Abiad JM, Aziz K, Levin AS, McCarthy EM, Morris CD. Osseous Metastatic Disease to the Hands and Feet. Orthopedics. 2019;42:e197-e201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Muñoz-Mahamud E, Combalia A, Carreño A, Arandes JM. Five cases of acrometastasis to the hand from a carcinoma and review of the literature. Hand Surg Rehabil. 2017;36:12-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Morris G, Evans S, Stevenson J, Kotecha A, Parry M, Jeys L, Grimer R. Bone metastases of the hand. Ann R Coll Surg Engl. 2017;99:563-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Mavrogenis AF, Mimidis G, Kokkalis ZT, Karampi ES, Karampela I, Papagelopoulos PJ, Armaganidis A. Acrometastases. Eur J Orthop Surg Traumatol. 2014;24:279-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Seltzer VL, Beckman EM. Pathologic fracture of the ulna secondary to metastatic vulvar carcinoma: report of a case. Gynecol Oncol. 1976;4:232-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 37. | Heisterberg L, Johansen TS. Treatment of pathological fractures. Acta Orthop Scand. 1979;50:787-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 38. | Kerin R. Metastatic tumors of the hand. A review of the literature. J Bone Joint Surg Am. 1983;65:1331-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 86] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 39. | Madjidi A, Cole P, Laucirica R. Digital acrometastasis: a rare initial sign of occult pulmonary squamous cell carcinoma. J Plast Reconstr Aesthet Surg. 2009;62:e365-e367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 40. | Dijstra S, Wiggers T, van Geel BN, Boxma H. Impending and actual pathological fractures in patients with bone metastases of the long bones. A retrospective study of 233 surgically treated fractures. Eur J Surg. 1994;160:535-542. [PubMed] |
| 41. | Choi M, Probyn L, Rowbottom L, McDonald R, Bobrowski A, Chan S, Zaki P, Turner A, Chow E. Clinical presentations of below knee bone metastases: a case series. Ann Palliat Med. 2017;6:S85-S89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 42. | Hekmat S, Ghaedian T, Barati H, Movahed M. Solitary metastasis of gastric cancer to fibula: a case report. Iran J Radiol. 2012;9:161-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 43. | Akram M, Zaheer S, Hussain A, Siddiqui SA, Afrose R, Khalid S. Solitary fibular metastasis from nonsmall cell lung carcinoma. J Cytol. 2017;34:113-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 44. | Dieckmann R, Ahrens H, Streitbürger A, Budny TB, Henrichs MP, Vieth V, Gebert C, Hardes J. Reconstruction after wide resection of the entire distal fibula in malignant bone tumours. Int Orthop. 2011;35:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Piccioli A, Maccauro G, Scaramuzzo L, Graci C, Spinelli MS. Surgical treatment of impending and pathological fractures of tibia. Injury. 2013;44:1092-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |