Published online Jul 18, 2019. doi: 10.5312/wjo.v10.i7.268
Peer-review started: April 8, 2019
First decision: June 12, 2019
Revised: June 29, 2019
Accepted: July 8, 2019
Article in press: July 8, 2019
Published online: July 18, 2019
Processing time: 103 Days and 22.1 Hours
Triclosan-coated vicryl plus suture (Ethicon, Inc.) was developed to reduce microbial colonisation during surgical procedures. However, its effect on wound healing and surgical site infections remain unclear after hip and knee arthro-plasty surgery.
To determine the effect of triclosan-coated sutures (TCS) vs non-coated sutures on wound healing, following primary hip and knee arthroplasties.
A single-centred, double-blind randomised controlled trial (RCT) was undertaken. We randomly allocated patients to receive either the triclosan-coated sutures (TCS vicryl plus) or non-coated sutures (NCS vicryl) during the closure of unilateral primary hip and knee arthroplasties. We utilised the ASEPSIS wound scoring system to evaluate wound healing for the first 6 weeks post-operatively.
One hundred and fifty patients undergoing primary total hip or knee arthroplasty over a one-year period were included. Eighty-one were randomised to the TCS group and 69 to the NCS group. Despite no statistically significant difference in the ASEPSIS scores among the study groups (P = 0.75), sensitivity analysis using the Mann Whitney test (P = 0.036) as well as assessment of the wound complications at 6 weeks follow up, demonstrated significantly higher wound complication rates in the TCS group (8 vs 1, P = 0.03).
No clear advantage was demonstrated for using the TCS. However, larger multi-centred RCTs are required to validate their use in hip and knee arthroplasty surgery.
Core tip: This randomised controlled trial does not support the hypothesis that triclosan-coated sutures are superior to non-coated sutures in wound healing and wound complications, following primary hip and knee arthroplasty surgery.
- Citation: Sukeik M, George D, Gabr A, Kallala R, Wilson P, Haddad FS. Randomised controlled trial of triclosan coated vs uncoated sutures in primary hip and knee arthroplasty. World J Orthop 2019; 10(7): 268-277
- URL: https://www.wjgnet.com/2218-5836/full/v10/i7/268.htm
- DOI: https://dx.doi.org/10.5312/wjo.v10.i7.268
Periprosthetic infections after hip and knee arthroplasties remain a challenging problem with rates ranging between one to two percent for primary and up to five percent for revision procedures[1,2]. Management of such infections often requires a prolonged course of treatment; is associated with a cost to the healthcare system estimated at four times the cost of a primary procedure without infection and leads to dissatisfied patients with poor function[2,3].
Methods to prevent, diagnose and treat infection must be optimised in order to reduce both direct and indirect costs to patients and healthcare systems. Preventative measurements have so far been the single most effective method in managing such infections[4].
Intra-operative wound contamination is the main route for contracting a post-operative infection[5], with various bacteria contaminating the surgical wound and the suture material[6,7]. To prevent microbial colonisation of the suture material, the triclosan-coated vicryl plus suture (Ethicon, Inc.) was developed. Triclosan is a broad-spectrum antiseptic which has been widely used in humans for more than 30 years and is effective against Staphylococcus aureus and Staphylococcus epidermidis including methicillin-resistant and vancomycin-resistant strains[8,9].
Triclosan-coated sutures (TCS) have demonstrated favourable outcomes, in terms of wound healing[10,11] and reducing surgical site infections (SSIs) in general surgery[12-14], neurosurgery[15] and cardiac surgery[16]. In orthopaedic surgery, there have only been two recent RCTs which investigated the rates of SSIs in hip and knee arthroplasty surgery and showed conflicting results of TCS effect on rates of SSIs[17,18].
We therefore hypothesised that TCS may be associated with better wound healing characteristics and fewer infections than non-coated sutures (NCS), and as a result may potentially be more appropriate for total hip and total knee arthroplasty wound closures.
The local Research and Development department within our institute and the Regional Ethics Committee (REC) approved the trial (REC reference number: 11/LO/0196) which was also registered with an International Standard Randomised Controlled Trials Number (ISRCTN) 21430045. Written informed consent was obtained from all patients.
This single-centred, double-blind RCT included adult patients (≥ 18 years old) undergoing primary total hip (THA) or knee arthroplasty (TKA) under the care of one surgical team at our institute.
Patients were excluded if they met one of the following criteria: (1) Unilateral primary total hip or knee arthroplasty performed for trauma; (2) Revision procedure or a previous incision in the operative field; (3) History of tendency for keloid formation; (4) Allergy to triclosan/vicryl; (5) Bleeding tendency (e.g., haemophilia and platelet disorders) or being on regular anticoagulation treatment (e.g., warfarin, treatment dose of low molecular weight heparin (LMWH) or conventional heparin); (6) Underlying malignancy and immunocompromised status; and (7) Dementia and mental illnesses preventing informed consent, and children (age < 18 years).
The operations were performed according to the senior surgeon’s default procedure, which include using a medial parapatellar approach and cement for knee arthroplasty, and a posterior approach and uncemented prostheses for hip arthroplasty.
Closure of the TKA wounds included using interrupted 1 vicryl or vicryl plus for the medial parapatellar incisions and 2-0 vicryl or vicryl plus for the subcutaneous tissues followed by skin clips. Closure for the THA wounds included using interrupted 1 vicryl or vicryl plus for the fascia lata and 2-0 vicryl or vicryl plus for the subcutaneous tissues followed by skin clips. For TKAs, a tourniquet was only inflated at the time of cementation and was released after dressing the wound. No drains were used.
Antibiotic prophylaxis included 3 doses of cefuroxime 750mg or alternatively 2 doses of teicoplanin 400mg if the patient was allergic to cefuroxime, with the first dose given at induction of anaesthesia and the rest within the first 24 hours from the operation. All patients received anti-embolism stockings as well as low molecular weight heparin (LMWH) for thromboprophylaxis. Perioperative care plans were similar for each type of operation.
Participants were randomly assigned to two groups. “Cases” received coated polyglactin 910 sutures with triclosan (Vicryl Plus; Ethicon, Inc.), whilst “controls” received the coated polyglactin 910 sutures (Vicryl; Ethicon, Inc.).
Randomisation and blinding were performed by SealedEnvelope Ltd. with assignment of letter codes to cases and controls. The suture type corresponding to a particular letter code was known only to the member of team who received the codes and was not part of the operating surgeons or the operating room nurses. An equal number of cases and control letter code cards were prepared and placed individually in sealed envelopes.
The nurses used consecutive allocation, which was concealed from all professionals delivering patient care including the surgeons and the team involved in assessment of the wounds. Patients, surgeons and the team assessing the wounds were all blinded to treatment assignment (double-blinded study), because both sets of sutures are indistinguishable after removal of the package labelling by the nurses.
Block randomisation was used, with unequal block sizes in order to keep the sizes of treatment groups similar. Randomisation codes were only broken in a case of a serious adverse event.
The primary outcome was the ASEPSIS wound scoring system[19]. This quantitative wound scoring method is calculated using objective criteria based both on visual characteristics of the wound and the consequences of infection[20-23]. A score of > 10 indicates an increasing probability and severity of infection (Table 1). Surgical wounds were inspected two or three days after the operation, and again on days four or five if the patient was still in hospital. The proportion of each wound exhibiting erythema, serous discharge, purulent discharge or dehiscence was recorded. At each post-operative visit, the notes and drug charts of each patient were inspected. The diagnosis of a wound infection by a medical practitioner, the prescription of prophylactic or therapeutic antibiotics, and the opening of a wound or drainage of an abscess was recorded.
| ASEPSIS Score calculation | |
| Points | Criterion |
| Additional treatment | |
| 10 | Antibiotics |
| 5 | Drainage of pus under local anaesthetic |
| 10 | Debridement of wound under general anaesthetic |
| 0-5 | Serous discharge |
| 0-5 | Erythema |
| 0-10 | Purulent exudate |
| 0-10 | Separation of deep tissues |
| 10 | Isolation of bacteria |
| 5 | Stay in hospital over 14 d |
| Breakdown of ASEPSIS score | |
| 0-10 | No infection, normal healing |
| 11-20 | Disturbance of healing |
| 21-30 | Minor infection |
| 31-40 | Moderate infection |
| > 40 | Severe infection |
Infection was considered superficial if resolved with oral antibiotics only and deep if not controlled with oral antibiotics or required a washout/debridement or revision surgery.
At the time of discharge patients were given a simple “yes/no” questionnaire regarding their wound, which they have been asked to complete and return in a pre-paid envelope two months later. Patients were contacted by telephone if no postal questionnaire was returned. The questionnaire was used to ascertain whether a wound infection had been diagnosed since discharge, whether antibiotics had been prescribed for the wound, whether any further surgery had been necessary and whether the hospital stay had been longer than 14 d. Additionally, each patient attended our arthroplasty clinic at 2 and 6 wk postoperatively for assessment of the wound, and received any additional treatment if necessary.
The secondary outcomes included: (1) Time for wound closure, defined as the time period in minutes after insertion of the prosthesis and commencing closure of the fascia in case of THAs or retinaculum for TKAs until completion of skin clips insertion; (2) Length of operation in minutes; (3) Length of hospital stay in days; (4) Pain assessment using the visual analogue scale scores (1-10) measured at 1, 3 and 5 d postoperatively; and (5) Post-operative complications.
Patient demographics and co-morbidities were collected for baseline comparison of the study groups through attendance of pre-assessment clinics, operative lists and follow-up clinic appointments. This included patient age, gender, body mass index, diabetes, smoking and performance level classified according to the American Society of Anaesthesiologists grade[24].
A clinically important difference would be the TCS reducing the ASEPSIS score by 10. A preliminary audit suggested that if the TCS reduced all patients with a score of 11 to 20 to 10 and below and everyone else to a score 10 lower, then we would expect 97.5% of patients to score 10 and below. Sample size calculation was undertaken using Stata 11 based upon the following assumptions: a two group RCT with equal group sizes, 90% of patients with the NCS to have a score of ten and below and 97.5% of patients with the TCS to have a score of 10 and below. Therefore, we required 210 patients in each group to demonstrate a two-sided 5% significance, with 80% power, and 10% dropout rate.
The two study groups’ baseline characteristics were compared using means and standard deviations (SDs) for continuous data and frequency counts and percentages for categorical data. The data was analysed using a chi-squared test, Mann-Whitney U test, or Fisher exact test where appropriate. Furthermore, we undertook a logistic regression to determine what patient and operative factors, if any, were independently risks of developing a post-operative complication. All statistical analyses were performed with SPSS version 21.0 software (SPSS, Inc.). A P-value < 0.05 was deemed statistically significant.
The proportion of dropouts from the study was reported. Data analysis was done on an intention to treat basis.
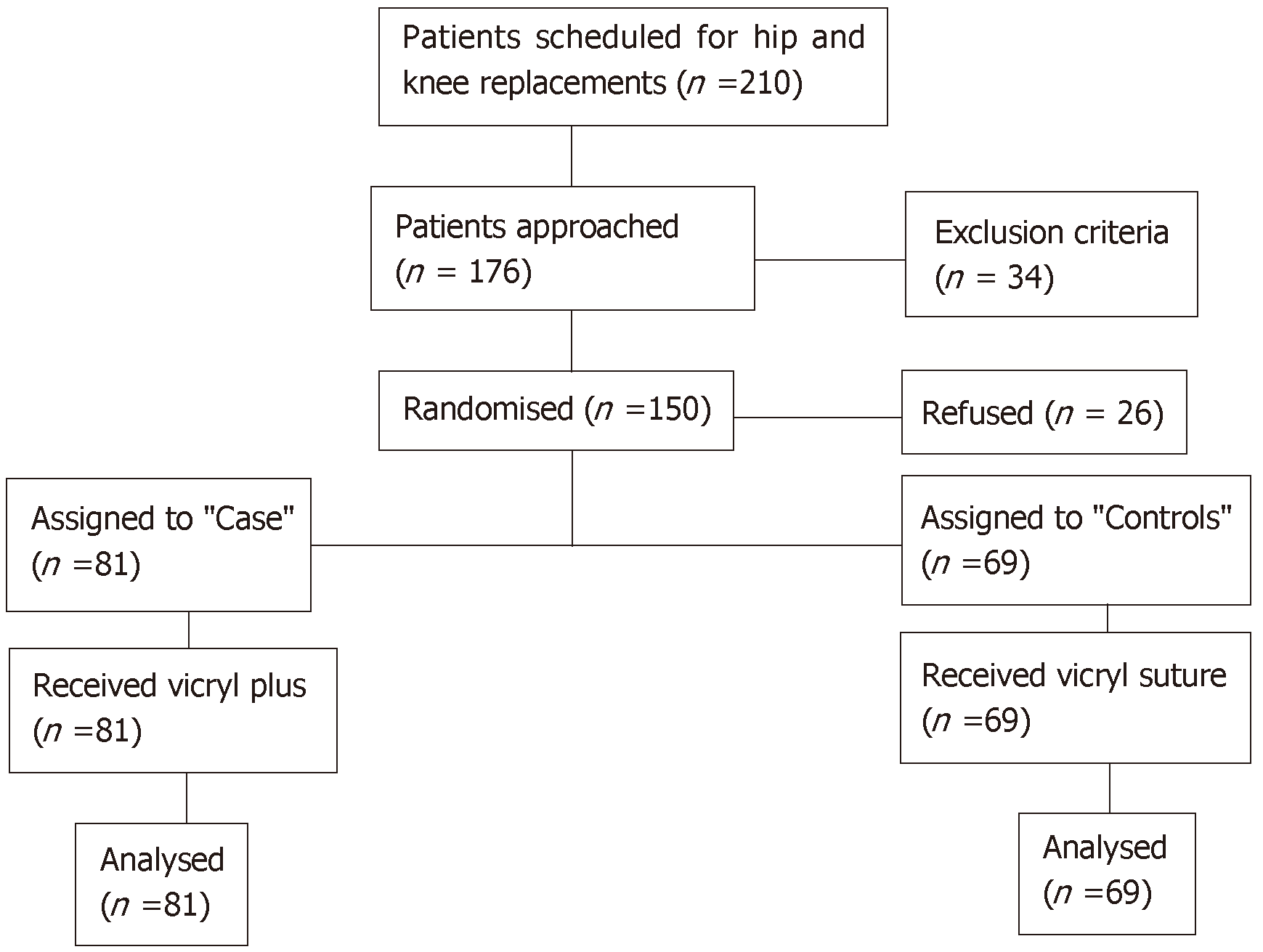
Patients were recruited between November 2013 and December 2014. During this period, there were 210 patients scheduled for primary hip and knee arthroplasty. Thirty-four patients were excluded for various reasons such as history of previous trauma accounting for the osteoarthritis, revision surgery or being on warfarin. Twenty-six patients refused to take part in the study. Therefore, the study consisted of 150 participants, 81 were randomised to the TCS group (cases) and 69 were randomised to the NCS group (controls) (Figure 1).
After December 2014, our institute terminated the contract with Ethicon to move to another supplier and hence the sutures were no longer available and the trial had to be ended prematurely with inclusion of 150 out of the 420 intended patients and the results analysed.
The patient cohort included 49 males and 101 females, with a mean age of 68 years (SD 10). The primary indication for surgery was osteoarthritis in 145 (96%) patients. Ninety-six THAs and 54 TKAs were performed; with a mean length of hospital stay of 6 days (SD 4). One hundred and forty-four patients (96%) completed the full follow-up needed for the study. The demographics were comparable for the two groups (Table 2).
| Trial group | P value | |||
| Controls (n = 69) | Cases (n = 81) | |||
| Age | mean (SD) | 67.85 (9.85) | 68.65 | 0.44 |
| (10.90) | ||||
| Diagnosis | OA | 68 | 77 | 0.33 |
| SUFE | 0 | 2 | ||
| AVN | 1 | 0 | ||
| Hip dysplasia | 0 | 1 | ||
| Perthes | 0 | 1 | ||
| Gender | Male | 24 | 25 | 0.73 |
| Female | 45 | 56 | ||
| BMI | mean (SD) | 28.70 (5.13) | 29.14 (4.97) | 0.54 |
| Smoker | Yes | 6 | 6 | 0.64 |
| Never | 42 | 57 | ||
| Ex-smoker | 13 | 12 | ||
| Diabetic | Yes | 4 | 10 | 0.26 |
| No | 57 | 64 | ||
| ASA Grade | 1 | 9 | 9 | 0.68 |
| 2 | 47 | 52 | ||
| 3 | 13 | 20 | ||
There were 96 THAs and 54 TKAs performed during the study. Table 3 demonstrates the difference between the operative data between cases and controls.
| Trial group | P value | |||
| Controls (n = 69) | Cases (n = 81) | |||
| Site | Hip | 42 | 54 | 0.5 |
| Knee | 27 | 27 | ||
| Surgeon | Consultant | 25 | 29 | 0.63 |
| Registrar | 38 | 41 | ||
| Fellow | 6 | 11 | ||
| Anaesthetic | General | 45 | 50 | 0.56 |
| Regional | 17 | 26 | ||
| Both | 3 | 2 | ||
| Local anaesthetic | Yes | 67 | 77 | 0.38 |
| No | 1 | 4 | ||
| Length of operation | mean (SD) | 88.44 (23.84) | 91.24 (26.5) | 0.67 |
| mean (SD) | 3.75 (0.87) | 3.53 (0.81) | 0.12 | |
| Number of sutures used | 2 | 1 | 7 | 0.26 |
| 3 | 23 | 29 | ||
| 4 | 30 | 30 | ||
| 5 | 6 | 8 | ||
| > 5 | 1 | 0 | ||
| Prosthesis hip | Synergy – R3 | 37 | 48 | 0.30 |
| Trifit – Trinity | 3 | 3 | ||
| Exeter | 0 | 3 | ||
| Knee | Triathalon | 21 | 23 | 0.70 |
| Saiph knee | 5 | 3 | ||
| Wound closure (min) | mean (SD) | 14.64 (5.51) | 13.89 (5.13) | 0.47 |
| VAS score (mean, SD) | Day 1 | 6.47 (2.62) | 6.20 (2.35) | 0.34 |
| Day 3 | 4.75 (2.33) | 4.18 (2.33) | 0.15 | |
| Day 5 | 4.67 (1.75) | 2.92 (2.87) | 0.18 | |
| Length of stay (d) | mean (SD) | 6.13 (4.23) | 6.23 (4.11) | 0.95 |
No statistically significant difference was seen between the two study groups when comparing an ASEPSIS score of ≤ 10 compared to > 11 (P = 0.75). However, a score of greater than 10 was seen in only 6 cases and 4 controls. On the other hand, a statistically significant difference was demonstrated when comparing the overall mean ASEPSIS scores among the study groups (cases = 2.5, controls = 1.4, P = 0.036) (Table 4).
| Trial group | P value | |||
| Controls (n = 69) | Cases (n = 81) | |||
| ASEPSIS scores | ||||
| 0-10 | 65 | 75 | 0.75 | |
| > 10 | 4 | 6 | ||
| mean (SD range) | 1.41 | 2.54 | 0.036 | |
| (0.38-2.43) | (1.41-3.68) | |||
| Follow-up outcomes (2-wk) | ||||
| Site of follow-up | Hospital | 35 | 37 | 0.21 |
| Community | 27 | 28 | ||
| Inpatient | 2 | 10 | ||
| Did not attend | 5 | 6 | ||
| Wound complications | Yes | 1 | 6 | 0.22 |
| No | 63 | 69 | ||
| Superficial SSI | 1 | 2 | ||
| Erythema | 0 | 3 | ||
| Serous discharge | 0 | 1 | ||
| Follow-up outcomes (6-wk) | ||||
| Attended hospital | Yes | 61 | 65 | 0.189 |
| No | 8 | 16 | ||
| Wound complications | Yes | 1 | 8 | 0.03 |
| No | 60 | 57 | ||
| Superficial SSI | 1 | 3 | ||
| Wound dehiscence | 0 | 1 | ||
| Irritation from suture | 0 | 2 | ||
| Serous discharge | 0 | 1 | ||
| Deep SSI | 0 | 1 | ||
| Systemic complications | Nausea and vomiting | 0 | 2 | 0.12 |
| Dizziness | 0 | 0 | 1.00 | |
| Bleeding(not from wound) | 1 | 2 | 0.26 | |
| Stiffness | 4 | 5 | 0.30 | |
| Neurovascular injury | 0 | 0 | 1.00 | |
| DVT | 1 | 0 | 0.18 | |
| PE | 0 | 1 | 0.19 | |
| Chest infection | 1 | 2 | 0.26 | |
| MI | 0 | 0 | 1.00 | |
| CVA | 0 | 0 | 1.00 | |
| Fracture | 0 | 2 | 0.12 | |
| Dislocation | 0 | 0 | 1.00 | |
| Loosening | 0 | 0 | 1.00 | |
| Mortality | 0 | 0 | 1.00 | |
| Missing data | 8 | 16 | ||
This RCT was undertaken to compare the wound healing characteristics and wound complications, following wound closure with triclosan-coated and non-coated sutures in primary THA and TKA surgery. Despite the premature termination of this study, there was evidence that the TCS were associated with more wound complications than NCS, rejecting our hypothesis (P = 0.03).
Triclosan-coated sutures have recently gained popularity in Orthopaedics, due to its perceived advantages seen in other surgical specialities. Whilst the majority of evidence in the literature supports the use of TCS in surgical wound closures including recent meta-analyses[25-29], there have been several recent studies questioning its efficacy and complication rates.
Mattavelli et al[30] demonstrated no advantage in reducing SSI rates after colorectal surgery in a multi-centred RCT, which included 281 patients (P = 0.564). The overall incision complication rate was noted to be more in the TCS group (45.7%), compared to the control group (38.3%; P = 0.208). Other RCTs concluded similar findings following abdominal wall closure[31], colorectal surgery[32], and leg wound closure following graft harvest in coronary artery bypass patients[11].
The two RCTs published on TCS and rates of SSIs in hip and knee arthroplasty surgery show conflicting results[17,18]. Lin et al[18] randomised 102 patients to TCS or a control group in TKA surgery and concluded that none of the patients in the TCSs group developed a superficial infection whereas 2 patients in the control group (3.9%) developed superficial infections. They also reported lower serum interleukin-6 levels and lower local skin temperature recorded at 3 mo using infrared thermography in the TCS group[18]. On the other hand, Sprowson et al[17] conducted the largest multi-centred double-blinded quasi-RCT to date including 2546 patients who underwent either hip or knee arthroplasty surgery and concluded that TCSs did not lead to a reduction in the rate of SSIs (0.7% TCS vs 0.8% control groups). Despite the difference in the primary outcome measured between our study and this RCT, our study findings support higher wound complication rates related to the TCS. Additionally, it is worth noting that this was a quasi-randomised trial conducted at 3 sites with a large number of contributing surgeons and different. Furthermore, the surgeons were not blinded to the type of suture utilised, but both the patients and assessors of outcomes were.
Considering the lack of significant improvement in wound complications, we cannot advocate the continued use of TCS in these procedures. Whilst not the objective of this study, one has to also consider the financial implications of the two suture types. The price per suture will vary amongst institutions, but based upon National Health System (NHS) Supply Chain data[33], the TCS is typically 20% more expensive than the standard NCS [£3.82 compared to £2.99 respectively]. Whilst suture costs are minimal when reviewing the overall costs of arthroplasty procedures, if no benefit has been shown between the two in reducing rates of infection, there may be significant savings should the standard NCS be the suture of choice.
Strengths of this RCT include the robust inclusion and exclusion criteria and randomisation process, the double blinding of the study including the surgeons performing the operations and inclusion of a single surgeon’s cohort of patients to reduce bias in the surgical technique in wound closure among various surgeons.
The incidence of wound infection and poor wound healing, is multifactorial due to a combination of both patient and provider risk factors. In this study, the baseline demographics were similar for both groups, having all undergone pre-operative optimisation prior to surgery. However, after the surgery we were unable to control patient factors following their discharge from hospital, which no doubt may have had an impact on wound healing. Other limitations of this study included the premature termination of the trial due to the unavailability of the sutures after December 2014. This may have resulted in a type II error due to the study being underpowered; hence the binary variable ASEPSIS score ≤ 10 versus > 10 was insignificant. However, sensitivity analysis using the Mann Whitney test (P = 0.036) as well as assessment of the wound complications at the last follow up (P = 0.03), demonstrated significantly higher wound complication rates in the TCS group. Additionally, we utilised the ASEPSIS scoring system which addresses acute postoperative wound healing only, and therefore the protocol for wound surveillance continued for only 6 weeks post-operatively. We acknowledge that the results of this study cannot be applied to delayed onset periprosthetic joint infections.
This double-blinded RCT was undertaken according to a strict inclusion and exclusion criteria to address the intervention of interest. It is the first of its kind reporting the outcomes investigating the effect of TCS on wound healing after primary THA and TKA surgery.
In conclusion, our study has demonstrated that TCS are not associated with improved wound healing or reduction of infections, when compared to NCS. However, larger multi-centred RCTs are required, with adequate power, to fully validate the use TCS in hip and knee arthroplasty surgery.
Despite the lack of evidence that using triclosan-coated sutures has any benefits in hip and knee arthroplasty surgery, they have been used widely due to the potential benefit of improving wound healing and reducing surgical site infections.
We sought to compare the wound healing characteristics and wound complications associated with the use of triclosan-coated sutures and compared them to non-coated sutures in primary hip and knee arthroplasty surgery.
Our main objective was to investigate the potential benefits of using triclosan-coated sutures in hip and knee arthroplasty surgery using a well designed randomised controlled trial to guide future practice.
A single-centred double blinded randomised controlled trial was conducted according to strict inclusion and exclusion criteria and following the Research and Development and Regional Ethics Committee guidelines for conducting high-quality well-designed trials with the above objectives. Primary and secondary outcomes were defined, computer randomisation was performed through Sealed Envelope and statistical analysis including power calculation was planned and approved prior to conducting the trial.
Utilising the ASEPSIS scoring system, there were no significant differences between the triclosan-coated and non-coated sutures. However, wound complications were noted more frequently at the 2 and 6 wk follow up in the triclosan-coated sutures group. As the study has been terminated earlier than planned due to the unavailability of the sutures, further randomised controlled trials are still warranted to fully answer the question of whether triclosan-coated sutures provide any protection against wound complications and infections after hip and knee arthroplasty surgeries.
The current literature supports the use of triclosan-coated sutures in some disciplines of general surgery but the evidence in orthopaedic surgery especially in arthroplasty procedures remains inconclusive. This trial supports the findings from other studies that triclosan-coated sutures do not provide any benefits over non-coated sutures in protecting against wound complications and infections after hip and knee arthroplasty surgery. Therefore, we recommend against the routine use of those sutures and advise that efforts should continue to emphasise the benefits of preventative measures against infections and explore new modalities of reducing surgical site infections. The utilisation of a well-designed randomised controlled trial will help in answering whether any of those new modalities will stand the challenge of time and optimal outcomes.
Manuscript source: Invited manuscript
Specialty type: Orthopedics
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pavone P S-Editor: Gong ZML-Editor:A E-Editor: Wu YXJ
| 1. | Sukeik M. Thematic Issue: Management of Periprosthetic Joint Infections after Total Hip and Knee Replacements. Open Orthop J. 2016;10:577-578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Sukeik M, Haddad FS. Periprosthetic joint infections after total hip replacement: an algorithmic approach. SICOT J. 2019;5:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Vanhegan IS, Malik AK, Jayakumar P, Ul Islam S, Haddad FS. A financial analysis of revision hip arthroplasty: the economic burden in relation to the national tariff. J Bone Joint Surg Br. 2012;94:619-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 240] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 4. | Sukeik M, Haddad FS. Two-stage procedure in the treatment of late chronic hip infections--spacer implantation. Int J Med Sci. 2009;6:253-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Sukeik M, Haddad FS. Management of periprosthetic infection in total hip arthroplasty. Orthop Trauma 2009, 23: 342-349. . [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Uff CR, Scott AD, Pockley AG, Phillips RK. Influence of soluble suture factors on in vitro macrophage function. Biomaterials. 1995;16:355-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Rodeheaver GT, Kurtz LD, Bellamy WT, Smith SL, Farris H, Edlich RF. Biocidal braided sutures. Arch Surg. 1983;118:322-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Edmiston CE, Seabrook GR, Goheen MP, Krepel CJ, Johnson CP, Lewis BD, Brown KR, Towne JB. Bacterial adherence to surgical sutures: can antibacterial-coated sutures reduce the risk of microbial contamination? J Am Coll Surg. 2006;203:481-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 153] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 9. | Rothenburger S, Spangler D, Bhende S, Burkley D. In vitro antimicrobial evaluation of Coated VICRYL* Plus Antibacterial Suture (coated polyglactin 910 with triclosan) using zone of inhibition assays. Surg Infect (Larchmt). 2002;3 Suppl 1:S79-S87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Nakamura T, Kashimura N, Noji T, Suzuki O, Ambo Y, Nakamura F, Kishida A. Triclosan-coated sutures reduce the incidence of wound infections and the costs after colorectal surgery: a randomized controlled trial. Surgery. 2013;153:576-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 11. | Thimour-Bergström L, Roman-Emanuel C, Scherstén H, Friberg Ö, Gudbjartsson T, Jeppsson A. Triclosan-coated sutures reduce surgical site infection after open vein harvesting in coronary artery bypass grafting patients: a randomized controlled trial. Eur J Cardiothorac Surg. 2013;44:931-938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 12. | Ford HR, Jones P, Gaines B, Reblock K, Simpkins DL. Intraoperative handling and wound healing: controlled clinical trial comparing coated VICRYL plus antibacterial suture (coated polyglactin 910 suture with triclosan) with coated VICRYL suture (coated polyglactin 910 suture). Surg Infect (Larchmt). 2005;6:313-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Mingmalairak C, Ungbhakorn P, Paocharoen V. Efficacy of antimicrobial coating suture coated polyglactin 910 with tricosan (Vicryl plus) compared with polyglactin 910 (Vicryl) in reduced surgical site infection of appendicitis, double blind randomized control trial, preliminary safety report. J Med Assoc Thai. 2009;92:770-775. [PubMed] |
| 14. | Justinger C, Moussavian MR, Schlueter C, Kopp B, Kollmar O, Schilling MK. Antibacterial [corrected] coating of abdominal closure sutures and wound infection. Surgery. 2009;145:330-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Rozzelle CJ, Leonardo J, Li V. Antimicrobial suture wound closure for cerebrospinal fluid shunt surgery: a prospective, double-blinded, randomized controlled trial. J Neurosurg Pediatr. 2008;2:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 16. | Fleck T, Moidl R, Blacky A, Fleck M, Wolner E, Grabenwoger M, Wisser W. Triclosan-coated sutures for the reduction of sternal wound infections: economic considerations. Ann Thorac Surg. 2007;84:232-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Sprowson AP, Jensen C, Parsons N, Partington P, Emmerson K, Carluke I, Asaad S, Pratt R, Muller S, Ahmed I, Reed MR. The effect of triclosan-coated sutures on the rate of surgical site infection after hip and knee arthroplasty: a double-blind randomized controlled trial of 2546 patients. Bone Joint J. 2018;100-B:296-302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Lin SJ, Chang FC, Huang TW, Peng KT, Shih HN, Lee MS. Temporal Change of Interleukin-6, C-Reactive Protein, and Skin Temperature after Total Knee Arthroplasty Using Triclosan-Coated Sutures. Biomed Res Int. 2018;2018:9136208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Wilson AP, Treasure T, Sturridge MF, Grüneberg RN. A scoring method (ASEPSIS) for postoperative wound infections for use in clinical trials of antibiotic prophylaxis. Lancet. 1986;1:311-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 270] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 20. | Bruce J, Russell EM, Mollison J, Krukowski ZH. The quality of measurement of surgical wound infection as the basis for monitoring: a systematic review. J Hosp Infect. 2001;49:99-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Wilson AP, Gibbons C, Reeves BC, Hodgson B, Liu M, Plummer D, Krukowski ZH, Bruce J, Wilson J, Pearson A. Surgical wound infection as a performance indicator: agreement of common definitions of wound infection in 4773 patients. BMJ. 2004;329:720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 143] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 22. | Wilson AP, Weavill C, Burridge J, Kelsey MC. The use of the wound scoring method 'ASEPSIS' in postoperative wound surveillance. J Hosp Infect. 1990;16:297-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Wilson AP, Webster A, Gruneberg RN, Treasure T, Sturridge MF. Repeatability of asepsis wound scoring method. Lancet. 1986;1:1208-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Keats AS. The ASA classification of physical status--a recapitulation. Anesthesiology. 1978;49:233-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 512] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 25. | Daoud FC, Edmiston CE, Leaper D. Meta-analysis of prevention of surgical site infections following incision closure with triclosan-coated sutures: robustness to new evidence. Surg Infect (Larchmt). 2014;15:165-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 26. | de Jonge SW, Atema JJ, Solomkin JS, Boermeester MA. Meta-analysis and trial sequential analysis of triclosan-coated sutures for the prevention of surgical-site infection. Br J Surg. 2017;104:e118-e133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 27. | Henriksen NA, Deerenberg EB, Venclauskas L, Fortelny RH, Garcia-Alamino JM, Miserez M, Muysoms FE. Triclosan-coated sutures and surgical site infection in abdominal surgery: the TRISTAN review, meta-analysis and trial sequential analysis. Hernia. 2017;21:833-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Konstantelias AA, Andriakopoulou CS, Mourgela S. Triclosan-coated sutures for the prevention of surgical-site infections: a meta-analysis. Acta Chir Belg. 2017;117:137-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Leaper DJ, Edmiston CE, Holy CE. Meta-analysis of the potential economic impact following introduction of absorbable antimicrobial sutures. Br J Surg. 2017;104:e134-e144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 30. | Mattavelli I, Rebora P, Doglietto G, Dionigi P, Dominioni L, Luperto M, La Porta A, Garancini M, Nespoli L, Alfieri S, Menghi R, Dominioni T, Cobianchi L, Rotolo N, Soldini G, Valsecchi MG, Chiarelli M, Nespoli A, Gianotti L. Multi-Center Randomized Controlled Trial on the Effect of Triclosan-Coated Sutures on Surgical Site Infection after Colorectal Surgery. Surg Infect (Larchmt). 2015;16:226-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 31. | Diener MK, Knebel P, Kieser M, Schüler P, Schiergens TS, Atanassov V, Neudecker J, Stein E, Thielemann H, Kunz R, von Frankenberg M, Schernikau U, Bunse J, Jansen-Winkeln B, Partecke LI, Prechtl G, Pochhammer J, Bouchard R, Hodina R, Beckurts KT, Leißner L, Lemmens HP, Kallinowski F, Thomusch O, Seehofer D, Simon T, Hyhlik-Dürr A, Seiler CM, Hackert T, Reissfelder C, Hennig R, Doerr-Harim C, Klose C, Ulrich A, Büchler MW. Effectiveness of triclosan-coated PDS Plus versus uncoated PDS II sutures for prevention of surgical site infection after abdominal wall closure: the randomised controlled PROUD trial. Lancet. 2014;384:142-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 136] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 32. | Huszár O, Baracs J, Tóth M, Damjanovich L, Kotán R, Lázá G, Mán E, Baradnai G, Oláh A, Benedek-Tóth Z, Bogdán-Rajcs S, Zemanek P, Oláh T, Somodi K, Svébis M, Molnár T, Horváth Ö P. [Comparison of wound infection rates after colon and rectal surgeries using triclosan-coated or bare sutures -- a multi-center, randomized clinical study]. Magy Seb. 2012;65:83-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | DHL Supply Chain Limited (NHS Supply Chain),NHS Business Services Authority,NHS Catalogue version 3.6.1.156. Available from:https://my.supplychain.nhs.uk/catalogue/. |