Copyright
©2013 Baishideng Publishing Group Co.
World J Orthop. Oct 18, 2013; 4(4): 207-217
Published online Oct 18, 2013. doi: 10.5312/wjo.v4.i4.207
Published online Oct 18, 2013. doi: 10.5312/wjo.v4.i4.207
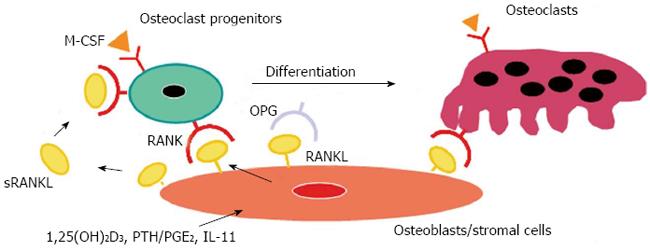
Figure 1 A model illustrating a mechanism by which osteoblasts/stromal cells regulate osteoclast differentiation and activation.
Three distinct signals stimulated by 1,25(OH)2D3, PTH/PGE2, and interleukin (IL)-11 induce receptor activator of nuclear factor-κB ligand (RANKL) expression on osteoblasts/stromal cells. RANKL mediates a signal for osteoclastogenesis through RANK produced on osteoclast progenitors. Osteoprotegerin (OPG) inhibits osteoclastogenesis by interrupting the binding of RANKL and RANK. Macrophage colony-stimulating factor (M-CSF) produced by osteoblasts/stromal cells is also indispensable for proliferation and differentiation of osteoclast progenitors. Soluble RANKL (sRANKL) is produced by digestion of RANKL with metalloproteinases and may regulate osteoclastogenesis.
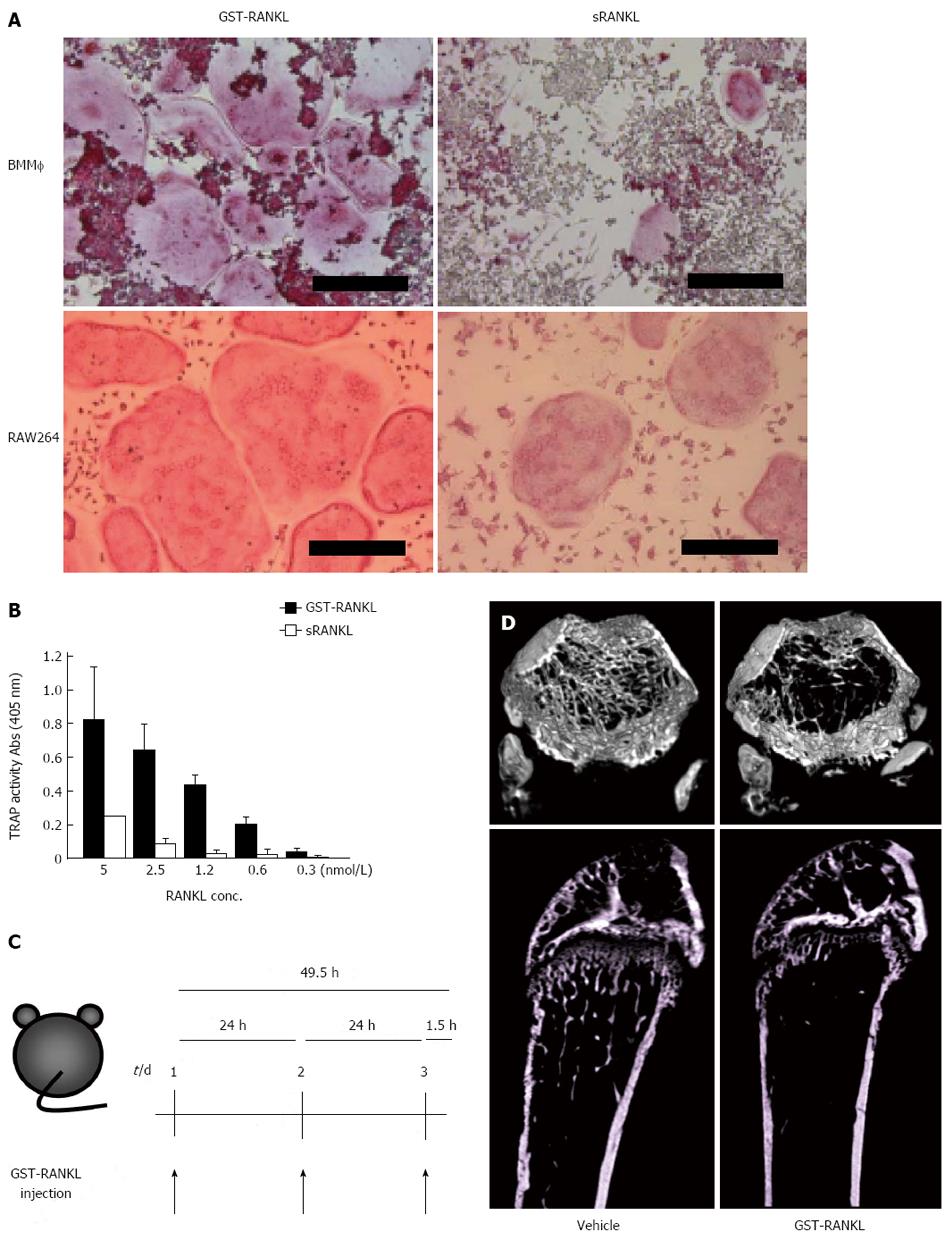
Figure 2 Establishment of GST-RANKL-injected bone loss model.
A: Osteoclasts were generated with 5 nmol/L glutathione-S transferase-receptor activator of nuclear factor-κB ligand (GST-RANKL) or sRANKL from bone marrow macrophages (BMM) and RAW264 cells in the presence and absence of M-CSF, respectively. The cells were fixed and stained for tartarate-resistant acid phosphatase (TRAP). Bars = 250 m; B: Activities of GST-RANKL and sRANKL were compared using in vitro osteoclastogenesis assay with RAW264 cells by TRAP solution assay. The TRAP activity represents the number of osteoclasts. C: Experimental design. PBS (vehicle) or GST-RANKL was injected intraperitoneally at 24-h intervals for 3 d into 7-wk-old female mice. Mice were sacrificed 90 min after the last injection; D: Micro computer tomography 3D images of femurs of mice treated with vehicle or 2 mg/kg GST-RANKL. The upper and lower panels are 3D cross-sectional and longitudinal images, respectively.
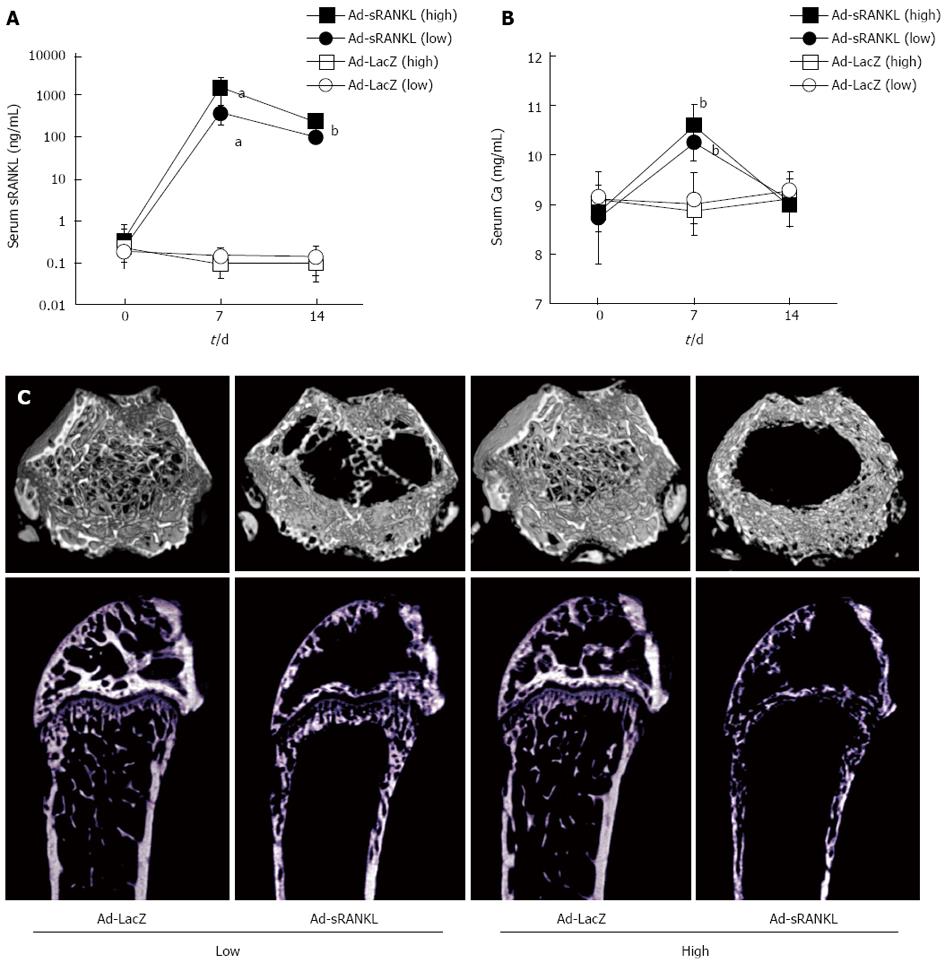
Figure 3 Establishment of Ad-sRANKL-injected osteoporosis/hypercalcemia model.
Time-dependent changes in serum soluble receptor activator of nuclear factor-κB ligand (sRANKL) (A) and Ca (B) levels. Adenovirus vector harboring mouse sRANKL cDNA (Ad-sRANKL) or Ad-LacZ was injected intraperitoneally into 6-wk-old male C57BL/6 mice at 1.0x109 pfu/mouse in high-dose groups and 3.0 x 108 pfu/mouse in low-dose groups. Serum levels of sRANKL and Ca were measured on day 0, 7 and 14 after injection. Data are presented as the mean ± SD (n = 5 or 6). Ad-sRANKL high dose (filled squares), Ad-sRANKL low dose (filled circles), Ad-LacZ high dose (open squares), Ad-LacZ low dose (open circles). aP < 0.05, bP < 0.01 vs Ad-LacZ control; C: Micro computer tomography images of femurs in each group of mice. All mice were sacrificed on day 14 after injection and femurs were collected. The upper and lower panels are 3D cross-sectional and longitudinal images, respectively.
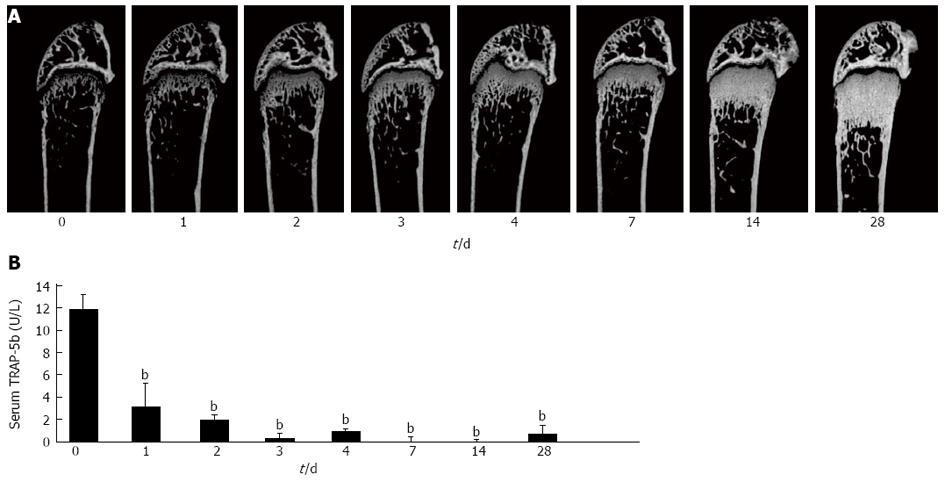
Figure 4 Establishment of anti-RANKL-injected osteopetrosis model.
A: Time course of the effect of anti-mouse receptor activator of nuclear factor-κB ligand (RANKL) neutralizing antibody (clone, OYC1) on bone mass (day 0-28). OYC1 (5 mg/kg) was administered subcutaneously to 6-wk-old female mice (n = 5 or 6) on day 0. Mice were sacrificed on day 0, 1, 2, 3, 4, 7, 14, and 28. Bone structure in the trabecular area was analyzed by micro computer tomography; B: Serum TRAP-5b levels were measured by ELISA. Data are shown as the mean ± SD. bP < 0.01 (ANOVA) vs value on day 0.
- Citation: Yasuda H. RANKL, a necessary chance for clinical application to osteoporosis and cancer-related bone diseases. World J Orthop 2013; 4(4): 207-217
- URL: https://www.wjgnet.com/2218-5836/full/v4/i4/207.htm
- DOI: https://dx.doi.org/10.5312/wjo.v4.i4.207