Published online Oct 10, 2015. doi: 10.5306/wjco.v6.i5.174
Peer-review started: April 28, 2015
First decision: June 29, 2015
Revised: July 17, 2015
Accepted: August 4, 2015
Article in press: August 7, 2015
Published online: October 10, 2015
Processing time: 174 Days and 9.3 Hours
Liposarcoma of the breast is a very rare malignant tumor. It can clinically manifest as a palpable breast mass and mimic primary breast cancer. We report an unusual case of a 51-year-old female who presented with an asymptomatic right breast mass, which was histologically diagnosed as well differentiated liposarcoma arisen within malignant phyllodes tumor. The patient underwent breast conserving surgery, received no adjuvant treatment and is disease-free after 2 years. Radiological and histopathological features are presented and described in detail. Data from the literature are presented and therapy recommendations discussed.
Core tip: Liposarcoma is a very rare malignant tumor of the breast and may mimic invasive breast cancer on imaging studies. The definite pathological diagnosis may be challenging.
- Citation: Banys-Paluchowski M, Burandt E, Quaas A, Wilczak W, Geist S, Sauter G, Krawczyk N, Pietzner K, Paluchowski P. Liposarcoma of the breast arising in a malignant phyllodes tumor: A case report and review of the literature. World J Clin Oncol 2015; 6(5): 174-178
- URL: https://www.wjgnet.com/2218-4333/full/v6/i5/174.htm
- DOI: https://dx.doi.org/10.5306/wjco.v6.i5.174
Breast cancer is the most common female malignancy worldwide[1]. In rare cases, the histopathological workup of a suspicious breast mass shows not epithelial (carcinoma) but sarcomatous differentiation. One of such rare malignant tumors is a liposarcoma, which may present as a pure liposarcoma or arise within a phyllodes tumor (PT). Upon imaging studies, liposarcoma often resembles primary invasive breast carcinoma. Given the rarity of the disease, there are no randomized trials specifically addressing treatment modalities in breast sarcoma and therapy guidelines are based on data from non-breast soft tissue sarcoma trials. In the following, we report an unusual case of a 51-year-old female with a well differentiated liposarcoma arisen within malignant PT and present current data and evidence-based therapy recommendations for breast liposarcoma.
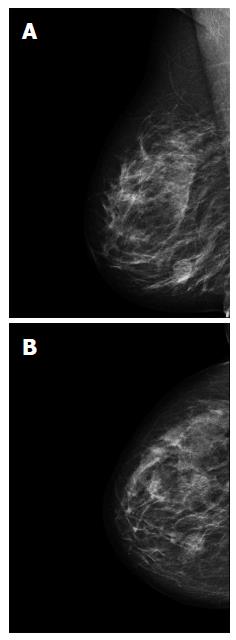
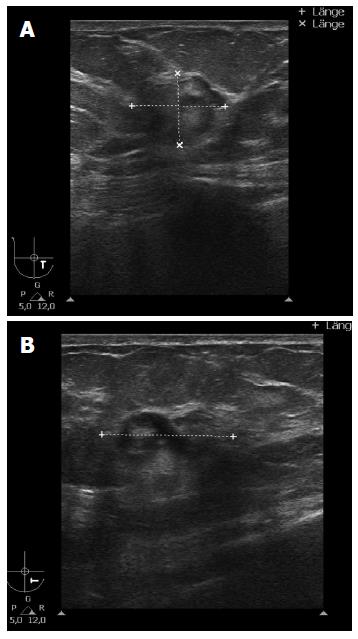
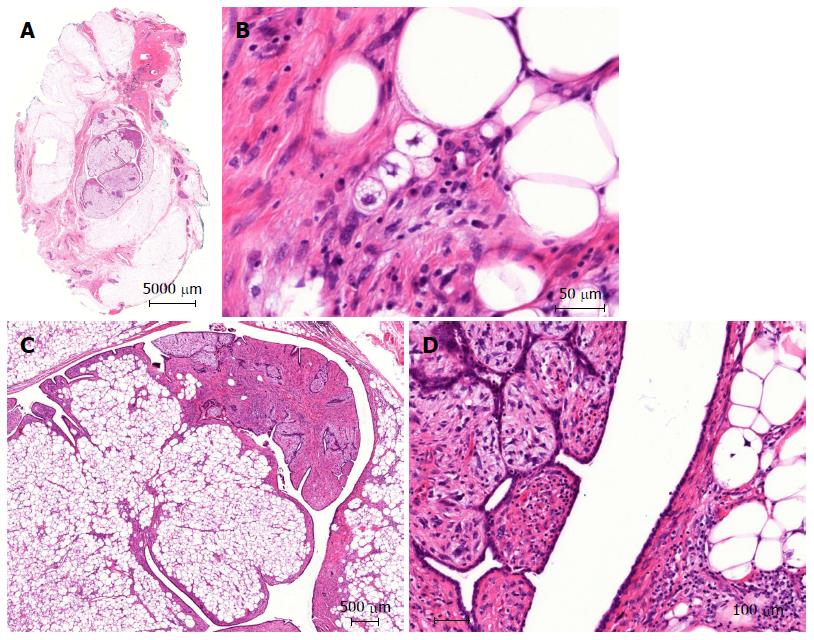
A 51-year-old Caucasian postmenopausal female presented at the certified Breast Cancer Center, Klinikum Pinneberg, Germany, with a newly diagnosed palpable, asymptomatic mass located in the lower inner quadrant of her right breast. Clinical examination showed a nodular movable mass of 2 cm diameter; the overlying skin was unremarkable. She had no concomitant diseases at time of presentation beside obesity (BMI 31 kg/m2); her previous surgeries included cholecystectomy and she was a nonsmoker. She denied any first- or second degree family medical history of cancer of any type and she never received radiotherapy. At mammography, the lesion was scored BI-RADS 5. Breast ultrasound and mammograms are presented in Figures 1 and 2, respectively. Axillary lymph nodes were unremarkable on sonography. Ultrasound guided minimal-invasive 14-gauge core biopsy revealed a biphasic tumor of the phyllodes type with suspicious stroma. We conducted a lumpectomy; histopathological workup described a malignant PT of 21 mm diameter with a specific heterologous component identified as well differentiated liposarcoma; mitotic rate was 21/10 high-power field (Figure 3). The case was discussed in the interdisciplinary tumor board. Because of close margins (min. 1 mm) a wide excision was recommended, which was conducted 4 wk after the lumpectomy and showed no further malignant lesion (resection margins after wide excision > 10 mm). The case was discussed again in the tumor board, which recommended further follow-up care including clinical examinations, mammography and breast sonography at regular intervals. Neither chemotherapy nor radiotherapy was recommended. The patient had an uneventful recovery, received no further therapy and is free of disease since surgery (two years).
Soft tissue sarcomas (STS) amount to less than 1% of all malignant tumors with an incidence estimated at 2-5 cases per 100000 yearly[2]. The exact diagnosis may pose a significant challenge since there are over fifty subtypes of STS, which determine their prognostic and therapeutic features[3]. Eight percent to 14% of all newly diagnosed STS have liposarcomatous differentiation making primary liposarcoma a common subtype. Lucas et al[4] reported on 58 consecutive cases of well differentiated liposarcoma treated at the Mayo Clinic; of these, the majority involved the extremities (32 cases) and the retroperitoneum (20), followed by the scrotum (4), the abdominal wall (1) and the cheek (1). Liposarcoma localized in the breast has been reported in the literature before but remains a very rare neoplasm.
Primary sarcomas of the breast account for 0.1% of all malignant breast tumors. A thorough review on breast sarcomas, along with a series of 25 cases, was published by Adem et al[5]. The incidence of liposarcoma among breast sarcomas vary in the literature from 2% to 10%[6-10]. Its definite pathological diagnosis is challenging and may require a cooperation with a reference center. Liposarcomas of the breast may occur either as pure primary liposarcoma or arise in cystosarcomas phyllodes. The patient in our case report presented with a suspicious breast mass that was revealed as malignant PT with heterologous liposarcomatous differentiation. Liposarcomatous differentiation is rarely diagnosed in PTs; the malignant stroma transformation of PT usually shows fibrosarcomatous differentiation and rarely heterologous sarcomatous elements[11]. Other uncommon sarcomatous stromal elements may include leiomyosarcoma, osteosarcoma, angiosarcoma, chondrosarcoma and rhabdomyosarcoma. PT with liposarcomatous differentiation may resemble breast cancer on imaging studies. The prognosis is strongly influenced by histologic subtype: dedifferentiated liposarcomas are aggressive tumors with high metastatic potential while well differentiated and myxoid types generally have a more favorable outcome[12]. Further features associated with favorable survival include complete surgical excision of tumor with tumor-free margins[13].
Given the rarity and heterogeneity of the disease, there are no prospective randomized trials on the surgical and systemic treatment of breast sarcomas and the optimal therapy remains yet to be defined. Current recommendation of the European Society for Medical Oncology (ESMO) and the European Sarcoma Network Working Group[14] is to treat non-radiation induced breast sarcomas as other STS by performing breast conserving surgery (e.g., wide excision as in our case), with the exception of angiosarcoma because of its high local recurrence rates[15,16]. Since an adequate resection margin is the most important prognostic factor, negative margins are crucial for long-term survival[8,17]. Given clear margins of resection, survival rates after mastectomy and breast conserving surgery are similar[18]. Sarcomas tend to spread by direct local invasion or hematogenously. Since lymphatic dissemination is rare, neither axillary lymph node dissection nor sentinel node biopsy are recommended in the absence of clinical evidence of lymph node involvement[14,19,20]. In the retrospective analysis, Shabahang et al[21] found no positive nodes in ten patients treated with axillary lymph node dissection for primary breast sarcoma. The role of adjuvant radiotherapy for breast sarcoma remains unclear due to the rarity of the disease and lack of randomized trials. Data from single institution studies are contradictory: some observational studies suggest improved local control[9,19] while others reported no benefit of radiotherapy[18,20,22,23]. The subgroup that might particularly benefit from adjuvant radiotherapy consists of patients with large tumors (> 5 cm), high-grade sarcoma and positive margins. The patient presented in the case report had a small tumor (2.1 cm) removed with clear margins of > 1 cm; based on the available data, the interdisciplinary tumor board did not recommend adjuvant radiation. As far as chemotherapy is concerned, since there are no trials specifically addressing breast sarcoma, current recommendations are based on randomized trials conducted in patients with non-breast STS. In the current ESMO guidelines, adjuvant chemotherapy is not standard treatment in adult-type STS[14]. The benefit of chemotherapy must be discussed on an individual basis, taking into account the tumor size, histologic subtype and grade. Patients with whom a chemotherapy should be discussed are those with high-risk primary sarcomas (tumor size > 5 cm, high-grade or lymph node positive). Due to their particularly poor prognosis, chemotherapy may be offered to angiosarcoma patients presenting with smaller tumor size as well (e.g., 3-5 cm). In the present case report, tumor board decided against adjuvant chemotherapy for well differentiated small (< 3 cm) liposarcoma. Another systemic option typically used in breast cancer, the endocrine therapy, is not recommended in breast sarcoma due to the lack of efficacy since these tumors tend to be hormone receptor negative. Regarding adjuvant options, one should keep in mind that neither radiotherapy nor chemotherapy can compensate for inadequate surgery, and re-excision to obtain clear margins should be pursued whenever possible. Surgical treatment of breast sarcoma should be carried out in centers specialized in oncological breast surgery[14].
Liposarcoma of the breast arising within a malignant PT is a rare neoplasm and may mimic breast cancer on clinical and radiological examination. Malignant stroma may be present in only part of the tumor, so thorough sampling is essential. Surgery is a potentially curative modality; the role of adjuvant chemo- and radiotherapy remains yet to be clarified.
An 51-year-old female presented with an asymptomatic breast mass.
Nodular movable mass of 2 cm diameter in the lower inner quadrant of the right brass, the overlying skin unremarkable.
Invasive breast carcinoma.
Mammography: suspicious round lesion with smooth margins measuring 2.6 cm in the lower inner quadrant (BI-RADS 5). Breast ultrasound: irregular structure of complex echogenicity measuring 2.4 cm × 2.0 cm × 1.6 cm (BI-RADS 5), axillary lymph nodes unremarkable.
Core biopsy revealed a biphasic tumor of the phyllodes type with suspicious stroma. Lumpectomy showed a malignant phyllodes tumor (PT) with a specific heterologous component identified as well differentiated liposarcoma.
The patient was treated by a lumpectomy and subsequent wide excision.
This case report describes a rare malignant tumor and emphasizes the importance of thorough histopathological workup in case of PT with suspicious heterologous component.
This is a well-written manuscript. It defines a rare case of liposarcoma arising from PT of the breast.
P- Reviewer: Gromov P, Higa GM, Houvenaeghel G, Kanat O, Vu HN S- Editor: Ji FF L- Editor: A E- Editor: Jiao XK
| 1. | World Cancer Report 2014. World Health Organisation. Edited by Bernard W Stewart and Christopher P Wild. Available from: http://www.iarc.fr/en/publications/books/wcr/. |
| 2. | Wibmer C, Leithner A, Zielonke N, Sperl M, Windhager R. Increasing incidence rates of soft tissue sarcomas? A population-based epidemiologic study and literature review. Ann Oncol. 2010;21:1106-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 162] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 3. | Fletcher CDM, Bridge JA, Hogendoorn P, Mertens F. WHO Classification of Tumours of Soft Tissue and Bone, vol. 5, 4 edn. IARC, 2013. . |
| 4. | Lucas DR, Nascimento AG, Sanjay BK, Rock MG. Well-differentiated liposarcoma. The Mayo Clinic experience with 58 cases. Am J Clin Pathol. 1994;102:677-683. [PubMed] |
| 5. | Adem C, Reynolds C, Ingle JN, Nascimento AG. Primary breast sarcoma: clinicopathologic series from the Mayo Clinic and review of the literature. Br J Cancer. 2004;91:237-241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 214] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 6. | Callery CD, Rosen PP, Kinne DW. Sarcoma of the breast. A study of 32 patients with reappraisal of classification and therapy. Ann Surg. 1985;201:527-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 121] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Barnes L, Pietruszka M. Sarcomas of the breast: a clinicopathologic analysis of ten cases. Cancer. 1977;40:1577-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Zelek L, Llombart-Cussac A, Terrier P, Pivot X, Guinebretiere JM, Le Pechoux C, Tursz T, Rochard F, Spielmann M, Le Cesne A. Prognostic factors in primary breast sarcomas: a series of patients with long-term follow-up. J Clin Oncol. 2003;21:2583-2588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 137] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | Johnstone PA, Pierce LJ, Merino MJ, Yang JC, Epstein AH, DeLaney TF. Primary soft tissue sarcomas of the breast: local-regional control with post-operative radiotherapy. Int J Radiat Oncol Biol Phys. 1993;27:671-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Gutman H, Pollock RE, Ross MI, Benjamin RS, Johnston DA, Janjan NA, Romsdahl MM. Sarcoma of the breast: implications for extent of therapy. The M. D. Anderson experience. Surgery. 1994;116:505-509. [PubMed] |
| 11. | Powell CM, Rosen PP. Adipose differentiation in cystosarcoma phyllodes. A study of 14 cases. Am J Surg Pathol. 1994;18:720-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Crago AM, Singer S. Clinical and molecular approaches to well differentiated and dedifferentiated liposarcoma. Curr Opin Oncol. 2011;23:373-378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 196] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 13. | Austin RM, Dupree WB. Liposarcoma of the breast: a clinicopathologic study of 20 cases. Hum Pathol. 1986;17:906-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | ESMO/EuropeanPVESarcomaPVENetwork Working Group. Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25 Suppl 3:iii102-iii112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 379] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 15. | Scow JS, Reynolds CA, Degnim AC, Petersen IA, Jakub JW, Boughey JC. Primary and secondary angiosarcoma of the breast: the Mayo Clinic experience. J Surg Oncol. 2010;101:401-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 16. | Monroe AT, Feigenberg SJ, Mendenhall NP. Angiosarcoma after breast-conserving therapy. Cancer. 2003;97:1832-1840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 144] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | Pandey M, Mathew A, Abraham EK, Rajan B. Primary sarcoma of the breast. J Surg Oncol. 2004;87:121-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | North JH, McPhee M, Arredondo M, Edge SB. Sarcoma of the breast: implications of the extent of local therapy. Am Surg. 1998;64:1059-1061. [PubMed] |
| 19. | McGowan TS, Cummings BJ, O’Sullivan B, Catton CN, Miller N, Panzarella T. An analysis of 78 breast sarcoma patients without distant metastases at presentation. Int J Radiat Oncol Biol Phys. 2000;46:383-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 112] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Barrow BJ, Janjan NA, Gutman H, Benjamin RS, Allen P, Romsdahl MM, Ross MI, Pollock RE. Role of radiotherapy in sarcoma of the breast--a retrospective review of the M.D. Anderson experience. Radiother Oncol. 1999;52:173-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 88] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Shabahang M, Franceschi D, Sundaram M, Castillo MH, Moffat FL, Frank DS, Rosenberg ER, Bullock KE, Livingstone AS. Surgical management of primary breast sarcoma. Am Surg. 2002;68:673-677; discussion 677. [PubMed] |
| 22. | Bousquet G, Confavreux C, Magné N, de Lara CT, Poortmans P, Senkus E, de Lafontan B, Bolla M, Largillier R, Lagneau E. Outcome and prognostic factors in breast sarcoma: a multicenter study from the rare cancer network. Radiother Oncol. 2007;85:355-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 23. | Pollard SG, Marks PV, Temple LN, Thompson HH. Breast sarcoma. A clinicopathologic review of 25 cases. Cancer. 1990;66:941-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |